Simple Summary
Mucosal morphology and microbiota composition are key components of the gut development of calves. Adverse health events during pre-weaning and diet changes at weaning can create a lasting impact on those components. Phytogenic feed additives such as the Macleaya cordata plant extract preparations (MCE) improved the gut morphology and microbiota composition of pigs and poultry in previous studies. In this study, MCE was fed to dairy × beef crossbred calves before (6 to 45 d) and after weaning (45 to 90 d). The cumulative impact on the ruminal and small intestinal morphology and microbiota composition were evaluated at 95 d of age. Despite the minor changes in ruminal and intestinal microbial populations, MCE increased the rumen papillae length and villus height: crypt depth in the small intestine, suggesting the potential to enhance gut health and development.
Abstract
The objective was to determine the impact of feeding MCE on ruminal and intestinal morphology and microbiota composition of calves. A total of 10 male and 10 female crossbred (dairy × beef) calves (6 d of age) were assigned randomly to control (CTL; n = 10) or MCE-supplemented (TRT; n = 10) groups. The MCE was fed in the milk replacer and top-dressed on the calf starter during pre-weaning (6 to 49 d) and post-weaning (50 to 95 d) periods, respectively. Calves were slaughtered at 95 d to collect rumen and intestinal samples to determine volatile fatty acid (VFA) profile, mucosal morphology, and microbiota composition. The effects of MCE were analyzed by accounting for the sex and breed effects. Feeding MCE increased rumen papillae length (p = 0.010) and intestinal villus height: crypt depth (p < 0.030) compared to CTL but did not affect rumen VFA profile. The TRT had a negligible impact on microbial community composition in both the rumen and the jejunum. In conclusion, feeding MCE from birth through weaning can improve ruminal and small intestinal mucosa development of calves despite the negligible microbiota composition changes observed post-weaning.
1. Introduction
Macleaya cordata, commonly called plume poppy, is a plant native to China and Japan, where it is used for medicinal purposes. M. cordata belongs to the tribe Chelidonieae of the family Papaveraceae, which produces colored latex rich in alkaloids [1]. Among those alkaloids, sanguinarine and chelerythrine are predominant and possess antimicrobial and anti-inflammatory properties [2] that have been shown to modulate gut microbiota and improve small intestinal morphology to enhance growth [3]. Therefore, plant extracts rich in those alkaloids can substitute antibiotics aiming to promote the growth of young animals [3,4,5]. In support, dietary supplementations of MCE enhanced intestinal morphology (e.g., longer villi) and increased the average daily gain (ADG) of pigs and chickens [3,6,7]. Similar effects could be hypothesized for calves, as Lima et al. [8] demonstrated that MCE improved the ruminal epithelial development in lambs. Dietary MCE has been also shown to affect VFA profiles in the cecum of broiler chickens [9], suggesting an ability of MCE to modulate gut microbiota composition, an important component of gut development. Liu et al. [10] demonstrated that dietary MCE increased the abundance of beneficial bacteria such as Lactobacillus and Bifidobacterium in the caecum, whereas it decreased the abundance of pathogenic bacteria such as E. coli and Salmonella in the ileum of piglets. The impact of MCE on the gut microbiota composition of cattle is unknown. Given the unique complexity of the ruminal gastrointestinal tract, the findings of monogastric animals cannot be extrapolated directly to cattle. On the other hand, ruminal VFA profiles can reflect the changes in ruminal bacteria composition. For instance, increased propionate and butyrate concentrations were associated with an increased abundance of Lactobacillaceae in the rumen of calves [11]. High propionate and butyrate concentrations in the rumen can stimulate ruminal papillae development and feed intake [12,13]. Interestingly, ruminal papillae development can be positively associated with the small intestinal mucosal development of calves [14]. Calf diarrhea and weaning stresses can impede gastrointestinal development of calves, and nutritional interventions are key to ameliorate those effects [15,16]. Hence, it was hypothesized that feeding MCE from birth through weaning would improve the ruminal and small intestinal development of calves. The study objective was to determine the effects of feeding MCE in milk replacers (pre-weaning) and in starter feed (post-weaning) on the mucosal morphology and microbiota composition in the rumen and the small intestine of weaned calves.
2. Materials and Methods
2.1. Animals and Treatments
All animal-related procedures were conducted under the approval of the Animal Care and Use Committee of Iowa State University (IACUC-19-204). The experiment was conducted at the Dairy Research and Teaching Farm at Iowa State University (Ames, IA). Forty dairy (Holstein) × beef (Angus or Simmental) crossbred calves (20 male and 20 female) at 5 d of age were balanced for sex, and assigned to four doses (0, 2, 5 and 10 g/d pre-weaning, and 0, 4, 10 and 20 g/d post-weaning) of an MCE preparation (Sangrovit®, Phytobiotics Futterzusatzstoffe GmbH, Eltville, Germany). Dose-dependent effects of MCE on feed intake, growth, and safety are reported in Matulka et al. [17]. Given the linear increase in calf starter intake with MCE dose [17] and the positive relationship between starter intake and gut development of calves [18], the gut morphology and microbiota analyses were performed only on calves in 0 g/d (CTL) and the highest dose (10 calves per treatment group). The latter is referred to as the MCE-feeding group (TRT) in this paper, reporting only the results of those two analyses. Further, a statistical power analysis (power = 80% and α = 0.05) using the data from Alves Costa et al. [19] revealed that the sample size = 10 could be adequate to capture 10 and 15% change from average rumen papillae length and villus height of crossbred calves, respectively. The sample size = 10 was assumed to be adequate to capture treatment differences in gut microbiota community composition, as Wang et al. [20] captured such differences in rats in response to feeding an MCE with a sample size = 6.
The baseline (5 d of age) serum total protein by refractometer (mean = 7.30% and SD = 0.77%) and the body weight (mean = 45.3 kg and SD = 6.5 kg) were similar between CTL and TRT. Calves were housed individually in outdoor calf hutches (2.4 m × 1.4 m × 1.3 m) bedded with straw throughout the study. The MCE preparation (0 or 10 g/d) was fed in milk replacer during the pre-weaning period (up to 49 d). The feeding rate of MCE was doubled (20 g/d) to be in line with the dry matter intake increase [16] and fed with a calf starter during the post-weaning period (50 to 95 d). The daily dose of MCE was top-dressed on a calf starter by mixing with the topmost layer (about 1–2” deep) of the starter in the morning (0700 h). When needed, the starter buckets were refilled in the afternoon by placing the morning feed on top of the new feed to ensure complete consumption of the MCE dose. The composition of the milk replacer (Land O’Lakes®, St. Louis, MI, USA) and the calf starter (Farmers Win Coop®, Houston, MN, USA) is given in Table 1.

Table 1.
Ingredient and nutrient composition of milk replacer and calf starter.
2.2. Feeding and General Management
2.2.1. Pre-Weaning
A total of 6.0 L of liquid milk replacer (12% solids) was fed daily at two feedings (3.0 L per feeding at 0730 and 1930 h) until the beginning of weaning. One-half of the MCE dose was hand-mixed with 3.6 kg of dry milk replacer, which was then reconstituted with 27.0 kg of lukewarm water (40 to 45 °C) to prepare the liquid milk replacer at a single feeding. In addition to the MCE dose, 10 g of a coccidiostat containing decoquinate (Zoetis, Parsippany-Troy Hills, NJ, USA) was added to dry milk replacer of both CTL and TRT until calves were 28 d of age. The weaning process was started at 42 d of age by eliminating the morning milk replacer feeding. All the calves then received only 3.0 kg/d liquid milk replacer until weaned completely at 49 d of age. For TRT calves, the total MCE dose was fed in 3.0 kg/d liquid milk replacer during weaning (d 42 to 49). The weights of liquid milk replacer offered to and left over by individual calves were recorded daily.
2.2.2. Post-Weaning
The MCE dose was top-dressed on calf starter (110% of previous day intake) and offered to calves at 0700 h during the post-weaning period. To avoid losses due to wind and animal activities (e.g., coughing and exhaling), the dose was gently mixed into the topmost layer (about one inch deep) of the calf starter before offering to the animals. The amounts of calf starter offered, and leftover were recorded daily. Multiple batches of starter feed were used during the study. Representative samples of each batch were analyzed for nutrient composition using the standard procedures (Cumberland Valley Analytical Services, Waynesboro, PA, USA). The average composition is presented in Table 1.
2.3. Ruminal and Intestinal Sample Collection
At the end of the study (95 d of age), calves were slaughtered to collect digesta samples and segments of the rumen and the small intestine for microbiota analysis and morphometry analysis, respectively. The slaughtering involved stunning with a captive bolt pistol followed by immediate exsanguination at the livestock infectious disease isolation facility of Iowa State University (Ames, IA, USA). About 300 g of rumen content of the ventral sac and about 50 g of digesta from the jejunum were retrieved and placed immediately in dry ice until transfer to a −80 °C freezer. The jejunum was specifically chosen for the microbiome analysis (described below), as both digesta and mucosa of the jejunum were shown to have a greater and similar density of bacteria (numbers per gram wet weight) compared to those of the duodenum and ileum in cattle, respectively [21]. Additionally, 4.0–6.0 cm2 segments of the rumen (ventral sac), and 2–3 cm long segments of the duodenum, jejunum (15 and 80 cm distal to pyloric sphincter, respectively), and ileum (15 cm proximal to ileocecal junction) were obtained. All samples were flushed with saline to remove any digesta and were fixed in 10% neutral-buffered formaldehyde (Shandon Formal-Fixx®, Thermo Scientific, Waltham, MA, USA) for the morphometric analysis described below.
2.4. Morphometry Analysis of Rumen and Small Intestinal Mucosa
The rumen, duodenum, jejunum, and ileum samples were fixed in 10% neutral buffered formalin and submitted for tissue sectioning and imaging at the Veterinary Diagnostic Laboratory at Iowa State University (Ames, IA, USA). After trimming into plastic cassettes, specimens were routinely processed overnight (Tissue-Tek VIP 6, Sakura Finetek, Tokyo, Japan), paraffin-embedded, and cut to 4-micron-thick sections on an automated microtome (Leica Microtome RM2255, Leica Biosystems, Deer Park, IL USA). Tissue sections were placed on glass slides (Surgipath Snowcoat clipped corner slides, Leica Biosystems, Deer Park, IL USA) and stained with hematoxylin and eosin on an automated stainer (Tissue-Tek Prisma Autostainer, Sakura Finetek, Tokyo, Japan) and glass coverslipped (G2 Automated Glass Coverslipper, Sakura Finetek, Tokyo, Japan). The images of tissue sections were obtained at 10× magnification using an Olympus DP73 camera mounted onto an Olympus B53 microscope (Olympus Corporation, Shinjuku City, Tokyo, Japan). Papillae length and width, villus height, and crypt depth in three non-overlapping fields of each tissue cross-section were measured using the ImageJ software (National Institutes of Health, Bethesda, MD, USA). The arbitrary units obtained from the ImageJ software were converted to mm by using a reference image obtained from the microscope. The length and width of rumen papillae and villus height and crypt depth of the small intestine were averaged across the three fields of each image representing a sample from an individual animal. The villus height was divided by corresponding crypt depth to calculate villus height to crypt depth ratio.
2.5. Rumen VFA Analysis
Rumen content samples were thawed on ice and squeezed through four layers of cheesecloths to collect rumen fluid (~15 mL per sample). All solids and approximately 5 mL of rumen fluid were stored again at −80 °C for the microbiota composition analyses. The remaining liquid was centrifuged at 4 °C and 9000× g for 15 min. Then, 5 mL of the supernatant was mixed with 1.0 mL of 25% metaphosphoric acid and centrifuged again for 10 min at 4000× g at room temperature, and the supernatant was separated. Solutions of acetate, propionate, butyrate, isobutyrate, valerate, and isovalerate, each at 1:1, 1:2, 1:4, and 1:6 dilutions were prepared using deionized water and used as the standards. 100 µL of 2-ethyl butyric acid was added as an internal standard to all samples as well as the standards. Concentrations of VFA in samples were analyzed using Varian CP-3800 Gas Chromatograph (Varian Medical Systems, Palo Alto, CA, USA).
2.6. DNA Extraction and Sequencing
DNA was extracted from 0.25 g of each rumen liquid and solid and jejunal digesta and mucosa samples using the Qiagen DNeasy Powersoil Powerlyzer Kit® (Qiagen Sciences Inc., Germantown, MD, USA) according to the protocol provided by the manufacturer. The process included a bead-beating step for one minute at 22 °C using a FisherbrandTM Bead Mil 24 Homogenizer (Fisher Scientific, Portsmouth, NH, USA) for mechanical lysis of the cells. The concentration of DNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) and the concentrations were adjusted to 25 to 50 ng of DNA/µL using PCR-grade water. All samples were stored at −80 °C until being submitted to Iowa State University DNA Facility (Ames, IA, USA) for sequencing.
DNA sequencing was conducted at the Iowa State University DNA Facility using an Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA) and a protocol designed to amplify the 16S rRNA genes of bacteria and archaea. Template DNA from each sample (one replicate per sample) was amplified using the PlatinumTM Hot Start PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) with the 515F forward-barcoded primer (GTGYCAGCMGCCGCGGTAA) [22], and the 806R reverse primer (GGACTACNVGGGTWTCTAAT) [23]. All samples underwent an initial denaturation step at 94 °C for 2 min, followed by denaturation at 94 °C for 45 s, annealing at 50 °C for 20 s, and extension at 72 °C for 90 s. Those steps were repeated 35 times. Amplicons from each sample were run on an agarose gel with an expected band size ranging from 300 to 350 bp. Amplicon pools were cleaned using Qiagen’s QIAquick PCR Purification Kit (Qiagen Sciences Inc., Germantown, MD, USA). Raw sequencing data from each sample location was analyzed separately using the mothur software (v1.43.0) [24]. The number of raw reads per sample varied from 13,742 to 473,268. All reads were subjected to quality control using the “screen.seqs” command to remove contigs with ambiguous bases, homopolymers greater than eight bases in length, as well as contigs below a minimum length of 252 bases. Sequences were aligned against the SILVA SSU database (v138) and sequences outside the target variable region were removed [25]. Possible chimeric sequences were detected and removed using the “chimera.vsearch” command and the SILVA.gold reference database provided by the mothur website (https://mothur.org/wiki/silva_reference_files/, accessed on 15 January 2021). The remaining 9,482,307 high-quality sequences were clustered into de novo operational taxonomic units (OTU) with a 99% similarity threshold using the “cluster.split” command. The OTU abundance data were imported into R v3.6.2 for further processing. OTUs represented by fewer than 10 reads were removed, resulting in a total of 2606 OTUs in jejunal content, 1984 OTUs in jejunal mucosa, 2761 OTUs in rumen solid and 2895 OTUs in rumen liquid samples. Alpha diversity parameters including Chao 1, Shannon, Simpson, and inverse-Simpson indices were determined by using the Phyloseq R package (v1.32, https://github.com/joey711/phyloseq, accessed on 15 January 2021) [26]. Beta diversity was analyzed by using the Adonis and beta-dispersion tests with the R package vegan. Genera abundance heatmaps were generated with ggplot2 (v3.2.1, https://CRAN.R-project.org/package=ggplot2, accessed on 15 January 2021) using a color palette from the viridis package (v0.5.1, https://CRAN.R-project.org/package=viridis, accessed on 15 January 2021) in the R scripting language (The R Foundation, Indianapolis, IN, USA).
2.7. Statistical Analysis
The MCE dose effect on gut morphology and rumen VFA measurements, alpha-diversity indices of gut microbiota composition, and relative abundance of 50 most abundant genera or 100 most abundant OTU were analyzed by using the GLM procedure of SAS 9.4 (SAS Institute Inc., Cary, NC, USA) with the following model:
where Yijklm = the response variable of interest, μ = overall mean, Ti = treatment effect (i = CTL and TRT), Sj = effect of sex, Bk = effect of breed, (T × S)ij = interaction effect between treatment and sex, and eijklm = the residual error. When analyzing the villus height, crypt depth, and villus height to crypt depth ratio, the fixed effect of the region (duodenum, jejunum, or ileum) was included in the model, and the treatment effect within each region was assessed with the slice option of the SAS. The significance of treatment effects on the microbiota abundance was assessed by adjusting the raw p-values for false discovery rates (FDR) and by calculating FDR-adjusted p-values (q-values) according to Benjamini and Hochberg [27].
Yijkl = μ + Ti + Sj + Bk + (T × S)ij + eijkl
3. Results and Discussion
3.1. Effects of MCE on Gut Morphology and Rumen VFA
The effects of feeding MCE on rumen papillae and small intestinal morphology are presented in Table 2. In alignment with weaned piglets [28], TRT increased villus height and villus height: crypt depth in duodenum and ileum (p < 0.070), and villus height: crypt depth in the jejunum (p = 0.022) of the small intestine. Additionally, TRT increased crypt depth in the jejunum compared to CTL (p = 0.033). The data did not support an effect of sex (p = 0.250), sex × treatment (p = 0.130), or breed (p = 0.760) on any intestinal morphology parameters across the duodenum, jejunum, and ileum. Taller villi represent increased digestive and absorptive capacities, whereas deeper crypts can indicate inflammations in the intestinal epithelium [29]. Moreover, high villus height to crypt depth ratios can be a positive indicator of intestinal barrier function, reducing pathogen and toxin transfer from intestinal digesta to the bloodstream [29,30]. Feeding MCE also increased papillae length in the rumen (p = 0.013), while papillae width remained similar between CTL and TRT suggesting an elevated capacity for VFA absorption. In support, Lima et al. [8] demonstrated an increased surface area of rumen papillae in MCE-fed sheep. The data did not support an effect of sex (p = 0.649) or sex × treatment (p = 0.197) on papillae length. The papillae width of male calves was greater than that of female calves (535 vs. 485 mm, p = 0.002). Nevertheless, the consistent responses of both rumen papillae and intestinal villi to MCE agreed with Górka et al. [31], demonstrating simultaneous responses of the rumen and small intestine development to nutritional interventions in dairy calves. The improvements in the ruminal and intestinal morphology can be a result of sanguinarine and chelerythrine in MCE that inhibited the programmed cell death of intestinal mucosa of rats [32] and enhanced the recovery from enteritis in broiler chicks [33]. Given the high risk of intestinal damage and the responsiveness of the intestine to dietary bioactive compounds in pre-weaning calves [34], the improved intestinal morphology observed post-weaning could be a result of a response to MCE pre-weaning. A comprehensive evaluation representing both pre- and post-weaning stages could better explain the effects of MCE on the gut morphology of calves.

Table 2.
The least-square means and associated standard error of the mean (SEM) for parameters representing gastrointestinal morphology in control (CTL) and MCE-fed calves (TRT).
The effects of MCE on VFA concentrations and the molar percentages in the rumen are presented in Table 3. The MCE in the diet did not affect (p > 0.100) the individual or total VFA concentrations, which are net outcomes of VFA production and VFA absorption in the rumen. High molar percentages of butyrate and propionate have been shown to stimulate the morphological and metabolic developments of the rumen [14]. Due to the greater VFA absorption potential indicated by longer papillae of TRT than CTL, one could speculate an increased VFA production in response to feeding MCE. The sum of propionate and butyrate molar percentages increased (25%) only numerically in TRT compared to CTL (p = 0.154). MCE did not affect the molar percentages of acetate, valerate, isovalerate, or isobutyrate (p > 0.550). On the contrary, Aguilar-Hernandez et al. [35] observed increased molar percentage of acetate and decreased molar percentages of valerate and isovalerate in the rumen of steers fed an MCE preparation. Differences in the basal diet composition (calf starter vs. total mixed ration), MCE feeding duration (90 vs. 21 d), and MCE feeding frequency (once vs. twice per d) between two studies might explain the discrepancy. Overall, the VFA data do not corroborate the MCE-induced rumen papillae development. Potentially, the current sampling time (13 wk) missed the fermentation responses that preceded the papillae development response to feeding MCE. van Niekerk et al. [36] observed significant changes in rumen VFA profiles at an earlier age (7–9 wk of age) than the age they could capture papillae development changes (12 wk of age) in calves weaned at 6 wk of age. Moreover, the present data did not support an effect of sex (p = 0.330), sex × treatment (p = 0.120), or breed (p = 0.270) on concentration or molar percentage of VFA in the rumen of post-weaned calves.

Table 3.
The least-square means and associated standard error of the mean (SEM) for volatile fatty acid (VFA) concentrations and molar percentages of VFA in the rumen of control (CTL) and MCE-fed calves (TRT).
3.2. Digesta- and Mucosa-Associated Microbiota in the Jejunum of Weaned Calves
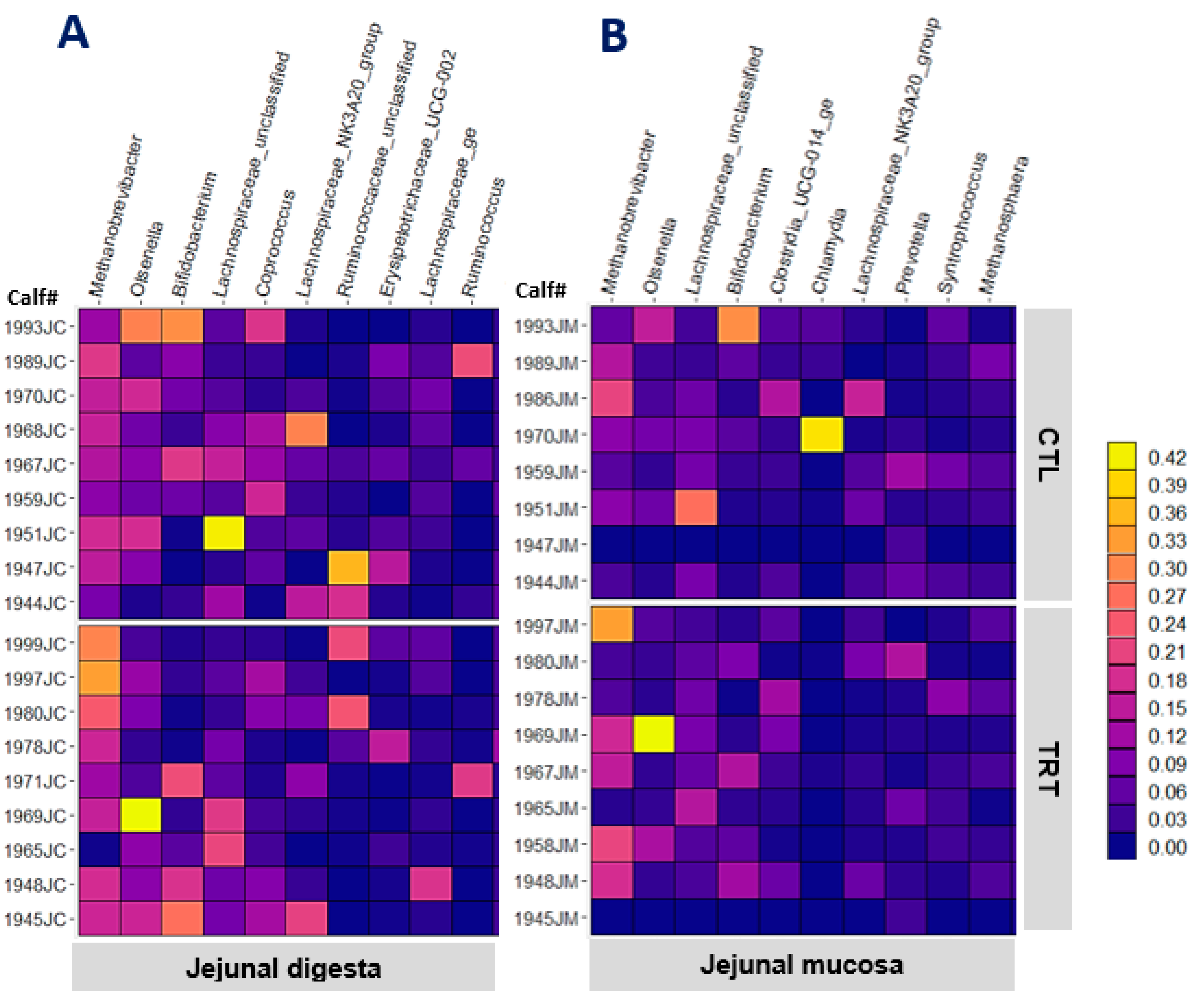
Feeding MCE did not affect any of the alpha diversity indices of digesta- or mucosa-associated microbiota in the jejunal digesta or jejunal mucosa (p > 0.220). The heat map in Figure 1 depicts the relative abundance of the 10 most abundant genera in jejunal mucosa and jejunal digesta. Methanobrevibacter, Olsenella, Lachnospiraceae_UC, Bifidobacterium_UC, and Clostridia_UCG-014_ge were the most abundant genera common across the digesta and mucosa of the jejunum. The genera previously reported to be the most abundant in the intestinal mucosa of pre-weaned calves were different from that of the weaned calves in this study [21]. This discrepancy corroborates the notion that diet change at weaning is a predominant factor shaping the gut microbiota composition of calves [34,37]. Table 4 presents the top genera having different relative abundance between CTL and TRT calves according to raw p-values from the statistical analysis (p ≤ 0.050). Feeding MCE decreased the abundance of Erysipelotrichaceae_UC in the digesta (p = 0.016) and Lachnospiraceae_NK3A20_group in the mucosa (p = 0.037). The high FDR-adjusted p-values (q > 0.80).), however, point out a lack of strong evidence to support those effects. Table 5 presents OTUs among the 100 most abundant, showing different relative abundances between CTL and TRT calves based on the raw p-values (p ≤ 0.050). The abundance of two digesta OTUs closely related to Eubacterium pyruvativoran (p = 0.025), and Gastranaerophilales_UC (p = 0.045) seem to be different between TRT and CTL calves. The abundance of four mucosa-associated OTUs also seem to be different between CTL and TRT calves (p < 0.050). Nevertheless, the high FDR-adjusted p-values (q > 0.550) signify the lack of strong evidence to support an effect of feeding MCE from birth on the microbiota composition of jejunal mucosa of weaned calves. The digesta and mucosa samples from a greater number of animals at multiple time points (e.g., both pre-weaning and post-weaning) could have increased the likelihood of deriving robust conclusions about the MCE effects. Nonetheless, studies such as this one investigating the microbiota composition of the calf small intestine are limited in literature. Most of the available studies rely on fecal samples to describe intestinal microbiota composition.
Figure 1.
The relative abundance of the 10 most abundant genera in jejunal digesta (A) and jejunal mucosa (B) of weaned calves (95 d old) in control (CTL) and MCE-fed (TRT) groups.

Table 4.
Genera within the 50 most abundant showing differences (p ≤ 0.05) between control (CTL) and MCE-fed (TRT) calves.

Table 5.
Operational taxonomic units (OTU) within the 100 most abundant showing differences (p ≤ 0.05) between control (CTL) and MCE-fed (TRT) calves.
3.3. Liquid- and Solid-Associated Microbiota in the Rumen of Weaned Calves
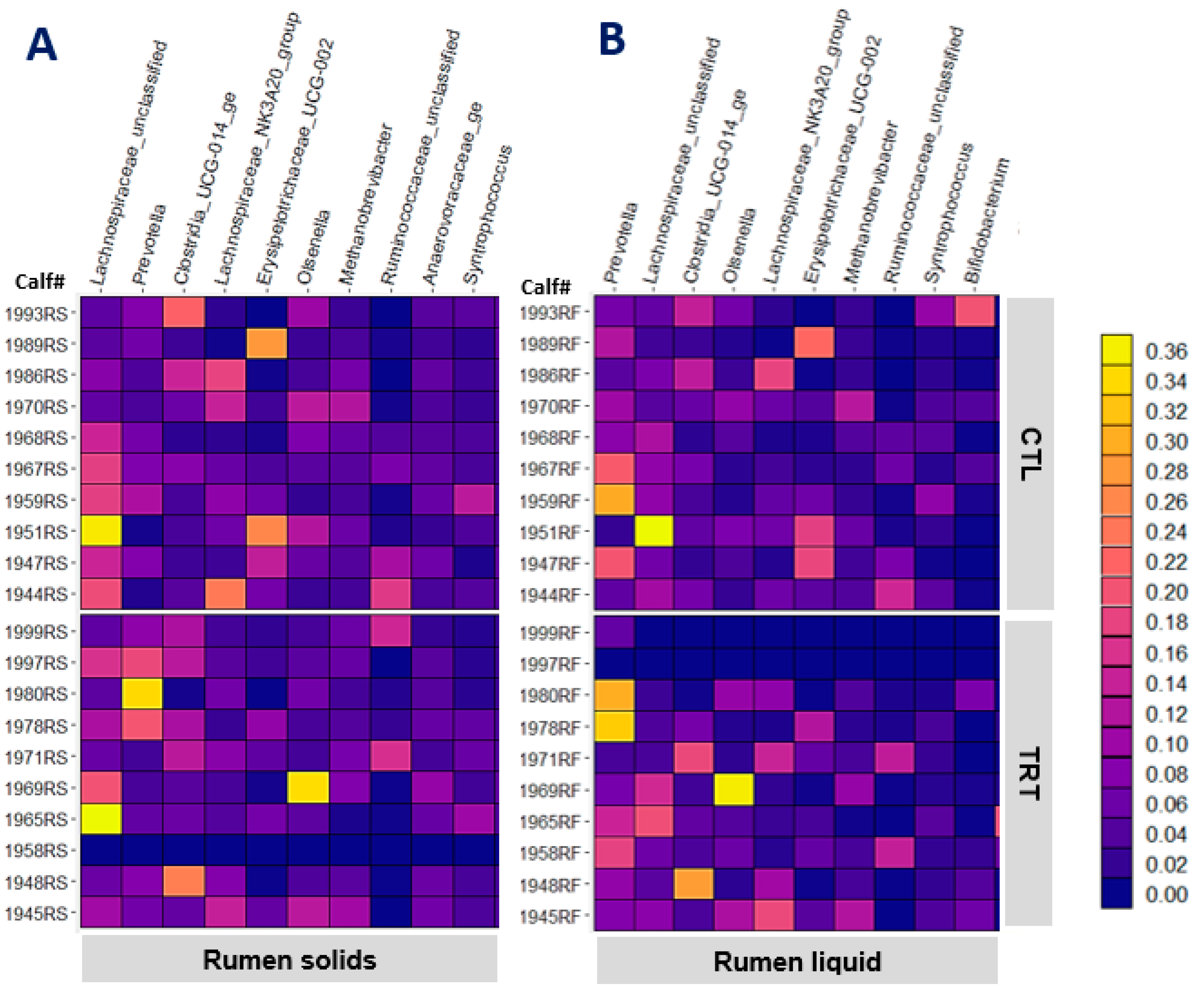
Feeding MCE did not affect any of the alpha diversity indices of liquid- or solid-associated taxa in the rumen (p > 0.230). The heat maps in Figure 2A,B depict the relative abundance of the 10 most abundant genera in rumen solid and liquid, respectively. The most abundant jejunal genera, Lachnospiraceae_UC, Lachnospiraceae_NK3A20_group, Olsenella, and Methanobrivibacter were also among the most abundant bacteria in the rumen. Of the 50 most abundant genera in each solid or liquid-associated microbiota, feeding MCE was confirmed with strong evidence (p = 0.002 and q = 0.110, Table 4) to have an impact on the abundance of only the genus Lachnospira in rumen solid. Feeding MCE since birth decreased the abundance of Lachnospira in the rumen solid of weaned calves. The low abundance of Lachnospira could be beneficial as Huang et al. [38] demonstrated a lower abundance of Lachnospira in the rumen of cows with high feed efficiency than those with low feed efficiency. Based on the raw p-values, the abundance of OTU60 (rumen solid) and OTU89 (rumen liquid) closely related to Blautia caecimuris decreased in response to feeding MCE. Regardless of the q-values not supporting robust conclusions (q ≥ 0.390), the abundance reduction consistent in both rumen solid and liquid corroborates an inhibitory effect of MCE on Blautia caecimuris in the rumen.
Figure 2.
The relative abundance of the 10 most abundant genera in rumen solids (A) and rumen liquid (B) of weaned calves (95 d old) in control (CTL) and MCE-fed (TRT) groups.
4. Conclusions
Feeding MCE from birth through weaning increased papillae length in the ventral sac of the rumen, villus height in the duodenum and ileum, and villus height: crypt depth ratio in the duodenum, jejunum, and ileum of weaned calves (13 wk of age). Feeding MCE, however, did not change the rumen VFA profile and had negligible effects on microbial community composition in the rumen and the jejunum of those calves. Future studies with periodic sampling covering both pre-weaning and post-weaning stages are warranted to better understand the effects of MCE on the gut development of calves.
Author Contributions
The authors contributed to each of the following areas of the preparation of this study and manuscript: Conceptualization, J.D., F.R.B.R. and R.A.; methodology, J.D., F.R.B.R., P.J.G., J.W., C.J.A., S.S.-E. and R.A.; software, S.S.-E. and R.A.; validation, R.A. and. S.S.-E.; formal analysis, J.W. and C.J.A.; investigation, J.W., C.J.A. and C.A.K.; resources, R.A. and S.S.-E.; data curation, C.J.A. and R.A.; writing—original draft preparation, J.W. and R.A.; writing—review and editing, R.A., J.W., C.J.A. and S.S.-E.; visualization, S.R. and R.A.; supervision, R.A. and S.S.-E.; project administration, J.W., C.A.K. and P.J.G.; funding acquisition, J.D. and F.R.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded (ISU020627.1) by Phytobiotics Futterzusatzstoffe GmbH (Eltville, Germany). The funders had no role in design of the experiments and interpretation of the data.
Institutional Review Board Statement
The animal care and handling procedures used in this study were approved by the Institutional Animal Care and Use Committee of Iowa State University, Ames, IA, USA (IACUC-19-204).
Informed Consent Statement
Not applicable as this research did not involve humans.
Data Availability Statement
All datasets collected and analyzed during the current study are available from the corresponding author on fair request.
Acknowledgments
The authors are thankful to the staff at the Dairy Research and Teaching Farm of Iowa State University (Ames, IA, USA).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the experiments and interpretation of the data.
References
- Pěnčíková, K.; Urbanová, J.; Musil, P.; Táborská, E.; Gregorová, J. Seasonal variation of bioactive alkaloid contents in Macleaya microcarpa (Maxim.) Fedde. Molecules 2011, 16, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Kudera, T.; Doskocil, I.; Salmonova, H.; Petrtyl, M.; Skrivanova, E.; Kokoska, L. In Vitro Selective Growth-Inhibitory Activities of Phytochemicals, Synthetic Phytochemical Analogs, and Antibiotics against Diarrheagenic/Probiotic Bacteria and Cancer/Normal Intestinal Cells. Pharmaceuticals 2020, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Wang, X.-L.; Ou, S.-Q.; Hou, D.-X.; He, J.-H. Sanguinarine Modulate Gut Microbiome and Intestinal Morphology to Enhance Growth Performance in Broilers. PLoS ONE 2020, 15, e0234920. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Thames, C.H.; Pruden, A.; James, R.E.; Ray, P.P.; Knowlton, K.F. Excretion of antibiotic resistance genes by dairy calves fed milk replacers with varying doses of antibiotics. Front. Microbiol. 2012, 3, 139. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, B.; Zhao, Y.; Yao, K.; Fu, C. Effects of natural dietary supplementation with Macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Kantas, D.; Papatsiros, V.G.; Tassis, P.D.; Athanasiou, L.V.; Tzika, E.D. The Effect of a Natural Feed Additive (Macleaya cordata) containing Sanguinarine, on the Performance and Health Status of Weaning Pigs. Anim. Sci. J. 2015, 86, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.R.F.; Gallo, S.B.; Rosa, A.F.; Brochado, T.; Bezerra, H.V.A.; Putrino, S.M.; Leme, P.R. Effect of Macleaya cordata and Magnolia officinalis plant extracts on oxidative stress control in lambs fed a high-concentrate diet. Asian-Australas. J. Anim. Sci. 2020, 33, 913–920. [Google Scholar] [CrossRef]
- Zdunczyk, Z.; Gruzauskas, R.; Juskiewicz, J.; Semaskaite, A.; Jankowski, J.; Godycka-Klos, I.; Jarule, V.; Mieželiene, A.; Alencikiene, G. Growth performance, gastrointestinal tract responses, and meat characteristics of broiler chickens fed a diet containing the natural alkaloid sanguinarine from Macleaya cordata. J. Appl. Poult. Res. 2010, 19, 393–400. [Google Scholar] [CrossRef]
- Liu, G.; Guan, G.; Fang, J.; Martínez, Y.; Chen, S.; Bin, P.; Duraipandiyan, V.; Gong, T.; Tossou, M.C.B.; Al-Dhabi, N.A. Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets. BioMed Res. Int. 2016, 2016, 1069585. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Liu, X.; Zhao, X.; Liu, S.; Li, Y.; Zhang, Y. Growth, health, rumen fermentation, and bacterial community of Holstein calves fed Lactobacillus rhamnosus GG during the pre-weaning stage. J. Anim. Sci. 2019, 97, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Jesse, B. Effect of volatile fatty acid infusion on development of the rumen epithelium in neonatal sheep. J. Dairy Sci. 1997, 80, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; McLeod, K.; Klotz, J.; Heitmann, R. Rumen development, intestinal growth and hepatic metabolism in the pre-and postweaning ruminant. J. Dairy Sci. 2004, 87, 55–65. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Wu, X.; Chen, W.B.; Zhang, Y.W.; Jiang, Y.M.; Meng, Q.X.; Zhou, Z.M. Growth performance and development of internal organ, and gastrointestinal tract of calf supplementation with calcium propionate at various stages of growth period. PLoS ONE 2017, 12, e0179940. [Google Scholar] [CrossRef]
- Fischer, A.J.; Villot, C.; van Niekerk, J.K.; Yohe, T.T.; Renaud, D.L.; Steele, M.A. Invited Review: Nutritional regulation of gut function in dairy calves: From colostrum to weaning. Appl. Anim. Sci. 2019, 35, 498–510. [Google Scholar] [CrossRef]
- Wickramasinghe, H.K.J.P.; Kaya, C.A.; Baumgard, L.H.; Appuhamy, J.A.D.R.N. Early Step-down Weaning of Dairy Calves from a High Milk Volume with Glutamine Supplementation. J. Dairy Sci. 2022, 105, 1186–1198. [Google Scholar] [CrossRef]
- Matulka, R.A.; Wickramasinghe, J.; Dohms, J.; Ribeiro, F.R.B.; Appuhamy, R. Assessing Performance and Safety of Feeding a Standardized Macleaya Cordata Extract to Calves. Animals 2022, 12, 2875. [Google Scholar] [CrossRef]
- Yohe, T.T.; Dennis, T.S.; Buss, L.N.; Croft, E.J.D.; Quigley, J.D.; Hill, T.M.; Suárez-Mena, F.X.; Aragona, K.M.; Laarman, A.H.; Costa, J.H.C.; et al. Performance and Visceral Tissue Growth and Development of Holstein Calves Fed Differing Milk Replacer Allowances and Starch Concentrations in Pelleted Starter. J. Dairy Sci. 2022, 105, 4099–4115. [Google Scholar] [CrossRef]
- Costa, N.A.; Pansani, A.P.; de Castro, C.H.; Colugnati, D.B.; Xaxier, C.H.; Guimarães, K.C.; Rabelo, L.A.; Nunes-Souza, V.; Caixeta, L.F.S.; Ferreira, R.N. Milk Restriction or Oligosaccharide Supplementation in Calves Improves Compensatory Gain and Digestive Tract Development without Changing Hormone Levels. PLoS ONE 2019, 14, e0214626. [Google Scholar] [CrossRef]
- Wang, M.; Huang, X.; Liu, Y.; Zeng, J. Effects of Macleaya Cordata Extract on Blood Biochemical Indices and Intestinal Flora in Heat-Stressed Mice. Animals 2022, 12, 2589. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the Bacterial Microbiota across the Gastrointestinal Tracts of Dairy Cattle: Membership and Potential Function. Sci. Rep. 2015, 5, 16116. [Google Scholar] [CrossRef] [PubMed]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Schloss, P.; Westcott, S.; Ryabin, T.; Hall, J.; Hartmann, M.; Hollister, E.; Lesniewski, R.; Oakley, B.; Parks, D.; Robinson, C.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic. Acids. Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLOS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Wang, C.; Ding, L.; Zhao, F.; Wang, M.; Fu, J.; Wang, H. Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front. Microbiol. 2019, 10, 162. [Google Scholar] [CrossRef]
- Spreeuwenberg, M.A.M.; Verdonk, J.M.A.J.; Gaskins, H.R.; Verstegen, M.W.A. Small Intestine Epithelial Barrier Function Is Compromised in Pigs with Low Feed Intake at Weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hulst, R.R.; Von Meyenfeldt, M.F.; Van Kreel, B.K.; Thunnissen, F.B.; Brummer, R.J.M.; Arends, J.W.; Soeters, P.B. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition 1998, 14, 1–6. [Google Scholar] [CrossRef]
- Górka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Zabielski, R. Is rumen development in newborn calves affected by different liquid feeds and small intestine development? J. Dairy Sci. 2011, 94, 3002–3013. [Google Scholar] [CrossRef] [PubMed]
- Sukhotnik, I.; Bitterman, S.; Shahar, Y.B.; Pollak, Y.; Bitterman, N.; Halabi, S.; Coran, A.G.; Bitterman, A. Effect of Chelerythrine on Intestinal Cell Turnover Following Intestinal Ischemia-Reperfusion Injury in a Rat Model. Eur. J. Pediatr. Surg. 2017, 27, 36–43. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Suliman, G.M.; Abdullatif, A.A.; Abudabos, A.M. Effects of Phytobiotic Feed Additives on Growth Traits, Blood Biochemistry, and Meat Characteristics of Broiler Chickens Exposed to Salmonella Typhimurium. Poult. Sci. 2020, 99, 5744–5751. [Google Scholar] [CrossRef] [PubMed]
- Meale, S.J.; Chaucheyras-Durand, F.; Berends, H.; Guan, L.L.; Steele, M.A. From Pre- to Postweaning: Transformation of the Young Calf’s Gastrointestinal Tract. J. Dairy Sci. 2017, 100, 5984–5995. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, J.; Urías-Estrada, J.; López-Soto, M.; Barreras, A.; Plascencia, A.; Montaño, M.; González-Vizcarra, V.; Estrada-Angulo, A.; Castro-Pérez, B.; Barajas, R. Evaluation of isoquinoline alkaloid supplementation levels on ruminal fermentation, characteristics of digestion, and microbial protein synthesis in steers fed a high-energy diet. J. Anim Sci. 2016, 94, 267–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Niekerk, J.K.; Middeldorp, M.; Guan, L.L.; Steele, M.A. Preweaning to Postweaning Rumen Papillae Structural Growth, Ruminal Fermentation Characteristics, and Acute-Phase Proteins in Calves. J. Dairy Sci. 2021, 104, 3632–3645. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, H.K.J.P.; Anast, J.M.; Schmitz-Esser, S.; Serao, N.V.L.; Appuhamy, J.A.D.R.N. Beginning to offer drinking water at birth increases the species richness and the abundance of Faecalibacterium and Bifidobacterium in the gut of preweaned dairy calves. J. Dairy Sci. 2020, 103, 4262–4274. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ji, S.; Suen, G.; Wang, F.; Li, S. The Rumen Bacterial Community in Dairy Cows Is Correlated to Production Traits During Freshening Period. Front. Microbiol. 2021, 12, 630605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).