Structural and Functional Dynamics of the Ovary and Uterus during the Estrous Cycle in Donkeys in the Eastern Caribbean
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Animal Management
2.2. Study Design
2.3. Follicles and Follicular Waves
2.4. Ovulation and CL Structure and Function
2.5. Uterine Dynamics
2.6. Statistical Analysis
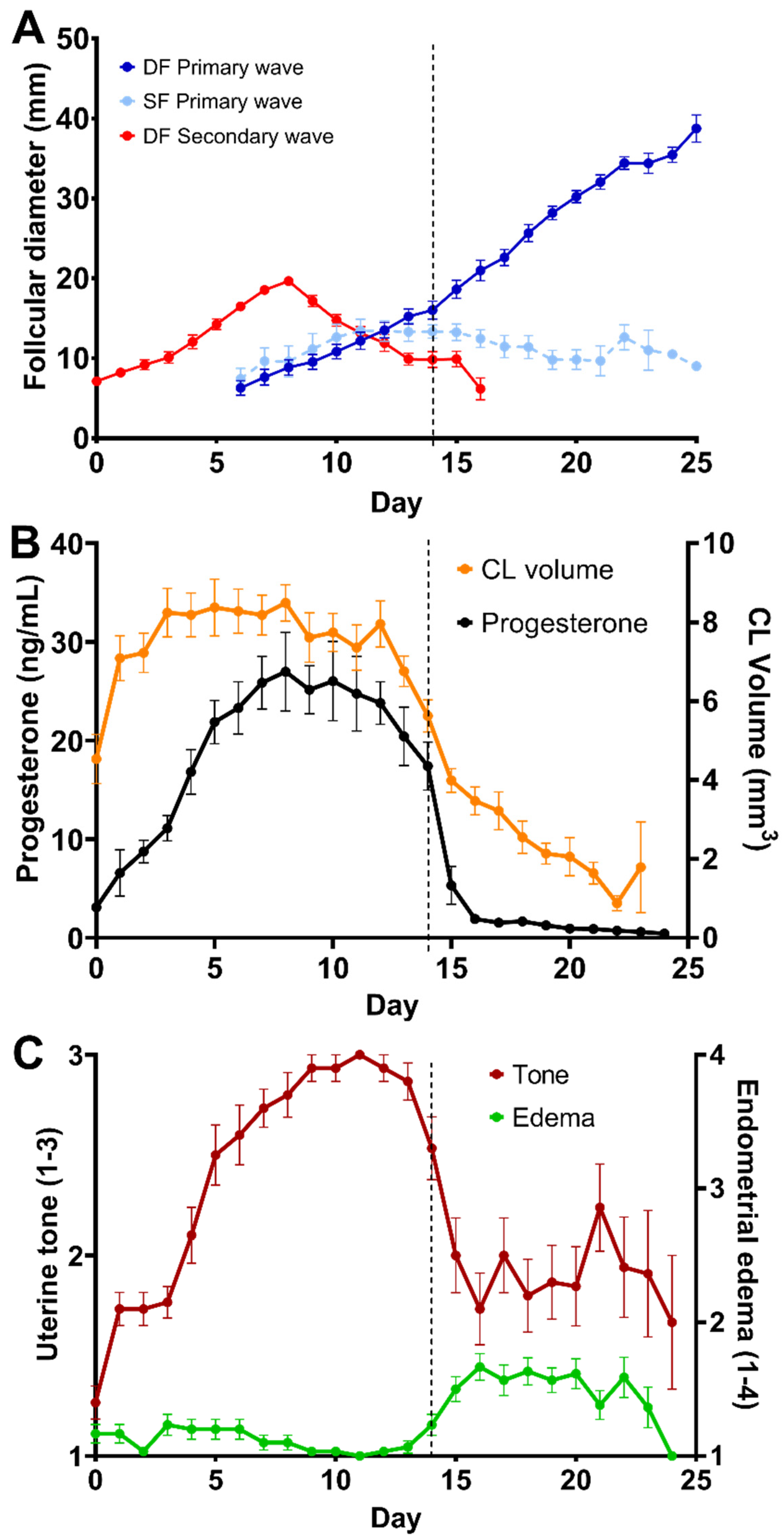
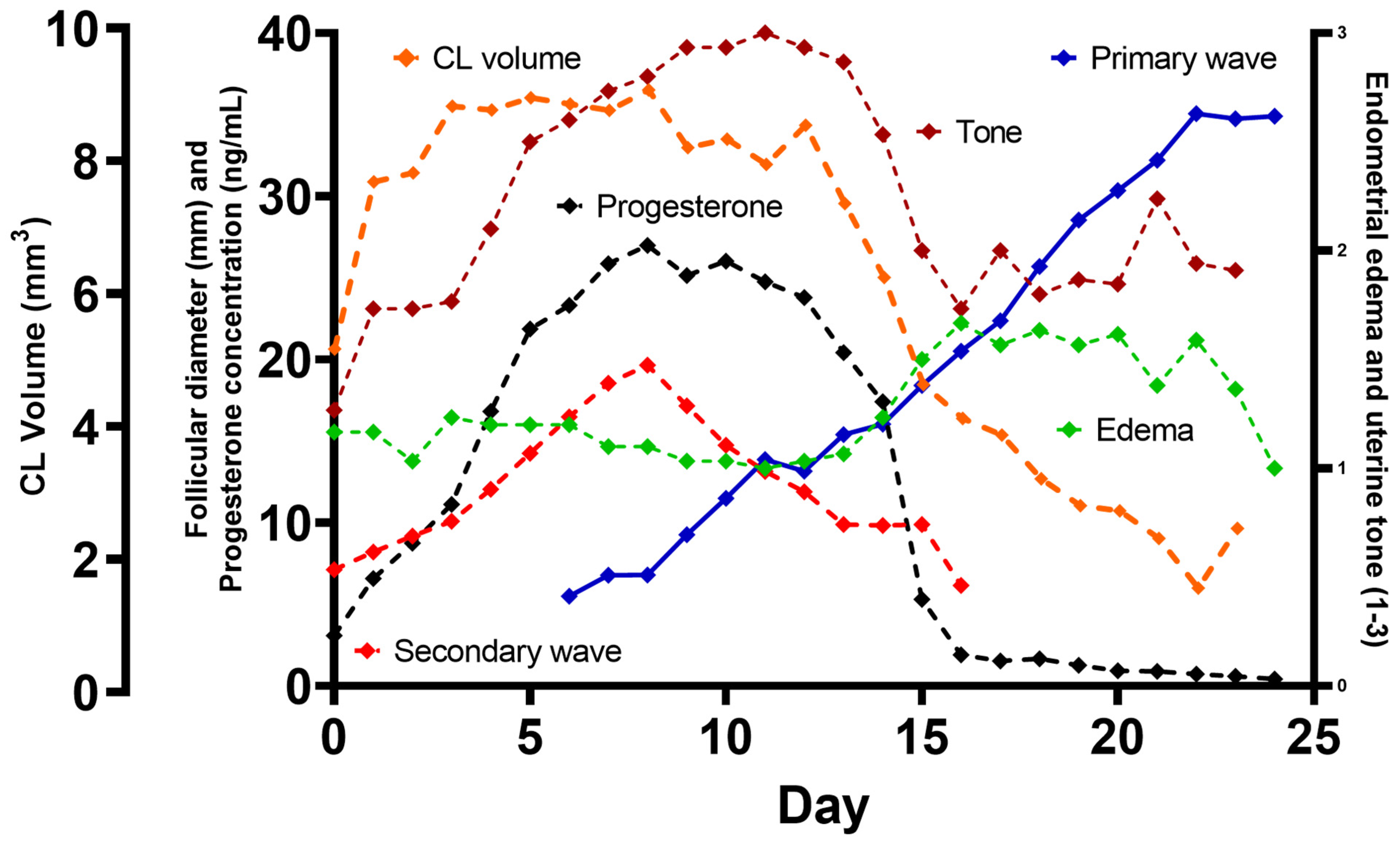
3. Results
- Periovulatory period temporal relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yılmaz, O.; Boztepe, S.; Ertu Grul, M. The domesticated donkey: III-Economic importance, uncommon usages, reproduction traits, genetics, nutrition and health care. Can. J. Appl. Sci. 2012, 2, 139. [Google Scholar] [CrossRef]
- Souroullas, K.; Aspri, M.; Papademas, P. Donkey milk as a supplement in infant formula: Benefits and technological challenges. Food Res. Int. 2018, 109, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Sarti, L.; Martini, M.; Brajon, G.; Barni, S.; Salari, F.; Altomonte, I.; Ragona, G.; Mori, F.; Pucci, N.; Muscas, G.; et al. Donkey’s milk in the management of children with cow’s milk protein allergy: Nutritional and hygienic aspects. Ital. J. Pediatr. 2019, 45, 102. [Google Scholar] [CrossRef] [PubMed]
- Kocic, H.; Stankovic, M.; Tirant, M.; Lotti, T.; Arsic, I. Favorable effect of creams with skimmed donkey milk encapsulated in nanoliposomes on skin physiology. Dermatol. Ther. 2020, 33, e13511. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Panzani, D.; Miró, J.; Ellerbrock, R.E. Key Aspects of Donkey and Mule Reproduction. Vet. Clin. N. Am.-Equine Pract. 2019, 35, 607–642. [Google Scholar] [CrossRef] [PubMed]
- Camillo, F.; Rota, A.; Biagini, L.; Tesi, M.; Fanelli, D.; Panzani, D. The current situation and trend of donkey industry in Europe. J. Equine Vet. Sci. 2018, 65, 44–49. [Google Scholar] [CrossRef]
- Miragaya, M.H.; Neild, D.M.; Alonso, A.E. A review of reproductive biology and biotechnologies in donkeys. J. Equine Vet. Sci. 2018, 65, 55–61. [Google Scholar] [CrossRef]
- Ginther, O.J.; Beg, M.A.; Gastal, M.O.; Gastal, E.L. Follicle dynamics and selection in mares. Anim. Reprod. 2004, 11, 45–63. [Google Scholar]
- Gastal, E.L.; Pastorello, M.; Godoi, D.B.; Gastal, M.O. Reproductive patterns and follicular waves in postpartum lactating versus non-postpartum cycling mares. J. Equine Vet. Sci. 2021, 107, 103732. [Google Scholar] [CrossRef]
- Ginther, O.J. Major and minor follicular waves during the equine estrous cycle. J. Equine Vet. Sci. 1993, 13, 18–25. [Google Scholar] [CrossRef]
- Gastal, E.L.; Gastal, M.O.; Bergfelt, D.R.; Ginther, O.J. Role of diameter differences among follicles in selection of a future dominant follicle in mares. Biol. Reprod. 1997, 57, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, M.; Gastal, M.O.; Godoi, D.B.; Gastal, E.L. Emergence and selection of the dominant follicle and gonadotropin dynamics in postpartum lactating versus non-postpartum cycling mares. Reprod. Biol. 2022, 22, 100618. [Google Scholar] [CrossRef] [PubMed]
- Gastal, E.L.; Gastal, M.O.; Beg, M.A.; Ginther, O.J. Interrelationships among follicles during the common-growth phase of a follicular wave and capacity of individual follicles for dominance in mares. Reproduction 2004, 128, 417–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gastal, E.L. Recent advances and new concepts on follicle and endocrine dynamics during the equine periovulatory period. Anim. Reprod. 2009, 6, 144–158. [Google Scholar]
- Contri, A.; Robbe, D.; Gloria, A.; De Amicis, I.; Veronesi, M.C.; Carluccio, A. Effect of the season on some aspects of the estrous cycle in Martina Franca donkey. Theriogenology 2014, 81, 657–661. [Google Scholar] [CrossRef]
- Derar, R.I.; Hussein, H.A. Ovarian follicular dynamics during the estrous cycle in jennies in upper Egypt. Vet. Med. Int. 2011, 2011, 860511. [Google Scholar] [CrossRef][Green Version]
- Li, N.; Yang, F.; Yu, J.; Yang, W.; Wu, S.; Ma, J.; Liu, B.; Zhang, R.; Zhou, X.; Losinno, L.; et al. Characteristics of follicular dynamics and reproductive hormone profiles during oestrous cycles of jennies over an entire year. Reprod. Domest. Anim. 2021, 56, 448–458. [Google Scholar] [CrossRef]
- Díaz-Duran, M.; Zarco, L.; Boeta, A.M. Ovarian dynamics and estrous cycle length in the donkey (Equus asinus). Theriogenology 2017, 103, 1–8. [Google Scholar] [CrossRef]
- Vandeplassche, G.M.; Wesson, J.A.; Ginther, O.J. Behavioral, follicular and gonadotropin changes during the estrous cycle in donkeys. Theriogenology 1981, 16, 239–249. [Google Scholar] [CrossRef]
- Kebede, H.; Lemma, A.; Negussie, H. Ultrasonographic studies on ovarian dynamics and associated estrus manifestations of jennies under controlled management, Ethiopia. Trop. Anim. Health Prod. 2012, 44, 1965–1970. [Google Scholar] [CrossRef]
- de Oliveira, S.N.; Canuto, L.; Segabinazzi, L.G.T.M.; Dell´Aqua Junior, J.A.; Papa, P.; Fonseca, M.; de Lisboa Ribeiro Filho, A.; Papa, F. Histrelin acetate-induced ovulation in Brazilian Northeastern jennies (Equus asinus) with different follicle diameters. Theriogenology 2019, 136, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Gastal, E.; Gastal, M.; Nogueira, G.; Bergfelt, D.; Ginther, O. Temporal interrelationships among luteolysis, FSH and LH concentrations and follicle deviation in mares. Theriogenology 1999, 53, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Gastal, E.L.; Bergfelt, D.R.; Nogueira, G.P.; Gastal, M.O.; Ginther, O.J. Role of luteinizing hormone in follicle deviation based on manipulating progesterone concentrations in mares. Biol. Reprod. 1999, 61, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Penitente-Filho, J.M.; Jimenez, C.R.; Zolini, A.M.; Carrascal-Triana, E.; Azevedo, J.L.; Silveira, C.O.; Oliveira, F.A.; Torres, C.A.A. Influence of corpus luteum and ovarian volume on the number and quality of bovine oocytes. Anim. Sci. J. 2015, 86, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Segabinazzi, L.G.T.M.; Roberts, B.N.; Peterson, E.W.; Ambrosia, R.; Bergfelt, D.; Samper, J.; French, H.; Gilbert, R.O. Early regnancy in jennies in the Caribbean: Corpus luteum development and progesterone production, uterine and embryo dynamics, conceptus growth and maturation. Animals 2022, 12, 127. [Google Scholar] [CrossRef]
- Ginther, O.J.; Utt, M.D.; Bergfelt, D.R.; Beg, M.A. Controlling interrelationships of progesterone/LH and estradiol/LH in mares. Anim. Reprod. Sci. 2006, 95, 144–150. [Google Scholar] [CrossRef]
- Hayes, K.E.N.; Ginther, O.J. Role of progesterone and estrogen in development of uterine tone in mares. Theriogenology 1986, 25, 581–590. [Google Scholar] [CrossRef]
- Mccue, P.; Scoggin, C.; Lindholm, A. Estrus. In Equine Reproduction; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Chichester, UK, 2011; Volume 1, pp. 1716–1727. ISBN 9788578110796. [Google Scholar]
- Blanchard, T.L.; Taylor, T.S.; Love, C.L. Estrous cycle characteristics and response to estrus synchronization in Mammoth Asses (Equus asinus americanus). Theriogenology 1999, 52, 827–834. [Google Scholar] [CrossRef]
- Taberner, E.; Medrano, A.; Peña, A.; Rigau, T.; Miró, J. Oestrus cycle characteristics and prediction of ovulation in Catalonian jennies. Theriogenology 2008, 70, 1489–1497. [Google Scholar] [CrossRef]
- Henry, M.; McDonnell, S.M.; Lodi, L.D.; Gastal, E.L. Pasture mating behaviour of donkeys (Equus asinus) at natural and induced oestrus. J. Reprod. Fertil. Suppl. 1991, 44, 77–86. [Google Scholar]
- Meira, C.; Ferreira, J.C.P.; Papa, F.O.; Tornero, M.T.T.; Bicudo, S.D. Study of the estrous cycle in donkeys (Equus asinus) using ultrasonography and plasma progesterone concentrations. Biol. Reprod. 1995, 52, 403–410. [Google Scholar] [CrossRef]
- Lemma, A.; Bekana, M.; Schwartz, H.J.; Hildebrandt, T. Ultrasonographic study of ovarian activities in the tropical jenny during the seasons of high and low sexual activity. J. Equine Vet. Sci. 2005, 25, 439–441. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Newcombe, J.R. Repeatability of preovulatory follicular diameter and uterine edema pattern in two consecutive cycles in the mare and how they are influenced by ovulation inductors. Theriogenology 2008, 69, 681–687. [Google Scholar] [CrossRef]
- Carnevale, E.M.; McKinnon, A.O.; Squires, E.L.; Voxx, J.L. Ultrasonographic characteristics of the preovulatory follicle preceding and during ovulation in mares. J. Equine Vet. Sci. 1988, 8, 428–431. [Google Scholar] [CrossRef]
- Koskinen, E.; Kuntsi, H.; Lindeberg, H.; Katila, T. Predicting ovulation in the mare on the basis of follicular growth and serum oestrone sulphate and progesterone levels. J. Vet. Med. Ser. A 1989, 36, 299–304. [Google Scholar] [CrossRef]
- Quintero, B.; Manzo, M.; Diaz, T.; Verde, 0.; Benacchio, N.; Sifontes, L. Seasonal changes in ovarian activity and estrous behavior of thoroughbred mares in a tropical environment. Biol. Reprod. 1995, 52, 469–474. [Google Scholar] [CrossRef]
- McKinnon, A.O.; Squires, E.L.; Vaala, W.E.; Varner, D.D. The estrous cycle. In Equine Reproduction; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Willey-Blackwell: Oxford, UK, 2011; pp. 1689–1770. [Google Scholar]
- Ginther, O.J.; Scraba, S.T.; Bergfelt, D.R. Reproductive seasonality of the jenney. Theriogenology 1987, 27, 587–592. [Google Scholar] [CrossRef]
- Perez-Marin, C.C.; Galisteo, I.; Perez-Rico, A.; Galisteo, J. Effects of breed, age, season, and multiple ovulations on cyclic, PGF2α-induced, and postpartum estrus characteristics in Spanish jennies. Theriogenology 2016, 85, 1045–1052. [Google Scholar] [CrossRef]
- Gastal, E.L.; Neves, A.P.; Mattos, R.C.; Petrucci, B.P.L.; Gastal, M.O.; Ginther, O.J. Miniature ponies: 1. Follicular, luteal and endometrial dynamics during the oestrous cycle. Reprod. Fertil. Dev. 2008, 20, 376–385. [Google Scholar] [CrossRef]
- Peña-Alfaro, C.E.; Barros, L.O.; Carneiro, G.F.; Gastal, M.O.; Gastal, E.L. Embryo transfer in Pega donkeys (Equus asinus) in Brazil. J. Equine Vet. Sci. 2014, 34, 185. [Google Scholar] [CrossRef]
- Rota, A.; Panzani, D.; Sabatini, C.; Camillo, F. Donkey jack (Equus asinus) semen cryopreservation: Studies of seminal parameters, post breeding inflammatory response, and fertility in donkey jennies. Theriogenology 2012, 78, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.V.; de Luna Freire Oliveira, P.V.; Melo e Oña, C.M.; Guasti, P.N.; Monteiro, G.A.; da Silva, Y.F.R.S.; de Mello Papa, P.; Alvarenga, M.A.; Dell’Aqua Junior, J.A.; Papa, F.O. Strategies to improve the fertility of fresh and frozen donkey semen. Theriogenology 2016, 85, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Marín, C.C.; Vizuete, G.; Galisteo, J.J. Embryo recovery results in Hispano-Arabe horse and Spanish donkey breeds. Livest. Sci. 2017, 206, 76–81. [Google Scholar] [CrossRef]
- Quaresma, M.; Payan-Carreira, R. Characterization of the estrous cycle of Asinina de Miranda jennies (Equus asinus). Theriogenology 2015, 83, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Duval, L.H.; Rechsteiner, S.M.F.; Gastal, G.D.A.; Gastal, M.O.; Mattos, R.C.; Gastal, E.L. Ovarian and uterine dynamics during the estrous cycle in Criollo breed mares. J. Equine Vet. Sci. 2022, 118, 104131. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.C.; Gastal, E.L.; Gastal, M.O.; Carvalho, G.R.; Beg, M.A.; Ginther, O.J. Temporal relationships and repeatability of follicle diameters and hormone concentrations within individuals in mares. Reprod. Domest. Anim. 2009, 44, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Driancourt, M.A. Use of ultrasonic echography in equine gynecology. Theriogenology 1980, 13, 203–216. [Google Scholar] [CrossRef]
- Gastal, M.O.; Pastorello, M.; Godoi, D.B.; Gastal, E.L. Dominant follicle and gonadotropin dynamics before ovulation in postpartum lactating mares. Mol. Reprod. Dev. 2022, 89, 113–124. [Google Scholar] [CrossRef]
- Gastal, E.L.; Gastal, M.O.; Ginther, O.J. The suitability of echotexture characteristics of the follicular wall for identifying the optimal breeding day in mares. Theriogenology 1998, 50, 1025–1038. [Google Scholar] [CrossRef]
- Tazawa, S.P.; Gastal, M.O.; Silva, L.A.; Evans, M.J.; Gastal, E.L. Preovulatory follicle dynamics, and ovulatory and endometrial responses to different doses of hCG and prediction of ovulation in mares. J. Equine Vet. Sci. 2017, 56, 40–51. [Google Scholar] [CrossRef]
- Gastal, E.L.; Gastal, M.O.; Ginther, O.J. Serrated granulosa and other discrete ultrasound indicators of impending ovulation in mares. J. Equine Vet. Sci. 2006, 26, 67–73. [Google Scholar] [CrossRef]
- Ginther, O.J.; Gastal, E.L.; Gastal, M.O.; Bergfelt, D.R.; Baerwald, A.R.; Pierson, R.A. Comparative study of the dynamics of follicular waves in mares and women. Biol. Reprod. 2004, 71, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Sirois, J.; Ball, B.A.; Fortune, J.E. Patterns of growth and regression of ovarian follicles during the oestrous cycle and after hemiovariectomy in mares. Equine Vet. J. 2010, 21, 43–48. [Google Scholar] [CrossRef]
- Ginther, O.J. Reproductive Biology of the Mare: Basic and Applied Aspects, 2nd ed.; Equiservices Publishing: Cross Plains, WI, USA, 1992; pp. 173–290. [Google Scholar]
- Dadarwal, D.; Tandon, S.N.; Purohit, G.N.; Pareek, P.K. Ultrasonographic evaluation of uterine involution and postpartum follicular dynamics in French Jennies (Equus asinus). Theriogenology 2004, 62, 257–264. [Google Scholar] [CrossRef]
- Segabinazzi, L.G.T.M.; Oba, E.; Alvarenga, M.A. The combination of hCG and GnRH analog to hasten ovulation in mares does not change luteal function and pregnancy outcome in embryo recipient mares. J. Equine Vet. Sci. 2021, 105, 103691. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, E.M.; Bergfelt, D.R.; Ginther, O.J. Aging effects on follicular activity and concentrations of FSH, LH, and progesterone in mares. Anim. Reprod. Sci. 1993, 31, 287–299. [Google Scholar] [CrossRef]
- Segabinazzi, L.G.T.M.; Canisso, I.F.; Podico, G.; Cunha, L.L.; Novello, G.; Rosser, M.F.; Loux, S.C.; Lima, F.S.; Alvarenga, M.A. Intrauterine blood plasma platelet-therapy mitigates persistent breeding-induced endometritis, reduces uterine infections, and improves embryo recovery in mares. Antibiotics 2021, 10, 490. [Google Scholar] [CrossRef]
- Berg, S.L.; Ginther, O.J. Effect of estrogens on uterine tone and life span of the corpus luteum in mares. J. Anim. Sci. 1978, 47, 203–208. [Google Scholar] [CrossRef]
- Hamer, J.M.; Taylor, T.B.; Evans, M.J.; Gason, L.M.; Irvine, C.H.G. Effect of administration of estradiol and progesterone, and bacterial contamination, on endometrial morphology of acyclic mares. Anim. Reprod. Sci. 1985, 9, 317–322. [Google Scholar] [CrossRef]
- Samper, J.C. A review of a practitioner’s perspective on endometrial edema. Pferdeheilkunde 2010, 26, 14–18. [Google Scholar] [CrossRef]
- Esteller-Vico, A.; Liu, I.K.M.; Vaughan, B.; Steffey, E.P.; Brosnan, R.J. Effects of estradiol on uterine perfusion in anesthetized cyclic mares affected with uterine vascular elastosis. Anim. Reprod. Sci. 2016, 164, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.A.; Glidewell-Kenney, C.; Jameson, J.L.; Moenter, S.M. Classical estrogen receptor α signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 2008, 149, 5328–5334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panigone, S.; Hsieh, M.; Fu, M.; Persani, L.; Conti, M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol. Endocrinol. 2008, 22, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Samper, J.C. Induction of estrus and ovulation: Why some mares respond and others do not. Theriogenology 2008, 70, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Pierson, R.A. Ultrasonic anatomy of equine ovaries. Theriogenology 1984, 21, 471–483. [Google Scholar] [CrossRef]
- McKinnon, A.O.; Squires, E.L.; Pickett, B.W. Equine reproductive ultrasonography. Fort Collins Color. State Univ. Anim. Reprod. Lab. 1988, 4, 31–40. [Google Scholar]
- Oliveira, S.N.; Segabinazzi, L.G.T.M.; Canuto, L.; Lisboa, F.P.; Medrado, F.E.; Dell’Aqua, J.A.; Aguiar, A.J.A.; Papa, F.O. Comparative efficacy of histrelin acetate and hCG for inducing ovulation in Brazilian Northeastern jennies (Equus africanus asinus). J. Equine Vet. Sci. 2020, 92, 103146. [Google Scholar] [CrossRef]
- Guinnefollau, L.; Bolwell, C.F.; Gee, E.K.; Norman, E.J.; Rogers, C.W. Horses’ physiological and behavioural responses during undergraduate veterinary practical teaching classes. Appl. Anim. Behav. Sci. 2021, 241, 105371. [Google Scholar] [CrossRef]
- Ille, N.; Aurich, C.; Aurich, J. Physiological stress responses of mares to gynecologic examination in Veterinary Medicine. J. Equine Vet. Sci. 2016, 43, 6–11. [Google Scholar] [CrossRef]
- Schönbom, H.; Kassens, A.; Hopster-Iversen, C.; Klewitz, J.; Piechotta, M.; Martinsson, G.; Kißler, A.; Burger, D.; Sieme, H. Influence of transrectal and transabdominal ultrasound examination on salivary cortisol, heart rate, and heart rate variability in mares. Theriogenology 2015, 83, 749–756. [Google Scholar] [CrossRef]
- Peterson, E.W.; Segabinazzi, L.G.T.M.; Gilbert, R.O.; Bergfelt, D.R.; French, H.M. Evaluation of stress accompanying immunocontraceptive vaccination in donkeys. Animals 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Segabinazzi, L.G.T.M.; Landers, M.; Kent, A.; Peterson, E.; Gilbert, R.; French, H. Efficacy and side effects of low single doses of cloprostenol sodium or dinoprost tromethamine to induce luteolysis in donkeys. J. Equine Vet. Sci. 2021, 107, 103774. [Google Scholar] [CrossRef] [PubMed]
- French, H.; Peterson, E.; Schulman, M.; Roth, R.; Crampton, M.; Conan, A.; Marchi, S.; Knobel, D.; Bertschinger, H. Efficacy and safety of native and recombinant zona pellucida immunocontraceptive vaccines in donkeys. Theriogenology 2020, 153, 27–33. [Google Scholar] [CrossRef] [PubMed]
- French, H.; Segabinazzi, L.; Middlebrooks, B.; Peterson, E.; Schulman, M.; Roth, R.; Crampton, M.; Conan, A.; Marchi, S.; Gilbert, T.; et al. Efficacy and safety of native and recombinant zona pellucida immunocontraceptive vaccines Formulated with Non-Freund’s Adjuvants in donkeys. Vaccines 2022, 10, 1999. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD |
|---|---|
| Interval (days) from | |
| Length of IOI | 22.9 ± 2.0 |
| Ovulation to follicular wave emergence | |
| Primary waves | 5.7 ± 3.6 |
| Secondary waves | 19.8 ± 2.9 |
| Ovulation to follicle deviation | 14.1 ± 2.9 |
| Primary wave emergence to deviation | 8.4 ± 2.1 |
| Follicle deviation to ovulation | 8.2 ± 1.4 |
| Follicle diameters (mm) | |
| Dominant follicle at deviation | 14.5 ± 2.6 |
| Largest subordinate follicle at deviation | 13.3 ± 1.8 |
| Dominant follicle maximum diameter prior to ovulation | 34.6 ± 2.9 |
| Follicle growth rate (mm/day) | |
| Emergence to deviation | |
| Dominant follicle | 1.5 ± 0.7 |
| Largest subordinate follicle | 1.6 ± 0.6 |
| Deviation to ovulation | |
| Dominant follicle | 2.6 ± 0.7 |
| Number of follicles per day | |
| Small (≥5 to ≤10 mm) | 8.3 ± 1.1 |
| Medium (11 to 19 mm) | 3.5 ± 1.0 |
| Large (≥20 mm) | 0.6 ± 0.5 |
| Total | 12.5 ± 1.7 |
| Corpus luteum | |
| Day of maximum size after ovulation | 5.4 ± 0.4 |
| Maximum size (mm3) | 8.5 ± 2.7 |
| Day of maximum progesterone concentration | 7.8 ± 0.8 |
| Maximum progesterone concentration (ng/mL) | 27.0 ± 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segabinazzi, L.G.T.M.; Gilbert, R.O.; Ambrosia, R.L.; Bergfelt, D.R.; Samper, J.C.; Peterson, E.W.; French, H.M. Structural and Functional Dynamics of the Ovary and Uterus during the Estrous Cycle in Donkeys in the Eastern Caribbean. Animals 2023, 13, 74. https://doi.org/10.3390/ani13010074
Segabinazzi LGTM, Gilbert RO, Ambrosia RL, Bergfelt DR, Samper JC, Peterson EW, French HM. Structural and Functional Dynamics of the Ovary and Uterus during the Estrous Cycle in Donkeys in the Eastern Caribbean. Animals. 2023; 13(1):74. https://doi.org/10.3390/ani13010074
Chicago/Turabian StyleSegabinazzi, Lorenzo G. T. M., Robert O. Gilbert, Rachael L. Ambrosia, Don R. Bergfelt, Juan C. Samper, Erik W. Peterson, and Hilari M. French. 2023. "Structural and Functional Dynamics of the Ovary and Uterus during the Estrous Cycle in Donkeys in the Eastern Caribbean" Animals 13, no. 1: 74. https://doi.org/10.3390/ani13010074
APA StyleSegabinazzi, L. G. T. M., Gilbert, R. O., Ambrosia, R. L., Bergfelt, D. R., Samper, J. C., Peterson, E. W., & French, H. M. (2023). Structural and Functional Dynamics of the Ovary and Uterus during the Estrous Cycle in Donkeys in the Eastern Caribbean. Animals, 13(1), 74. https://doi.org/10.3390/ani13010074





