Transcriptomic Analysis of Takifugu obscurus Gills under Acute Hypoxic Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Fish
2.3. Experimental Design and Sample Collection
2.4. RNA Extraction, Library Construction, and Transcriptomic Sequencing
2.5. Data Filtering, Read Mapping, and Differential Expression Analysis
3. Results
3.1. Summary of Sequencing Data Quality
3.2. Read Mapping
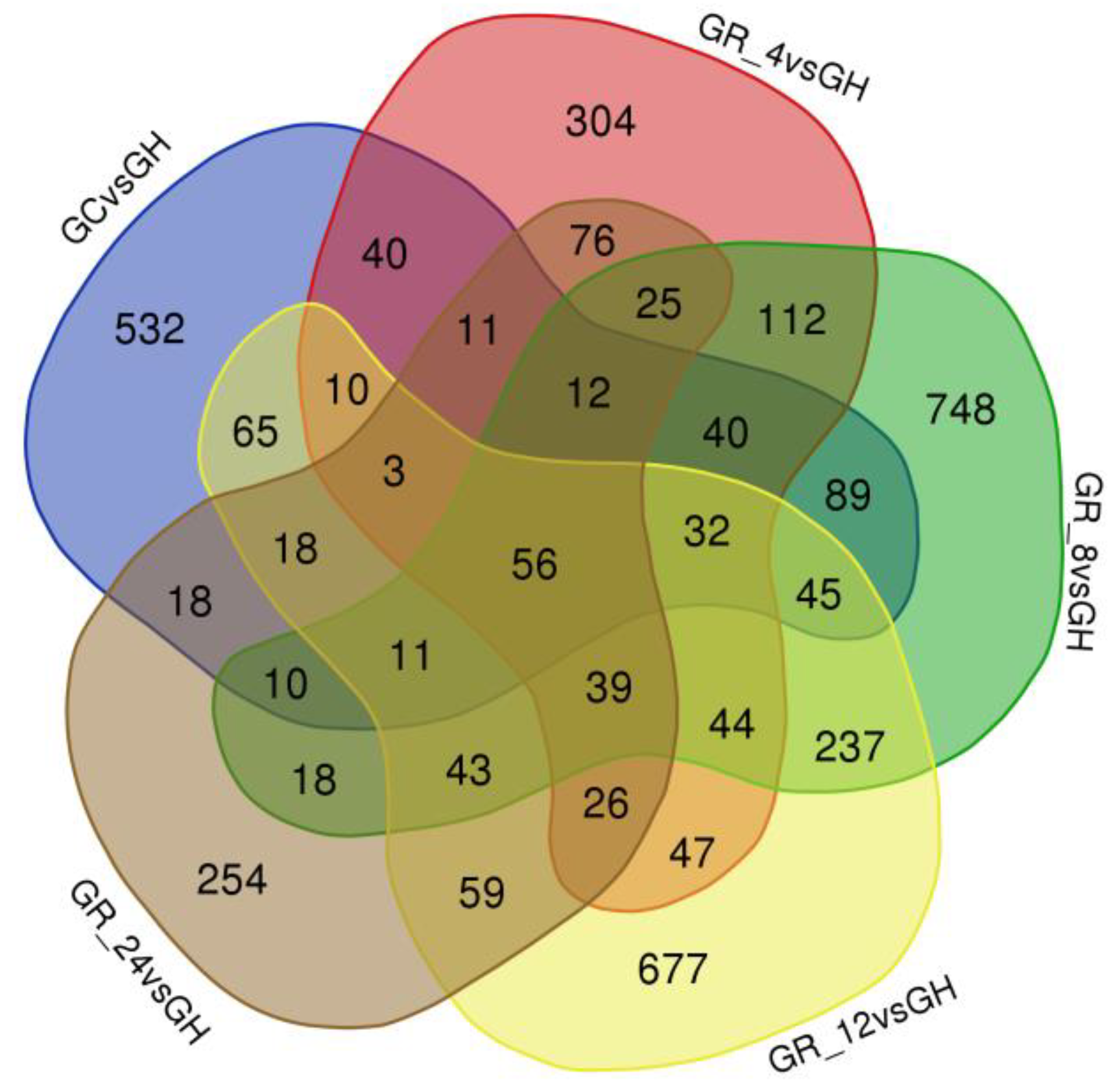
3.3. DEG Analysis
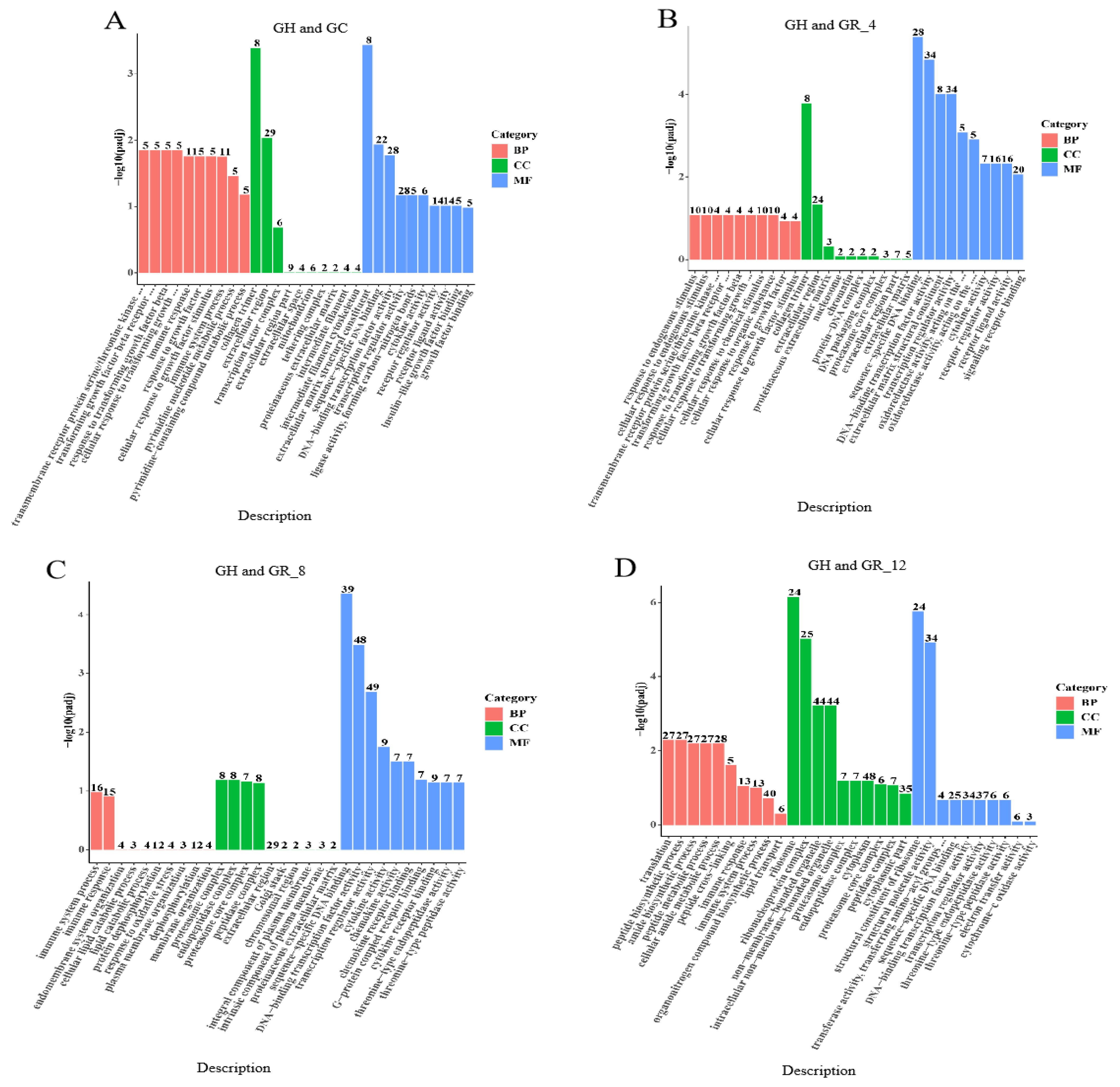
3.4. GO Enrichment Analysis of DEGs
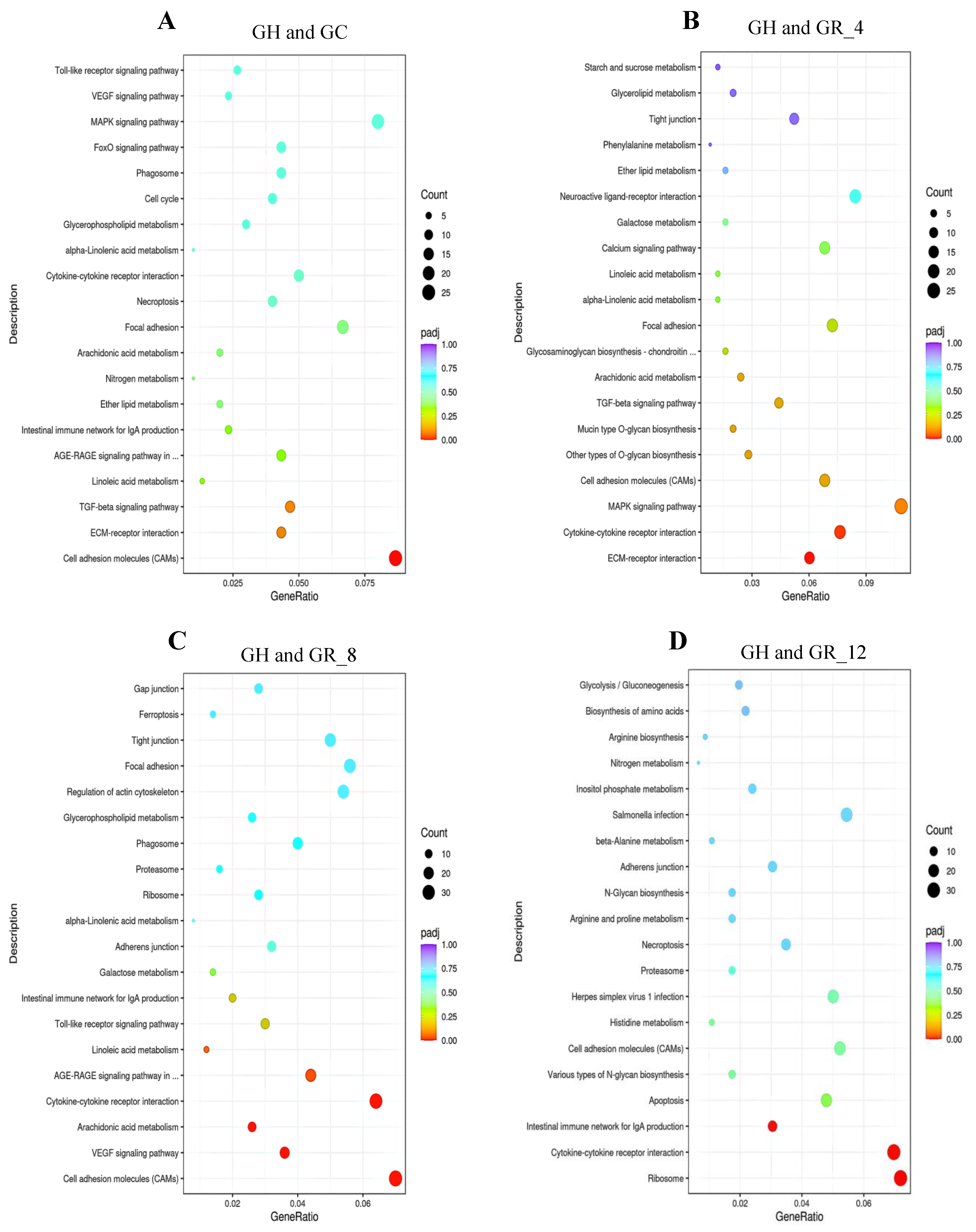
3.5. KEGG Enrichment Analysis of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.R. Effects of Hypoxic Stress on Energy Metabolism, Blood Indexes and Gene Expression of Takifugu obscurus; Nanjing Normal University: Nanjing, China, 2018. [Google Scholar]
- Chen, C.C.; Gong, G.C.; Shiah, F.K. Hypoxia in The East China Sea: One of The Largest Hypoxic Areas in The World. Mar. Environ. Res. 2007, 64, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.G. Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. J. Exp. Biol. 2011, 214, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.H.; Chen, Y.F.; Yang, Z. The combined effect of temperature and pH on embryonic development of obscure puffer Takifugu obscurus and its ecological implications. Biochem. Syst. Ecol. 2015, 58, 1–6. [Google Scholar] [CrossRef]
- Gong, D.B.; Xu, L.H.; Li, W.H.; Shang, R.J.; Chen, J.X.; Hu, F.Z.; Wang, S.; Liu, Q.F.; Wu, C.; Zhou, R.; et al. Comparative analysis of liver transcriptomes associated with hypoxia tolerance in the gynogenetic blunt snout bream. Aquaculture 2020, 523, 735163. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, W.W.; Yu, X.M.; Wang, J.R.; Feng, Y.Z.; Tong, J.G. Cardiac Transcriptomics Reveals That MAPK Pathway Plays an Important Role in Hypoxia Tolerance in Bighead Carp (Hypophthalmichthys nobilis). Animals 2020, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Kristensen, T.; Waagbø, R.; Rosseland, B.O.; Tollefsen, K.E.; Baeverfjord, G.; Berntssen, M.H.G. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 314–323. [Google Scholar] [CrossRef]
- Cappello, T.; Maisano, M.; Giannetto, A.; Parrino, V.; Mauceri, A.; Fasulo, S. Neurotoxicological effects on marine mussel Mytilus galloprovincialis caged at petrochemical contaminated areas (eastern Sicily, Italy): H NMR and immunohistochemical assays. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 169, 7–15. [Google Scholar] [CrossRef]
- Mcbryan, T.L.; Healy, T.M.; Haakons, K.L.; Schulte, P.M. Warm acclimation improves hypoxia tolerance in Fundulus heteroclitus. J. Exp. Biol. 2016, 219, 474–484. [Google Scholar] [CrossRef]
- Kanda, A.; Hirose, I.; Noda, K.; Murata, M.; Ishida, S. Glucocorticoid-transactivated TSC22D3 attenuates hypoxia- and diabetes-induced Müller glial galectin-1 expression via HIF-1α destabilization. J. Cell. Mol. Med. 2020, 24, 4589–4599. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.Y.; Song, X.L.; Cai, H.Y.; Chen, J.C.; Song, L.N.; Yang, R.; Lu, J. Upregulations of Glucocorticoid-Induced Leucine Zipper by Hypoxia and Glucocorticoid Inhibit Proinflammatory Cytokines under Hypoxic Conditions in Macrophages. J. Immunol. 2012, 188, 222–229. [Google Scholar] [CrossRef]
- Lau, L.F.; Lam, S.C.; Leu, S.J. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J. Biol. Chem. 2002, 277, 46248–46255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Mo, F.E.; Lau, L.F. The Angiogenic Factor Cyr61 Activates a Genetic Program for Wound Healing in Human Skin Fibroblasts. J. Biol. Chem. 2001, 276, 47237–47329. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.B.; Xu, Y.X. BMP/Smad signaling pathway and mammalian folliculogenesis. Hereditas 2009, 31, 245–254. [Google Scholar] [PubMed]
- Ripamonti, U.; Crooks, J.; Matsaba, T.; Tasker, J. Induction of endochondral bone formation by recombinant human transforming growth factor-beta2 in the baboon (Papio ursinus). Growth Factors 2000, 17, 269–285. [Google Scholar] [CrossRef]
- Zhou, D.Z.; Huang, G.J.; Liu, B.S.; Zhang, B.; Jiang, S.; Deng, Z.H.; Yu, D.H. Molecular cloning and expression analysis of BMP10 gene frompearl oyster (Pinctada fucata). South China Fish. Sci. 2016, 12, 83–90. [Google Scholar]
- Kim, H.S.; Asmis, R. Mitogen-activated protein kinase phosphatase 1 (MKP-1) in macrophage biology and cardiovascular disease. A redox-regulated master controller of monocyte function and macrophage phenotype. Free Radic. Biol. Med. 2017, 105, 75–83. [Google Scholar] [CrossRef]
- Mishra, O.P.; Delivoria-Papadopoulos, M. Effect of hypoxia on the expression and activity of mitogen-activated protein (MAP) kinase-phosphatase-1 (MKP-1) and MKP-3 in neuronal nuclei of newborn piglets: The role of nitric oxide. Neuroscience 2004, 129, 665–673. [Google Scholar] [CrossRef]
- Bernaudin, M.; Tang, Y.; Reilly, M.; Petit, E.; Sharp, F.R. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J. Biol. Chem. 2002, 277, 39728–39738. [Google Scholar] [CrossRef]
- Korhonen, R.; Turpeinen, T.; Taimi, V.; Nieminen, R.; Goulas, A.; Moilanen, E. Attenuation of the acute inflammatory response by dual specificity phosphatase 1 by inhibition of p38 MAP kinase. Mol. Immunol. 2011, 48, 2059–2068. [Google Scholar] [CrossRef]
- Ji, F.; Fu, S.J.; Shen, S.L.; Zhang, L.J.; Cao, Q.H.; Li, S.Q.; Peng, B.G.; Liang, L.J.; Hua, Y.P. The prognostic value of combined TGF-β1 and ELF in hepatocellular carcinoma. BMC Cancer 2015, 15, 116. [Google Scholar] [CrossRef][Green Version]
- Guillot, N.; Kollins, D.; Gilbert, V.; Xavier, S.; Chen, J.; Gentle, M.; Reddy, A.; Bottinger, E.; Jiang, R.; Rastaldi, M.P.; et al. BAMBI Regulates Angiogenesis and Endothelial Homeostasis through Modulation of Alternative TGFβ Signaling. PLoS ONE 2012, 7, e39406. [Google Scholar] [CrossRef] [PubMed]
- Dennler, S.; Goumans, M.J.; Ten, D.P. Transforming growth factor beta signal transduction. J. Leukoc. Biol. 2002, 7, 731–740. [Google Scholar] [CrossRef]
- Ping, H.L.; Wu, J.Y.; Xu, S.W.; Hu, K.S.; Duan, Z.G. Molecular Cloning and Expression Analysis of Transforming Growth Factor β1 (TGF β1) from Orange Spotted Grouper (Epinephelus coioides). Acta Sci. Nat. Univ. Sunyatseni 2011, 50, 92–98. [Google Scholar]
- Li, Y.M. Effect of the Degree and Frequency of Intermittent Hypoxia on Interleukin 6 and Interleukin 8 in the Sleep Apnea Pattern; Tianjin Medical University: Tianjin, China, 2010. [Google Scholar]
- Loboda, A.; Stachurska, A.; Florczyk, U.; Rudnicka, D.; Jazwa, A.; Wegrzyn, J.; Kozakowska, M.; Stalinska, K.; Poellinger, L.; Levonen, A.L.; et al. HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells. Antioxid. Redox Signal. 2009, 11, 1501–1517. [Google Scholar] [CrossRef]
- Geng, X.; Feng, J.B.; Liu, S.K.; Wang, Y.P.; Arias, C.; Liu, Z.J. Transcriptional regulation of hypoxia inducible factors alpha (HIF-alpha) and their inhibiting factor (FIH-1) of channel catfish (Ictalurus punctatus) under hypoxia. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 169, 38–50. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Kapczuk, P.; Kupnicka, P.; Gawrońska-Szklarz, B.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature. Int. J. Mol. Sci. 2021, 22, 843. [Google Scholar] [CrossRef]
- Yue, H.L.; Peng, D.Z. Structure and function of CCL20. Immunol. J. 2004, 20, 100–102. [Google Scholar]
- Bosco, M.C.; Puppo, M.; Santangelo, C.; Anfosso, L.; Pfeffer, U.; Fardin, P.; Battaglia, F.; Varesio, L. Hypoxia Modifies the Transcriptome of Primary Human Monocytes: Modulation of Novel Immune-Related Genes and Identification of CC-Chemokine Ligand 20 as a New Hypoxia-Inducible Gene. J. Immunol. 2006, 177, 1941–1955. [Google Scholar] [CrossRef]
- Yan, R.S.; Zhou, X.; Chen, X.; Fan, D.L.; Zhang, Y.M. Analysis of differentially expressed genes and related pathways in epidermal cells after short-term hypoxia treatment. Chin. J. Cell Stem Cell (Electron. Ed.) 2019, 9, 86–91. [Google Scholar]
- Li, Y.Q. Transcriptome Analysis and VEGFA Gene Expression Study on the Hypoxic Stress of Trachinotus blochii; Hainan University: Hainan, China, 2019. [Google Scholar]
- Sisakhtnezhad, S.; Alimoradi, E.; Akrami, H. External factors influencing mesenchymal stem cell fate in vitro. Eur. J. Cell Biol. 2016, 96, 13–33. [Google Scholar] [CrossRef]
- An, Q.X.; Meng, Y.L.; Lv, D.D. Advances in the study of mitogen-activated protein kinase signaling pathway. Heilongjiang J. Tradit. Chin. Med. 2016, 45, 65–66. [Google Scholar]
- Yu, R.; Ng, P.; Tan, T.; Chu, D.; Wu, R.; Kong, R. Enhancement of hypoxia-induced gene expression in fish liver by the aryl hydrocarbon receptor (AhR) ligand, benzo[a]pyrene (BaP). Aquat. Toxicol. 2008, 90, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Olfert, I.M.; Breen, E.C.; Mathieu-Costello, O.; Wagner, P.D. Chronic hypoxia attenuates resting and exercise-induced VEGF, flt-1, and flk-1 mRNA levels in skeletal muscle. J. Appl. Phys. 2001, 90, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; Lecouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.; Terova, G.; Ceccuzzi, P.; Marelli, S.; Antonini, M.; Saroglia, M. HIF-1α mRNA levels in Eurasian perch (Perca fluviatilis) exposed to acute and chronic hypoxia. Mol. Biol. Rep. 2012, 39, 4009–4015. [Google Scholar] [CrossRef]


| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate | GC Content (%) | Q30 (%) |
|---|---|---|---|---|---|---|---|
| GC1 | 43,559,868 | 6.53 G | 41,654,646 | 6.25 G | 0.03 | 50.49 | 92.97 |
| GC2 | 46,829,616 | 7.02 G | 42,629,596 | 6.39 G | 0.03 | 49.92 | 93.64 |
| GC3 | 46,140,914 | 6.92 G | 42,115,486 | 6.32 G | 0.03 | 50.10 | 93.85 |
| GH1 | 45,868,852 | 6.88 G | 42,349,752 | 6.35 G | 0.03 | 49.94 | 93.61 |
| GH2 | 46,227,668 | 6.93 G | 44,462,882 | 6.67 G | 0.03 | 50.62 | 93.41 |
| GH3 | 45,886,854 | 6.88 G | 43,746,966 | 6.56 G | 0.03 | 50.64 | 93.14 |
| GR1_4 | 45,403,326 | 6.81 G | 42,937,580 | 6.44 G | 0.03 | 50.69 | 93.36 |
| GR2_4 | 42,998,268 | 6.45 G | 40,784,166 | 6.12 G | 0.03 | 50.88 | 93.25 |
| GR3_4 | 45,506,236 | 6.83 G | 43,479,216 | 6.52 G | 0.03 | 51.25 | 93.10 |
| GR1_8 | 45,482,486 | 6.82 G | 42,964,718 | 6.44 G | 0.03 | 50.91 | 93.29 |
| GR2_8 | 46,235,922 | 6.94 G | 43,898,446 | 6.58 G | 0.03 | 51.01 | 93.15 |
| GR3_8 | 47,141,580 | 7.07 G | 43,991,278 | 6.60 G | 0.03 | 50.65 | 93.47 |
| GR1_12 | 44,758,330 | 6.71 G | 40,769,722 | 6.12 G | 0.03 | 50.28 | 93.32 |
| GR2_12 | 47,797,410 | 7.17 G | 44,347,812 | 6.65 G | 0.03 | 49.87 | 93.05 |
| GR3_12 | 42,904,626 | 6.44 G | 39,202,820 | 5.88 G | 0.03 | 50.52 | 93.29 |
| GR1_24 | 46,838,548 | 7.03 G | 45,699,722 | 6.85 G | 0.03 | 49.39 | 93.30 |
| GR2_24 | 46,183,646 | 6.93 G | 44,378,028 | 6.66 G | 0.03 | 50.60 | 93.07 |
| GR3_24 | 46,388,604 | 6.96 G | 44,297,968 | 6.64 G | 0.03 | 51.02 | 93.15 |
| Sample | Total Map | Unique Map | Multi Map |
|---|---|---|---|
| GC1 | 36,656,072 (88.0%) | 33,274,418 (79.88%) | 3,381,654 (8.12%) |
| GC2 | 36,636,834 (85.94%) | 33,391,350 (78.33%) | 3,245,484 (7.61%) |
| GC3 | 36,675,924 (87.08%) | 33,628,770 (79.85%) | 3,047,154 (7.24%) |
| GH1 | 36,779,197 (86.85%) | 34,025,598 (80.34%) | 2,753,599 (6.5%) |
| GH2 | 38,854,740 (87.39%) | 36,075,442 (81.14%) | 2,779,298 (6.25%) |
| GH3 | 38,481,642 (87.96%) | 35,928,246 (82.13%) | 2,553,396 (5.84%) |
| GR1_4 | 37,403,666 (87.11%) | 34,038,280 (79.27%) | 3,365,386 (7.84%) |
| GR2_4 | 35,918,644 (88.07%) | 32,457,035 (79.58%) | 3,461,609 (8.49%) |
| GR3_4 | 37,420,039 (86.06%) | 33,074,820 (76.07%) | 4,345,219 (9.99%) |
| GR1_8 | 37,468,050 (87.21%) | 34,102,188 (79.37%) | 3,365,862 (7.83%) |
| GR2_8 | 38,591,719 (87.91%) | 35,161,827 (80.1%) | 3,429,892 (7.81%) |
| GR3_8 | 38,103,192 (86.62%) | 35,332,509 (80.32%) | 2,770,683 (6.3%) |
| GR1_12 | 35,190,781 (86.32%) | 32,123,707 (78.79%) | 3,067,074 (7.52%) |
| GR2_12 | 38,460,120 (86.72%) | 34,765,756 (78.39%) | 3,694,364 (8.33%) |
| GR3_12 | 32,694,236 (83.4%) | 29,370,781 (74.92%) | 3,323,455 (8.48%) |
| GR1_24 | 39,216,187 (85.81%) | 36,171,393 (79.15%) | 3,044,794 (6.66%) |
| GR2_24 | 38,553,073 (86.87%) | 35,856,894 (80.8%) | 2,696,179 (6.08%) |
| GR3_24 | 37,729,741 (85.17%) | 34,958,208 (78.92%) | 2,771,533 (6.26%) |
| DEG Comparison | DEG Number | Upregulated Genes | Downregulated Genes |
|---|---|---|---|
| GC vs. GH | 992 | 426 | 566 |
| GR_4 vs. GH | 877 | 405 | 472 |
| GR_8 vs. GH | 1561 | 758 | 803 |
| GR_12 vs. GH | 1412 | 568 | 844 |
| GR_24 vs. GH | 679 | 325 | 354 |
| KEGG ID Pathway | Signaling Pathway | p | Genes |
|---|---|---|---|
| tru04514 | Cell adhesion molecules (CAMs) | 0.000013 | nrxn1, cldn33b, ptprm, itga8, vtcn1, itgb8, LOC115249662, LOC101064604, LOC101063645, LOC101063580 |
| tru04060 | Cytokine–cytokine receptor interaction | 0.009122 | bmp10, il-8, cxcr4, ackr3, amh, il2rg, LOC115248669, LOC101066952, LOC101063250 |
| tru04370 | VEGF signaling pathway | 0.04077 | vegfa, ptgs2, pla2g4a, pik3r3, LOC101073653, LOC101070662, LOC101069259 |
| tru04010 | MAPK signaling pathway | 0.04903 | dusp1, igf1, ntrk2, LOC101077057, LOC101065285, LOC101076406, LOC10106965, LOC101067669 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, R.; Wang, Y.; Zhou, J.; Xu, H.; Gou, M.; Ye, J.; Qiu, X.; Wang, X. Transcriptomic Analysis of Takifugu obscurus Gills under Acute Hypoxic Stress. Animals 2023, 13, 1572. https://doi.org/10.3390/ani13101572
Zhang H, Li R, Wang Y, Zhou J, Xu H, Gou M, Ye J, Qiu X, Wang X. Transcriptomic Analysis of Takifugu obscurus Gills under Acute Hypoxic Stress. Animals. 2023; 13(10):1572. https://doi.org/10.3390/ani13101572
Chicago/Turabian StyleZhang, Huakun, Run Li, Yaohui Wang, Jinxu Zhou, Hao Xu, Meng Gou, Jianhua Ye, Xuemei Qiu, and Xiuli Wang. 2023. "Transcriptomic Analysis of Takifugu obscurus Gills under Acute Hypoxic Stress" Animals 13, no. 10: 1572. https://doi.org/10.3390/ani13101572
APA StyleZhang, H., Li, R., Wang, Y., Zhou, J., Xu, H., Gou, M., Ye, J., Qiu, X., & Wang, X. (2023). Transcriptomic Analysis of Takifugu obscurus Gills under Acute Hypoxic Stress. Animals, 13(10), 1572. https://doi.org/10.3390/ani13101572






