Simple Summary
Hylidae is a rich family of Anura that is widely distributed across the world. Previous studies have shown that the Hyla and Dryophytos genera in the Hylidae used to belong to the same genus. The Eurasian species (including Hyla sanchiangensis) originated in North America and spread through the Beringian Land Bridge to Asia during the last ice age. Adaptation to low temperatures may require more energy expenditure. Mitochondria are the center of energy metabolism and, through oxidative phosphorylation, provide most of the ATP energy for the physiological and biochemical activities of the body. The mitogenome was used to investigate whether Hyla and Dryophytos genera are subject to positive selection. In addition, as a unique species in China, the distinction between Hyla sanchiangensis (Anura: Hylidae) and two different sites was also compared and analyzed in this paper.
Abstract
Hyla sanchiangensis (Anura: Hylidae) is endemic to China and is distributed across Anhui, Zhejiang, Fujian, Guangdong, Guangxi, Hunan, and Guizhou provinces. The mitogenomes of H. sanchiangensis from two different sites (Jinxiu, Guangxi, and Wencheng, Zhejiang) were sequenced. Phylogenetic analyses were conducted, including 38 mitogenomes of Hylidae from the NCBI database, and assessed the phylogenetic relationship of H. sanchiangensis within the analyzed dataset. Two mitogenomes of H. sanchiangensis showed the typical mitochondrial gene arrangement with 13 protein-coding genes (PCGs), two ribosomal RNA genes (12S rRNA and 16S rRNA), 22 transfer RNA (tRNA) genes, and one non-coding control region (D-loop). The lengths of the 12S rRNA and 16S rRNA genes from both samples (Jinxiu and Wencheng) were 933 bp and 1604 bp, respectively. The genetic distance (p-distance transformed into percent) on the basis of the mitogenomes (excluding the control region) of the two samples was calculated as 4.4%. Hyla sanchiangensis showed a close phylogenetic relationship with the clade of (H. annectans + H. tsinlingensis), which was supported by ML and BI analyses. In the branch-site model, five positive selection sites were found in the clade of Hyla and Dryophytes: Cytb protein (at position 316), ND3 protein (at position 85), and ND5 protein (at position 400) have one site, respectively, and two sites in ND4 protein (at positions 47 and 200). Based on the results, we hypothesized that the positive selection of Hyla and Dryophytes was due to their experience of cold stress in historical events, but more evidence is needed to support this conclusion.
1. Introduction
Amphibians are regarded as useful models for researching the elements that influence patterns of genetic differentiation and diversity. Firstly, they show relatively stable genetic traits as compared to other animals [1]. Secondly, they are very sensitive to climate conditions, and researchers can compare past and present climate changes via changes in various indicators of amphibians [2]. As a result, it is worthwhile to explore the relationship between amphibians and the environment. Hylidae is one of the largest families of frogs, currently comprising over a thousand species from around the world. There are 35 officially named species in the genus Hyla, 16 of which are found in Northern and Central America and 19 in Eurasia, including eight species found in China (http://www.amphibiachina.org/, accessed on 26 August 2022) [3]. The genus Hyla was shown by Li et al. [4] to have originated in North America and subsequently spread to China via Beringia during the Middle Eocene and Early Oligocene [5]. The genus Dryophytes and Hyla diverged from other Hylidae about 22.6 million years ago [6]. For both genera, the genus Hyla was generally thought to be distributed in Asia, and the genus Dryophytes was found mainly in North America [7]. The genera Dryophytes and Hyla are species of North American origin that were cold adapted during early historical events [4]. Therefore, it is considered to be better adapted to low temperatures than other species of Hylidae, so it may have undergone positive selection.
Hyla sanchiangensis [8] (Anura: Hylidae) was first described in 1929 from Wuyi Mountain, Fujian, China. The species is endemic to China and is distributed across Anhui, Zhejiang, Jiangxi, Hubei, Fujian, Guangdong, Guangxi, Hunan, and Guizhou provinces (http://www.amphibiachina.org/, accessed on 26 August 2022) [3]. All these distribution areas are discontinuous in China. These frogs inhabit paddy fields or can be found around fences. As a result of deforestation and habitat degradation, chemical pollution, and disease, H. sanchiangensis has declined substantially in Zhejiang province and is listed as a key species of wildlife under protection in Zhejiang province. Through the morphological analysis of Hyla sanchiangensis from Zhejiang and Guangxi, it was found that there were some differences in its morphological characteristics. Some studies have indicated that anuran larval development and variation in environmental factors can result in morphological changes that lead to increased intraspecific variation [9,10,11,12,13,14,15]. This means that a single species living in different areas may show significant differences.
Mitogenomes are currently used as an efficient tool for species identification and the analysis of phylogenetic relationships [16,17]. The length of frog mitogenomes is about 15–23 kb [18,19,20,21] and encodes 22 transfer RNAs (tRNAs), two ribosomal RNAs (12S and 16S RNAs), 13 protein-coding genes (PCGs), and the control region (CR) or D-loop region. As a result of their relatively conserved gene content, rapid evolutionary rate, small size, maternal inheritance, and limited recombination, mitogenomes have been widely used for studies of conservation biology, species delimitation, and the evolution of the Anura [16,22,23]. Using the mitogenome to study phylogenetic relationships is an efficient tool to analyze problems in taxonomy. In addition, further studies have found that mtDNA has evolved a variety of unique properties that enable this genome to be used as a cellular sentinel for genotoxic stress. [24]. Based on all of these factors, samples of H. sanchiangensis were collected from different sites to conduct a basal study and evaluate the specific differences in the characteristics of H. sanchiangensis from two different sites. These data allowed us to further explore the phylogenetic relationship of H. sanchiangensis among Hylidae.
Despite numerous studies, the phylogenetic relationships among Hylidae are still controversial, and full confirmation of the monophyly of the genus Hyla and the genus Dryophytes using mitogenomes still needs full confirmation. Dryophytes were long treated as a subgenus of Hyla [25], but Duellman et al. [6] suggested that the species belonging to the genus Hyla should now be divided into two genera: Hyla and Dryophytes; subsequent research by Zhang et al. also supported this view [26]. Therefore, it is necessary to further study whether Dryophytes and Hyla are monophyletic and show the relationships between these two clades. Li et al. [4] supported H. annectans and H. tsinlingensis as clustering into one clade. However, Lee et al. [27] found that H. tsinlingensis and H. suweoninsis were sister clades.
Despite the widespread belief that mitogenomes are in a neutral or nearly neutral state of selection [28], there is considerable evidence showing that positive selection acts on mitochondrial genes involved in environmental adaptation, which suggests that mitochondrial genes can be used to explore the relationship between environmental conditions and natural selection [17,29,30]. Mitogenomes of H. sanchiangensis from two different sites have been sequenced and compared with the mitogenome of a third site of H. sanchiangensis (MT561180), as recently reported from Lishui (LS), Zhejiang [31]. Hence, the current study generated and analyzed the mitogenome of H. sanchiangensis and conducted phylogenetic analyses of Hyla and Dryophytes to explore the relationships among these groups. In addition, positive selection sites in the Hyla and Dryophytes clades were analyzed to determine whether the clades of Hyla and Dryophytes were positively selected for cold origin in the mitogenome.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
Samples of H. sanchiangensis were collected from Jinxiu, Guangxi, China (JX) (24.14° N, 110.18° E) and Wencheng, Zhejiang, China (WC) (27.08° N, 120.08° E). According to the morphology of the frogs, they were preliminarily identified as H. sanchiangensis (Anura: Hylidae). Tissue samples were taken via toe clips and preserved at −40 °C in 100% ethanol for subsequent DNA extraction. Total genomic DNA was extracted using an Ezup Column Animal Genomic DNA Purification Kit (Sangon Biotech Company, Shanghai, China), following the manufacturer’s handbook.
2.2. Primer Design, PCR Amplification, and Sequencing
PCR amplification of mitogenomes by the polymerase chain reaction (PCR) used universal primers as described by Zhang et al. [32]. Sequence proofreading followed, and specific primers were designed using Primer Premier 5.0 (Primer Biosoft International) based on amplified fragments (Table S2). The reaction volume for PCR amplification was 50 μL. PCR amplification used standard methods as in Cai et al. [33]. The PCR products were identified by 1% agarose gel electrophoresis, and all PCR-purified products were sequenced at Sangon Biotech Company (Shanghai, China).
2.3. Mitogenome Annotation and Sequence Analyses
DNASTAR v.6.0 [34] was used to assemble the results from the two samples and obtain whole mitogenome sequences. MITOS (http://mitos.bioinf.uni-leipzig.de/index.py, accessed on 26 August 2022) [35] was used to determine all tRNA genes. We identified and annotated 12S rRNA, 16S rRNA, and the 13 protein-coding genes (PCGs) by Mega 11.0 [36] and compared the homology of genes [37]. We used tRNAscan-SE1.21 software [38] (http://lowelab.ucsc.edu/tRNAscan-SE/, accessed on 26 August 2022) to predict all the cloverleaf secondary structures of the tRNA genes. In addition, the secondary structure of the light-strand origin region was built by RNAalifold with default parameters (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAalifold.cgi, accessed on 26 August 2022) [39,40,41]. Mega 11.0 [36] was used to translate the PCG sequences into amino acids according to the vertebrate mitogenome genetic code and codon usage. The AT content and relative synonymous codon usage (RSCU) of the two newly sequenced mitogenomes were also calculated. Finally, AT-skew = (A − T)/(A + T) and GC-skew = (G − C)/(G + C) were used to calculate composition skewness [42].
2.4. Genetic Distance
The genetic distance of the two mitogenomes was determined using Mega 11.0 [36] and the p-distance approach. To further assess the genetic distance between Hylidae species, we downloaded the RAD-Seq reads from Borzée et al. [43] for three Hylidae species (Dryophytes suweonensis, Dryophytes immaculatus, and Dryophytes flaviventris) as shown in Table S4 and assembled the mitochondrial genes from this whole genome data using GetOrganelle 1.7.1 [44,45,46,47,48].
2.5. Phylogenetic Analyses
We downloaded 38 mitogenomes from the NCBI (a matrix of publicly available mitogenomes of the family Hylidae) to investigate the evolutionary connections of the two newly sequenced mitogenomes of H. sanchiangensis. Phylogenetic analyses were performed with 40 species from the Hylidae according to the methods of Zhou et al. [49], Yu et al. [50,51], and Zhang et al. [32]. These 40 species are shown with the GenBank accession numbers in Table 1. The mitogenomes of Dendrobates auratus, Mannophryne trinitatis, Odontophrynus occidentalis, and Rhinoderma darwinii were used for outgroup rooting [32] (Table 1). Clustal W in Mega 11.0 [36] was used to align the 13 protein-coding genes and translate the gene sequences into amino acids. Conserved regions were selected using Gblock 0.91b [52] with default settings. A dataset of 10,548 nucleotides and 3516 amino acids was obtained after final alignment. The saturation of the first, second, and third codons was analyzed using DAMBE [53]. The third codon positions were not saturated, and the datasets could be used to construct a phylogenetic tree (the results were shown in Table S1). The optimal partitions and best-fitting model of evolution were selected using PartitionFinder 1.1.1 [46] using the Bayesian information criterion (BIC) [54] (Table S3). RAxML allows only one rate heterogeneity model in partition analysis. The GTR + I + G model from RAxML 8.2.0 [55] was selected for maximum likelihood (ML) analysis, with each node branch supported for evaluation under 1000 ultra-fast replications. Partitioning results were used via MrBayes version 3.2 [56] for Bayesian inference (BI) analysis and ran four chains for 10 million generations, sampling every 1000 generations. The first 25% of runs were discarded as burn-in according to convergence (<0.01). The rest were used to build the BI phylogenetic tree.

Table 1.
Genus names, GenBank accession numbers, and their references for the species used to construct the phylogenetic tree.
2.6. Detecting Selective Pressure
The selection pressure of the mitogenomes was analyzed by the EasyCodeML program [67]. We use ω ratio to indicate the natural selection of all 13 PCGs. The ω ratio (dN/dS) is the rate of nonsynonymous (dN) versus synonymous (dS) substitution; ω = 1 denotes neutral mutations, ω < 1 denotes negative selection, and ω > 1 denotes positive selection [68].
The genera Hyla and Dryophytes were chosen as the foreground branches, whereas other frog species excluding outgroups were chosen as the background branches in order to investigate whether the clade of Hyla and Dryophytes is subject to positive selection. The branch model, branch-site model, and clade model were the three analysis methods utilized to investigate the association between mitochondrial genes and adaptive evolution. In order to obtain the difference between the foreground branch and the background branch, the branch model compared the results obtained by the one-ratio model (M0) and the two-ratio model, respectively. Additionally, the clade model was utilized to examine them in order to analyze numerous clades at once [58]. Finally, to understand the effect of forward selection on some sites in the foreground branch, the A model and the A null model in the branch-site model were tested. These models were also evaluated using LRT, and the posterior probability of positive selection sites was assessed using Bayesian Empirical Bayes (BEB). UniProt was used to collect the structural and functional details of positive selection genes [69]. Furthermore, SWISS-MODEL Workspace was used to construct the three-dimensional structure (3D) of the amino acid positive selection of the corresponding protein [70].
3. Results
3.1. Mitogenome Organization and Structure
In the present study, two mitogenomes of H. sanchiangensis were sequenced from Jinxiu (JX) and Wencheng (WC). The sequences were submitted to GenBank with accession numbers MK388867 and MK388868, respectively. Both are incomplete mitogenomes, missing a portion of the control region. The mitogenomes of H. sanchiangensis (WC) were 15,977 bp long, and those of H. sanchiangensis (JX) were 15,675 bp long. Both mitogenomes included 13 protein-coding genes (PCGs), two rRNAs (12S and 16S rRNAs), 22 tRNAs, and one control region (partially sequenced) (Figure 1 and Table 2). This arrangement is the same as that of other species of the genus Hyla.
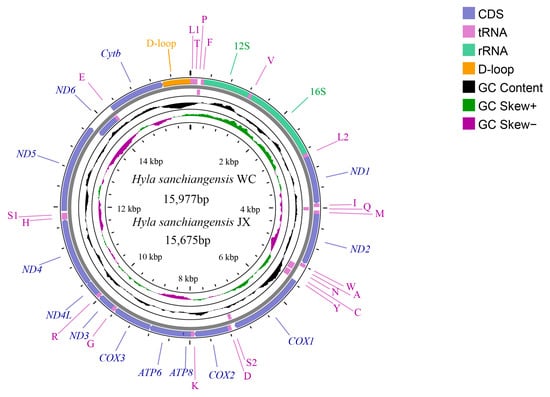
Figure 1.
Mitogenome map of H. sanchiangensis JX and H. sanchiangensis LS. The outermost two circles show the gene map (PCGs, rRNAs, tRNAs, and control region) and genes; the outer circle is encoded by the positive strand, the second circle is encoded by the negative strand, and the tRNAs are all represented by abbreviations. The third circle represents GC content, and the green and violet parts of the innermost circle are GC skew. The GC content and GC skew are calculated using the deviation of the mean value of the whole series.

Table 2.
Locations of features in the mtDNA of H. sanchiangensis JX and H. sanchiangensis WC.
The mitogenome compositions of H. sanchiangensis from Wencheng and Jinxiu are shown in Table 3, along with the mitogenome of another H. sanchiangensis sample reported recently (Genbank #MT561180). The AT% of the nucleotide composition of H. sanchiangensis from Wencheng was higher than Jinxiu, and both of them were higher than the H. sanchiangensis mitogenome of the individual from Lishui [31] (Table 3). The mitogenome of H. sanchiangensis from Lishui exhibited a negative GC-skew (−0.275) and a slightly positive AT-skew (0.001) [31], which was very similar to the genome from Jinxiu.

Table 3.
The mitogenome composition of H. sanchiangensis from Wencheng, Jinxiu, and Lishui.
3.2. Protein-Coding Genes and Codon Usages
The length of PCGs from Wencheng and Jinxiu were both 11,331 bp, whereas the length of PCGs from Lishui was 11,316 bp [31]. All PCGs, with the exception of ND6, were located on the heavy strand (H-strand). In the mitogenome of H. sanchiangensis, the start codons of most genes were ATG, but four genes had modified start codons. Complete stop codons were found in most genes. For three genes (COX2, ND1, and ND3), an incomplete stop codon (T) was identified (Table 2). All of the PCGs had the same start and termination codons in the three mitogenomes of H. sanchiangensis analyzed here.
The nucleotide AT% composition of the PCGs for both newly sequenced H. sanchiangensis mitogenomes (Wencheng and Jinxiu) was 59.6% and 59.8%, respectively. We calculated the relative synonymous codon usage (RSCU) of the 13 protein-coding genes from the two samples, excluding stop codons. This data is summarized in Figure 2, along with the mitogenome of H. sanchiangensis from Lishui. The RSCU showed that codons that had base A or T in the third position were consistently overused as compared to the other synonymous codons. The frequency of amino acid usage was almost the same among the three analyzed mitogenomes (Figure 2). For the mitogenomes of Wencheng and Jinxiu, Leu (CUR), Ala (GCR), and Ile (AUR) (>300) were the most frequently encoded amino acids, and Cys (UGR) (<45) was the least frequently used amino acid. This was similar to the mitogenome of H. sanchiangensis from Lishu [31]. Fourteen amino acids had two kinds of codons, whereas Ala, Arg, Leu, Ser, Val, Pro, Thr, and Gly had four kinds of codons. These results indicated that the RSCU of the three mitogenomes was highly conserved.
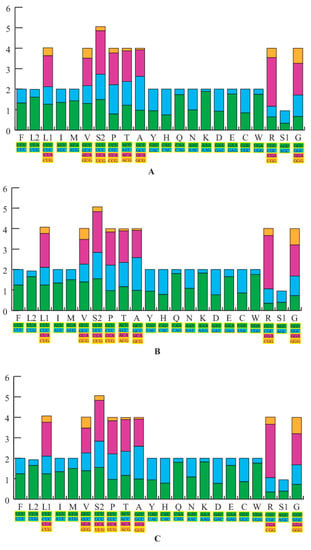
Figure 2.
The relative synonymous codon usage (RSCU) in three H. sanchiangensis mitogenomes. The RSCU of the mitogenome is characterized for the individuals of H. sanchiangensis from Wencheng (A), Jinxiu (B), and Lishui (C). The X-axis shows all the codons used and different combinations of synonymous codons, with different codons represented by different colors, and the Y-axis lists the RSCU values.
3.3. Ribosomal and Transfer RNAs
The two mitogenomes of H. sanchiangensis from Wencheng and Jinxiu contained two rRNAs and 22 tRNA genes. The positions of the 12S rRNA and 16S rRNA were the same as the mitogenome of H. sanchiangensis from Lishui [31], and their lengths were highly consistent. In all three sites of H. sanchiangensis, it was found that the content of A was higher than that of T, and the content of C was higher than that of G.
A total of 22 tRNAs that had a similar structure were detected in the newly sequenced mitochondrial genomes, and among them, individuals collected at Jinxiu and Wencheng had the same total tRNA length of 1533 bp. Excluding trnA, trnC, trnP, trnQ, trnN, trnY, trnS (UCN), and trnE, all other tRNAs were on the H-strand. The content of A + T was remarkably higher than that of G + C in all three samples from H. sanchiangensis.
Based on Figures S1 and S2, the secondary structures of the 22 tRNAs were found to be mainly typical clover structures, and all tRNA genes had equivalent lengths except trnK, which was 73 bp and 72 bp in the mitogenomes of the individuals collected at Jinxiu and Wencheng, respectively. In the mitogenome of the individual collected at Wencheng (Figure S1), trnC did not form a dihydrouridine (DHU) loop and trnS (AGN) had lost the DHU arm and could not form a complete cloverleaf secondary structure. In addition, some tRNA genes had unmatched base pairs, such as A-C in trnI, U-U in trnN, and A-A in trnD. In the mitogenome of the individual collected at Jinxiu (Figure S2), the cloverleaf secondary structure was very similar to that of H. sanchiangensis from Wencheng. The trnS (AGN), which lost the DHU arm, was a typical feature and had an unusual function. In addition, the trnC also lost the DHU loop. Furthermore, the secondary structure contained non-Watson-Crick base pairs in the structure of stems, such as A-A in trnL (UUN), U-U in trnN, and A-C in trnI.
3.4. Intergenic Regions and L-Strand Origin of Replication
There were eight non-coding regions (from 1 to 36 bp) and nine overlapping regions (from 1 to 25 bp) in the mitogenome of H. sanchiangensis from Wencheng and Jinxiu (Table 3), as was also described for the mitogenome of H. sanchiangensis from Lishui [31]. The longest overlapping region (25 bp) occurred between ATP8 and ATP6. The longest spacer region was 36 bp and occurred between trnS1 and ND5 in all three mitogenomes.
The most remarkable genomic feature observed in the mitogenomes of H. sanchiangensis was the presence of a distinctive insertion between trnN and trnC called the L-strand origin of replication (OL), which plays an important role in replication. The OL between trnN and trnC was about thirty bases and showed a similar secondary structure (stem-loop structure) (Figure 3). The structure of the OL was highly similar and symmetrical in the mitogenomes of the individuals collected at Jinxiu and Wencheng, and the mitogenome of the individual collected at Jinxiu only lacked an A in the circle of the stem-loop. The structures of the OL in the mitogenomes of the individuals from Wencheng and Lishui were exactly the same (Figure 3). Furthermore, the replication initiation region of the light strand (OL) in the mitogenome of the individual collected in Wencheng was one base longer than that of the mitogenome of the individual collected in Jinxiu. There were nine base pairs in the stem loop, including six pairs of C-G and three pairs of U-A.
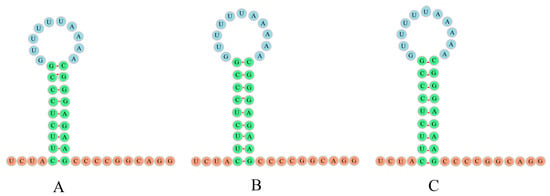
Figure 3.
The secondary structures for L-strand origin of replication (OL) for the individuals of H. sanchiangensis from Jinxiu (A), Jinxiu (B), and Lishui (C).
3.5. Genetic Distance
The amino acid alignment was the backbone to obtain the alignment of 10,983 nucleotide sites, with 5328 variable sites and 4061 parsimonious informative sites. The overall mitogenome genetic distance (excluding the control region) (Table S5) between individuals of H. sanchiangensis collected at Wencheng (MK388867) and Jinxiu (MK388868) was 4.4%, which was the same as the genetic distance observed between the mitogenomes of the individuals of H. sanchiangensis collected at Jinxiu (MK388868) and Lishui (MT561180). However, the mitogenome genetic distance between the individual H. sanchiangensis collected at Lishui (MT561180) and Wencheng (MK388867) was only 0.2%. On this basis, we concluded that the lower genetic distance between H. sanchiangensis collected at Lishui (MT561180) and Wencheng (MK388867) was due to their close geographical locations. Two parts of the COX1 gene from the known species of Hylaidae were used to calculate genetic distance (Tables S6 and S7), and we found that the highest genetic distance between Dryophytes suweonensis and Dryophytes flaviventris was 1.9%.
3.6. Phylogeny of Hylidae
The results of the two phylogenetic analyses (ML and BI) yielded a similar topological structure (Figure 4), and BI was used as the main one. The phylogenetic analyses supported the monophyly of Boana, Hyla, and Dryophytes. Two clades (clades A and B) were formed within Hylaidae. The clade A of (Nyctimystes + (Phyllomedusa + Pithecopus)) and the clade B of ((Bokermannohyla + Boana) + (Osteocephalus + (Pseudis + (Hyla + Dryophytes)))) were well supported. The results showed that Hyla tsinlingensis and H. annectans have sister-group relationships. Hyla sanchiangensis Wencheng (WC) was a sister clade to H. sanchiangensis Lishui (LS), and then the clade of (H. sanchiangensis WC + H. sanchiangensis LS) is a sister clade of H. sanchiangensis Mingxi (MX). Finally, this clade got together with H. sanchiangensis Jinxiu (JX).
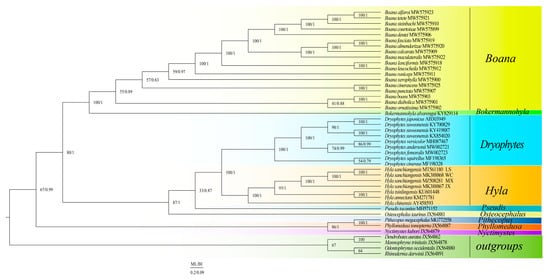
Figure 4.
BI and ML analyses were used to predict the phylogenetic relationships of the Hylidae based on the nucleotide data set encoded by 13 proteins. Species name information and GenBank number are marked in the figure. Species of different genera are distinguished by different colors, and outgroups are represented by one color.
3.7. Detecting Selective Pressure
The BI tree, which has the same topology as the ML tree, was used to analyze the selection pressure of the 13 PCGs. In the branch model, there was no role for selection. In this study, Hyla and Dryophytes were used as foreground clades, respectively, and the rest as background clades for selection pressure analyses. The results showed that in the branch-site model, forward selection occurs only when Hyla and Dryophytes act as foreground branches. In the branch-site model, model A vs. model A null was significant (p < 0.05), with five amino acid selection sites and BEB values >0.95 (amino acid residue 1595 in Cytb, 2308 in ND3, 2479, and 2632 in ND4, 3272 in ND5) (Table 4). The distinctive characteristics of these positive selection sites from the Hyla and Dryophytes clades were investigated to ascertain their functional importance. The results from other models are shown in Table S8. Table 5 lists the characteristics and descriptions of positive selection sites found in the mitochondrial PCGs of Hylidae species. It can be seen that four positive selection sites were located in the protein transmembrane domain: two were described as Proton_antipo_M, a second as Oxidored_q5_N, and the other as CYTB_CTER. All this evidence suggested that some amino acid sites in the clade of Hyla and Dryophytes might have been subjected to positive selection.

Table 4.
Adaptive analysis of the branch site model (BSM), in which Hyla and Dryophytes were used as foreground branches and other Hylidae species were used as background branches.

Table 5.
Characterization and description of positive selection sites of Hylidae species and comparison of amino acid sites in foreground and background branches.
4. Discussion
4.1. Mitogenome Structure
The gene arrangements were the same as the mitogenome patterns of other Hyla and Dryophytes species [26,27,31,59,60,61,62,63,64,65]. Comparing the two mitogenome sequences produced in our study to a previous mitogenome of H. sanchiangensis from Lishui (MT561180) [31], subtle differences were found. Some PCGs (e.g., COX2, ND1, and ND3) had an incomplete stop codon, as already identified in other frog species [27,31]. The secondary structure of tRNAs in the two mitogenome sequences contained non-Waston-Crick base pairs in the structure of stems, which was similar to the mitogenome of the individual collected in Lishui [31]. There were many overlapping regions in the three mitogenomes of H. sanchiangensis, with the longest overlapping region (25 bp) being between ATP8 and ATP6, which was also found in all mitogenome sequences of Hyla species [31,62,63,64,65], whereas only a 10 bp overlap region was found in the Dryophytes sequences [26,27,59,60,61]. This further illustrated the differences between the genera Hyla and Dryophytes and also supported the result expounded by Zhang et al. [26]. In addition, the most remarkable genomic feature was OL, which played an important role in replication when this region was a single chain [71].
4.2. Genetic Distance and Phylogeny of Hylidae
According to the results of the current study, the genetic distance between H. sanchiangensis individuals collected in Jinxiu and Wencheng or between Jinxiu and Lishui individuals was found to be 4.4%, which was much higher than the genetic distance reported between the species of Dryophytes [43]. This suggests that the intraspecific genetic distance of H. sanchiangensis was higher than the interspecific distance of Dryophytes species. Therefore, it was speculated that cryptic species may exist in H. sanchiangensis.
The BI and ML trees constructed from the mitogenomes showed similar topological structures. The clade formed by the individuals of H. sanchiangensis collected at Wencheng and Lishui clustered together and formed a sister clade to the H. sanchiangensis individual collected at Jinxiu and then formed together with H. sanchiangensis collected at Mingxi (Figure 4). This was the same as the results obtained from the mitogenome genetic distance data. The clade of all H. sanchiangensis forms the sister clade with (H. annectans + H. tsinlingensis), as already identified by Yan et al. [72], while the monophyly of Hyla and Dryophytes was still well supported in this study. Previously, Dryophytes had been treated as a subgenus of Hyla [25]. However, our data support the division of these frogs into two clades, Dryophytes and Hyla, according to their genetic distance and phylogenetic relationships, as well as the results of Huang et al. [73]. The overlap region (25 bp or 10 bp) between ATP8 and ATP6 could be used as a molecular characteristic to distinguish the two genera. Due to limited molecular data, our study provided a revised phylogenetic relationship for Hylidae and further promoted the continuing development of mitochondrial genomics.
4.3. Detecting Selective Pressure
Positive selection was found when Hyla and Dryophytes were used as foreground branches in the branch-site model. It was determined that Hyla and Dryophytes were a single genus in the past, originating from the Eocene to early Oligocene in North America, and the Eurasian species originated in North America and spread through the Beringian Land Bridge [4,5,25,74]. The hypothesis was that the frogs of these two genera were better adapted to low temperatures due to cold adaptation in low temperature regions during early historical events. However, this was just a hypothesis we put forward, and whether it is a fact needs to be proven by further studies.
Four mitochondrial PCGs were positively selected, showing two positive selection sites on ND4 and one positive selection site on each of the ND3, ND5, and Cytb genes. In addition, according to our branch-site model analysis, ND3, ND4, and ND5 proteins belonged to mitochondrial complex I, and Cytb proteins belonged to mitochondrial complex III; therefore, mitochondrial complexes I and III were the main protein complexes under selective pressure. To explore the function of these positive selection sites, all the positive selected amino acid sites are highlighted in Figure S3, showing the structure of the protein. Mitochondrial oxidative phosphorylation is an important life activity in organisms, providing energy for most cellular functions [75]. Mitochondrial Complex I (CI), the main entry point of nicotinamide adenine dinucleotide (NADH) electrons into the respiratory chain, is a large protein complex that is closely involved in energy metabolism [76]. In Complex I, the structural domain of Oxidored_q5_N was defined as NADH-ubiquinone oxidoreductase, which is the main entry point of electrons into the respiratory chain and closely related to energy metabolism [77]. Therefore, the mutation of ND series subunits could have a great impact on the transmission efficiency of mitochondria as well as on energy transfer and metabolic functions [78,79]. As for proton_antipo_M, containing Mnh1 and Mnh2, this family forms part of complex I, which is closely related to the translocation reaction of protons across the membrane and also affects energy transfer [80,81]. Mitochondrial Complex III (CIII) is a major multisubunit membrane-binding enzyme that is closely involved in ATP synthesis and respiratory energy transduction in many organisms [82]. Of the three catalytic subunits of CIII, only Cytb is a complete protein [83]. So Cytb mutations affect energy transduction in the cellular mitochondrial respiratory chain.
Due to the fact that positive selection only affects a small number of amino acid sites over a short evolutionary period, the signal of positive selection is frequently overwhelmed by successive negative selection at the majority of sites in a gene sequence, leading to the conclusion that there is no positive selection in the foreground branch when the branch model is analyzed [84]. However, the branch-site model is more likely to find that a foreground branch is subject to positive selection because it allows the amino acid site selection pressure to change [84,85]. Hence, the reason why positive selection sites were found only in the branch-site model in this study can also be explained by the above content.
Previous studies have also found that organisms exposed to low temperature stress may have engaged in positive selection of mitochondrial genes [86,87,88,89]. Sun et al. found that Tetranychus truncatus showed positive selection for ND4 in the process of adapting to low temperatures [90]. In addition, Xu et al. identified more positive selection sites under low temperature stress, using high-latitude Canadian Heptageniidae as foreground branches [30]. Similarly, our current study also recognized the influence of temperature on mitochondrial energy metabolism, but the influence was due more to cold adaptation in the past historical events of Hyla and Dryophytes. However, due to limited samples, the cause of this phenomenon still needs further analysis.
5. Conclusions
In this study, mitogenomes of Hyla sanchiangensis (except part of the D-loop) from two different sites were obtained. In addition, genetic distances and phylogenetic relationships between two different sites of Hyla sanchiangensis were also identified. This study also acknowledged the monophyly of Hyla and considered Hyla and Dryophytes to be sister groups. In the branch-site model, we found five positive selection sites with significant differences. According to the results, the cold adaptation experienced by Hyla and Dryophytes at historical events may be the cause of the positive selection of mitogenomes, possibly due to the need for more energy to adapt to low temperatures, but this conclusion needs to be confirmed by further research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13101593/s1, Figure S1. Secondary structures for 22 transfer RNAs in the mitogenome of the individual of H. sanchiangensis from Wencheng. (A) Leu (CUN), (B) Thr, (C) Pro, (D) Phe, (E) Val, (F) Leu (UUR), (G) Ile, (H) Gln, (I) Met, (J) Trp, (K) Ala, (L) Asn, (M) Cys, (N) Tyr, (O) Ser (UCN), (P) Asp, (Q) Lys, (R) Gly, (S) Arg, (T) His, (U) Ser (AGY), (V) Glu. Figure S2. Secondary structures for 22 transfer RNAs in the mitogenome of the individual of H. sanchiangensis from Jinxiu. (A) Leu (CUN), (B) Thr, (C) Pro, (D) Phe, (E) Val, (F) Leu (UUR), (G) Ile, (H) Gln, (I) Met, (J) Trp, (K) Ala, (L) Asn, (M) Cys, (N) Tyr, (O) Ser (UCN), (P) Asp, (Q) Lys, (R) Gly, (S) Arg, (T) His, (U) Ser (AGY), (V) Glu. Figure S3. The 85 amino acid sites at ND3, the 47 and 200 amino acid sites at ND4, the 400 amino acid sites at ND5, along with the 316 amino acid sites at Cytb. Table S1. The third codon saturation results are Iss < iss.csym, Iss < Iss. cAsym, p < 0.05, indicating that the third codon is unsaturated and the results are credible. Table S2. The codon number and relative synonymous codon usage in mitochondrial protein-coding genes. Table S3. The PartitionFinder program was used to obtain the best partitioning scheme and the best fit model. Table S4. Sequence Read Archive (SRA) of 13 species for calculating the genetic distance. Table S5. Genetic distances of Hyla sanchiangensis from different locations. Table S6. The genetic distance of COI part 1 (216 bp). Table S7. The genetic distance of COI part 2 (301 bp). Table S8. Adaptive analysis of the branch model (BM) and clade model (CM). Reference [91] are cited in the supplementary materials.
Author Contributions
Conceptualization, Y.-H.H., H.-M.H., Y.-P.Z. and D.-N.Y.; data curation, Y.-H.H., H.-M.H., L.W., K.B.S., J.-Y.Z., Y.-P.Z. and D.-N.Y.; formal analysis Y.-H.H., H.-M.H., L.W. and J.-Y.Z.; funding acquisition, J.-Y.Z. and D.-N.Y.; investigation, Y.-H.H., H.-M.H.; methodology, Y.-H.H., L.W., K.B.S., J.-Y.Z. and D.-N.Y.; project administration, Y.-P.Z. and D.-N.Y.; writing—original draft, Y.-H.H., H.-M.H. and L.W.; writing—review and editing, Y.-H.H., H.-M.H., L.W., K.B.S., J.-Y.Z., Y.-P.Z. and D.-N.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 31801963), the Zhejiang Province Natural Science Foundation (LQ16C030001), the College Students’ Innovation and Entrepreneurship Project of Zhejiang Province (202010345R119), and the New Talent Program of Zhejiang Province (2021R404067). The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Institutional Review Board Statement
All experimental designs and animal handling were approved by the Animal Research Ethics Committee of Zhejiang Normal University.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data to support this study are available from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov, accessed on 26 August 2022). The registration numbers are MK388867 and MK388868.
Acknowledgments
The authors are grateful for the contributions to sample collection and data analysis made by Yue Ma, Qing-Ping Chen, Ke-Ke Xu, Yao Tong, Zi-Yi Zhang, Jia-Yin Guan, and Shi-Qi Shen.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zeisset, I.; Beebee, T.J.C. Amphibian phylogeography: A model for understanding historical aspects of species distributions. Heredity 2008, 101, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, F.; Milinkovitch, M.C. Amphibians as indicators of early tertiary “out-of-India” dispersal of vertebrates. Science 2001, 292, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Wang, K. AmphibiaChina: An online database of Chinese Amphibians. Zool. Res. 2016, 37, 57–59. [Google Scholar]
- Li, J.T.; Wang, J.S.; Nian, H.H.; Litvinchuk, S.N.; Wang, J.; Li, Y.; Rao, D.Q.; Klaus, S. Amphibians crossing the Bering Land Bridge: Evidence from holarctic treefrogs (Hyla, Hylidae, Anura). Mol. Phylogenet. Evol. 2015, 87, 80–90. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H.; Moen, D.S.; Smith, S.A.; Reeder, T.W. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: Treefrog trees unearth the roots of high tropical diversity. Am. Nat. 2006, 168, 579–596. [Google Scholar] [CrossRef]
- Duellman, W.E.; Marion, A.B.; Hedges, S.B. Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa 2016, 4104, 1–109. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference, Version 5.4. 2010. Available online: http://research.amnh.org/vz/herpetology/amphibia (accessed on 26 August 2022).
- Pope, C.H. Four new frogs from Fukien Province, China. In American Museum Novitates; No. 352; The American Museum of National History: New York City, NY, USA, 1929. [Google Scholar]
- Kishida, O.; Nishimura, K. Multiple inducible defences against multiple predators in the anuran tadpole. Rana pirica. Evol. Ecol. Res. 2005, 7, 619–631. [Google Scholar]
- Ghioca-Robrecht, D.; Smith, L.; Densmore, L. Ecological correlates of trophic polyphenism in spadefoot tadpoles inhabiting playas. Can. J. Zool. 2009, 87, 229–238. [Google Scholar] [CrossRef]
- Székely, P.; Cogălniceanu, D.; Tudor, M. Effect of habitat drying on the development of the Eastern spadefoot toad (Pelobates syriacus) tadpoles. Amphib. Reptilia 2010, 31, 425–434. [Google Scholar] [CrossRef]
- Ledón-Rettig, C.C.; Pfennig, D.W. Emerging model systems in eco-evo-devo: The environmentally responsive spadefoot toad. Evol. Dev. 2011, 13, 391–400. [Google Scholar] [CrossRef]
- Johnson, J.B.; Saenz, D.; Adams, C.K.; Hibbitts, T.J. Naturally occurring variation in tadpole morphology and performance linked to predator regime. Ecol. Evol. 2015, 5, 2991–3002. [Google Scholar] [CrossRef] [PubMed]
- Van Buskirk, J. Spatially heterogeneous selection in nature favors phenotypic plasticity in anuran larvae. Evolution 2017, 71, 1670–1685. [Google Scholar] [CrossRef]
- Touchon, J.C.; Robertson, J.M. You cannot have it all: Heritability and constraints of predator-induced developmental plasticity in a Neotropical treefrog. Evolution 2018, 72, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, L.P.; Yu, D.N.; Storey, K.B.; Zheng, R.Q. Complete mitochondrial genomes of Nanorana taihangnica and N. yunnanensis (Anura: Dicroglossidae) with novel gene arrangements and phylogenetic relationship of Dicroglossidae. BMC Evol. Biol. 2018, 18, 26. [Google Scholar] [CrossRef]
- Cai, Y.T.; Li, Q.; Zhang, J.Y.; Storey, K.B.; Yu, D.N. Characterization of the mitochondrial genomes of two toads, Anaxyrus americanus (Anura: Bufonidae) and Bufotes pewzowi (Anura: Bufonidae), with phylogenetic and selection pressure analyses. PeerJ 2020, 8, e8901. [Google Scholar] [CrossRef] [PubMed]
- Roe, B.A.; Ma, D.P.; Wilson, R.; Wong, J. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J. Biol. Chem. 1985, 260, 9759–9774. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Wu, X.B.; Wang, Y.Q.; Zhou, K.Y.; Zhu, W.Q.; Nie, J.S.; Wang, C.L. Complete mitochondrial DNA sequence of Chinese alligator, Alligator sinensis, and phylogeny of crocodiles. Chin. Sci. Bull. 2003, 48, 2050–2054. [Google Scholar] [CrossRef]
- Sano, N.; Kurabayashi, A.; Fujii, T.; Yonekawa, H.; Sumida, M. Complete nucleotide sequence and gene rearrangement of the mitochondrial genome of the bell-ring frog, Buergeria buergeri (family Rhacophoridae). Genes Genet. Syst. 2004, 79, 151–163. [Google Scholar] [CrossRef]
- Kurabayashi, A.; Usuki, C.; Mikami, N.; Fujii, T.; Yonekawa, H.; Sumida, M.; Hasegawa, M. Complete nucleotide sequence of the mitochondrial genome of a Malagasy poison frog Mantella madagascariensis: Evolutionary implications on mitochondrial genomes of higher anuran groups. Mol. Phylogenetics Evol. 2006, 39, 223–236. [Google Scholar] [CrossRef]
- Kurabayashi, A.; Yoshikawa, N.; Sato, N.; Hayashi, Y.; Oumi, S.; Fujii, T.; Sumida, M. Complete mitochondrial DNA sequence of the endangered frog Odorrana ishikawae (family Ranidae) and unexpected diversity of mt gene arrangements in ranids. Mol. Phylogenetics Evol. 2010, 56, 543–553. [Google Scholar] [CrossRef]
- Wu, Z.; Sainz, A.G.; Shadel, G.S. Mitochondrial DNA: Cellular genotoxic stress sentinel. Trends Biochem Sci. 2021, 46, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M. A Checklist of North American Amphibians and Reptiles: The United States and Canada. Volume 1–Amphibians1. Ichthyol. Herpetol. 2014, 4, 762. [Google Scholar]
- Zhang, J.Y.; Luu, B.E.; Yu, D.N.; Zhang, L.P.; Al-Attar, R.; Storey, K.B. The complete mitochondrial genome of Dryophytes versicolor: Phylogenetic relationship among Hylidae and mitochondrial protein-coding gene expression in response to freezing and anoxia. Int. J. Biol. Macromol. 2019, 132, 461–469. [Google Scholar] [CrossRef]
- Lee, M.Y.; Jeon, H.S.; Min, M.S.; An, J. Sequencing and analysis of the complete mitochondrial genome of Hyla suweonensis (Anura: Hylidae). Mitochondrial DNA Part B 2017, 2, 126–127. [Google Scholar] [CrossRef]
- Ballard, J.W.O.; Kreitman, M. Is mitochondrial DNA a strictly neutral marker? Trends Ecol. Evol. 1995, 10, 485–488. [Google Scholar] [CrossRef]
- Wu, L.; Tong, Y.; Ayivi, S.P.G.; Storey, K.B.; Zhang, J.Y.; Yu, D.N. The Complete Mitochondrial Genomes of Three Sphenomorphinae Species (Squamata: Scincidae) and the Selective Pressure Analysis on Mitochondrial Genomes of Limbless Isopachys gyldenstolpei. Animals 2022, 12, 2015. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Guan, J.Y.; Zhang, Z.Y.; Cao, Y.R.; Cai, Y.Y.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. Insight into the Phylogenetic Relationships among Three Subfamilies within Heptageniidae (Insecta: Ephemeroptera) along with Low-Temperature Selection Pressure Analyses Using Mitogenomes. Insects 2021, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.E.; Lin, Y.F.; Ma, L.; Ding, G.H.; Lin, Z.H. Partial mitochondrial genome of the Sanchiang Tree Toad Hyla sanchiangensis (Anura: Hylidae). Mitochondrial DNA Part B 2020, 5, 2682–2683. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, D.; Mao, R.L.; Hillis, D.M.; Wake, D.B.; Cannatella, D.C. Efficient sequencing of anuran mtDNAs and a mitogenomic exploration of the phylogeny and evolution of frogs. Mol. Biol. Evol. 2013, 30, 1899–1915. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Shen, S.Q.; Lu, L.X.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. The complete mitochondrial genome of Pyxicephalus adspersus: High gene rearrangement and phylogenetics of one of the world’s largest frogs. PeerJ 2019, 7, e7532. [Google Scholar] [CrossRef] [PubMed]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Bioinf. Methods Protoc. 2000, 132, 71–91. [Google Scholar]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenetics Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Cameron, S. How to sequence and annotate insect mitochondrial genomes for systematic and comparative genomics research. Syst. Entomol. 2014, 39, 400–411. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Hofacker, I.L.; Fekete, M.; Stadler, P.F. Secondary structure prediction for aligned RNA sequences. J. Mol. Biol. 2002, 319, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Bernhart, S.H.; Hofacker, I.L.; Will, S.; Gruber, A.R.; Stadler, P.F. RNAalifold: Improved consensus structure prediction for RNA alignments. BMC Bioinform. 2008, 9, 474. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Borzee, A.; Messenger, K.R.; Chae, S.; Andersen, D.; Groffen, J.; Kim, Y.I.; An, J.; Othman, S.N.; Ri, K.; Nam, T.Y. Yellow sea mediated segregation between North East Asian Dryophytes species. PLoS ONE 2020, 15, e0234299. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; DePamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.Y.; Zheng, R.Q.; Yu, B.G.; Yang, G. Complete nucleotide sequence and gene organization of the mitochondrial genome of Paa spinosa (Anura: Ranoidae). Gene 2009, 447, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.N.; Zhang, J.Y.; Zheng, R.Q. The complete mitochondrial genome of Babina adenopleura (Anura: Ranidae). Mitochondrial DNA 2012, 23, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.N.; Zhang, J.Y.; Zheng, R.Q.; Shao, C. The complete mitochondrial genome of Hoplobatrachus rugulosus (Anura: Dicroglossidae). Mitochondrial DNA 2012, 23, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xie, Z. DAMBE: Software package for data analysis in molecular biology and evolution. J. Hered. 2001, 92, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Lima, N.G.d.S.; Carmo, A.O.d.; Martins, A.P.V.; Souza, R.C.C.d.; Kalapothakis, E.; Eterovick, P.C. Complete mitochondrial genome sequence of the high-altitude Brazilian tree frog Bokermannohyla alvarengai (Anura, Hylidae). Mitochondrial DNA Part B 2017, 2, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, A.; Marinho, P.; Réjaud, A.; Carvalho, T.R.; Caminer, M.A.; Jansen, M.; Rainha, R.N.; Rodrigues, M.T.; Werneck, F.P.; Lima, A.P. Systematics and biogeography of the Boana albopunctata species group (Anura, Hylidae), with the description of two new species from Amazonia. Syst. Biodivers. Sci. 2021, 19, 375–399. [Google Scholar] [CrossRef]
- Warwick, A.R.; Barrow, L.N.; Smith, M.L.; Means, D.B.; Lemmon, A.R.; Lemmon, E.M. Signatures of north-eastern expansion and multiple refugia: Genomic phylogeography of the Pine Barrens tree frog, Hyla andersonii (Anura: Hylidae). Biol. J. Linn. Soc. 2021, 133, 120–134. [Google Scholar] [CrossRef]
- Barrow, L.N.; Soto-Centeno, J.A.; Warwick, A.R.; Lemmon, A.R.; Moriarty Lemmon, E. Evaluating hypotheses of expansion from refugia through comparative phylogeography of south-eastern Coastal Plain amphibians. J. Biogeogr. 2017, 44, 2692–2705. [Google Scholar] [CrossRef]
- Borzee, A.; Didinger, C.; Jang, Y. Complete mitochondrial genome of Dryophytes suweonensis (Anura Hylidae). Mitochondrial DNA B Resour. 2017, 2, 5–6. [Google Scholar] [CrossRef]
- Ye, L.T.; Zhu, C.C.; Yu, D.N.; Zhang, Y.P.; Zhang, J.Y. The complete mitochondrial genome of Hyla annectans (Anura: Hylidae). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 1593–1594. [Google Scholar]
- Zhang, P.; Zhou, H.; Chen, Y.Q.; Liu, Y.F.; Qu, L.H. Mitogenomic perspectives on the origin and phylogeny of living amphibians. Syst. Biol. 2005, 54, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Kurabayashi, A.; Usuki, C.; Fujii, T.; Sumida, M.J.G. Complete mitochondrial genomes of three neobatrachian anurans: A case study of divergence time estimation using different data and calibration settings. Gene 2008, 407, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Sun, Z.L.; Guo, W.B.; Wu, J.; Qian, L.F.; Pan, T.; Wang, H.; Li, K.; Zhang, B.W. Sequencing of complete mitochondrial genome for Tsinling Tree Toad (Hyla tsinlingensis). Mitochondrial DNA B Resour. 2016, 1, 466–467. [Google Scholar] [CrossRef]
- Gatto, K.P.; Smith, J.J.; Lourenco, L.B. The mitochondrial genome of the endemic Brazilian paradoxical frog Pseudis tocantins (Hylidae). Mitochondrial DNA B Resour. 2018, 3, 1106–1107. [Google Scholar] [CrossRef]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2018, 46, 2699. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.A.; Clayton, D.A. Mechanism of mitochondrial DNA replication in mouse L-cells: Localization and sequence of the light-strand origin of replication. J. Mol. Biol. 1979, 135, 327–351. [Google Scholar] [CrossRef]
- Yan, P.; Pan, T.; Wu, G.Y.; Kang, X.; Ali, I.; Zhou, W.L.; Li, J.T.; Wu, X.B.; Zhang, B.W. Species Delimitation and Evolutionary History of Tree Frogs in the Hyla chinensis Group (Hylidae, Amphibian). Front. Ecol. Evol. 2020, 8, 234. [Google Scholar] [CrossRef]
- Huang, M.Y.; Duan, R.Y.; Tang, T.; Zhu, C.; Wang, Y. The complete mitochondrial genome of Hyla tsinlingensis (Anura: Hylidae). Mitochondrial DNA Part A 2016, 27, 4130–4131. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Stephens, P.R.; Wiens, J.J. Replicate patterns of species richness, historical biogeography, and phylogeny in Holarctic treefrogs. Evolution 2005, 59, 2433–2450. [Google Scholar] [PubMed]
- McKenzie, M.; Chiotis, M.; Pinkert, C.A.; Trounce, I.A. Functional respiratory chain analyses in murid xenomitochondrial cybrids expose coevolutionary constraints of cytochrome b and nuclear subunits of complex III. Mol. Biol. Evol. 2003, 20, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, M.A.; Willems, P.; van den Brand, M.; Valsecchi, F.; Kruse, S.; Palmiter, R.; Smeitink, J.; Nijtmans, L. Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum. Mol. Genet. 2012, 21, 115–120. [Google Scholar] [CrossRef]
- Bernardes, J.S.; Eberle, R.J.; Vieira, F.R.; Coronado, M.A. A comparative pan-genomic analysis of 53 C. Pseudotuberculosis strains based on functional domains. J. Biomol. Struct. Dyn. 2021, 39, 6974–6986. [Google Scholar] [CrossRef]
- Yu, D.N.; Zhang, J.Y.; Li, P.; Zheng, R.Q.; Shao, C. Do cryptic species exist in Hoplobatrachus rugulosus? An examination using four nuclear genes, the Cyt b gene and the complete MT genome. PLoS ONE 2015, 10, e0124825. [Google Scholar] [CrossRef]
- Da Fonseca, R.R.; Johnson, W.E.; O′Brien, S.J.; Ramos, M.J.; Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genom. 2008, 9, 119. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Vaish, M.; Price-Whelan, A.; Reyes-Robles, T.; Liu, J.; Jereen, A.; Christie, S.; Alonzo III, F.; Benson, M.A.; Torres, V.J.; Krulwich, T.A. Roles of Staphylococcus aureus Mnh1 and Mnh2 antiporters in salt tolerance, alkali tolerance, and pathogenesis. J. Bacteriol. 2018, 200, e00611–e00617. [Google Scholar] [CrossRef]
- Berry, E.A.; Guergova-Kuras, M.; Huang, L.; Crofts, A.R. Structure and function of cytochrome bc complexes. Annu. Rev. Biochem. 2000, 69, 1005–1075. [Google Scholar] [CrossRef]
- Lanciano, P.; Khalfaoui-Hassani, B.; Selamoglu, N.; Ghelli, A.; Rugolo, M.; Daldal, F. Molecular mechanisms of superoxide production by complex III: A bacterial versus human mitochondrial comparative case study. Biochim. Biophys. Acta. 2013, 1827, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Nielsen, R.; Yang, Z.H. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 2005, 22, 2472–2479. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Liang, L.; Zhu, Z.H.; Zhou, W.P.; Irwin, D.M.; Zhang, Y.P. Adaptive evolution of energy metabolism genes and the origin of flight in bats. Proc. Natl. Acad. Sci. USA 2010, 107, 8666–8671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.C.; Shen, X.J.; Irwin, D.M.; Shen, Y.Y.; Zhang, Y.P. Mitogenomic analyses propose positive selection in mitochondrial genes for high-altitude adaptation in galliform birds. Mitochondrion 2014, 18, 70–75. [Google Scholar] [CrossRef]
- Ben Slimen, H.; Awadi, A.; Tolesa, Z.G.; Knauer, F.; Alves, P.C.; Makni, M.; Suchentrunk, F. Positive selection on the mitochondrial ATP synthase 6 and the NADH dehydrogenase 2 genes across 22 hare species (genus Lepus). J. Zool. Syst. Evol. Res. 2018, 56, 428–443. [Google Scholar] [CrossRef]
- Shao′e, S.; Hui, M.; Wang, M.X.; Sha, Z.L. The complete mitochondrial genome of the alvinocaridid shrimp Shinkaicaris leurokolos (Decapoda, Caridea): Insight into the mitochondrial genetic basis of deep-sea hydrothermal vent adaptation in the shrimp. Comp. Biochem. Physiol. Part D: Genom. Proteom. 2018, 25, 42–52. [Google Scholar]
- Banguera-Hinestroza, E.; Sawall, Y.; Al-Sofyani, A.; Mardulyn, P.; Fuertes-Aguilar, J.; Cardenas-Henao, H.; Jimenez-Infante, F.; Voolstra, C.R.; Flot, J.F. mtDNA recombination indicative of hybridization suggests a role of the mitogenome in the adaptation of reef-building corals to extreme environments. bioRxiv 2019, 462069. [Google Scholar] [CrossRef]
- Sun, J.T.; Jin, P.Y.; Hoffmann, A.; Duan, X.Z.; Dai, J.; Hu, G.; Xue, X.F.; Hong, X.Y. Evolutionary divergence of mitochondrial genomes in two Tetranychus species distributed across different climates. Insect Mol. Biol. 2018, 27, 698–709. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).