Dietary Supplementation with a Blend of Hydrolyzable and Condensed Tannins Ameliorates Diet-Induced Intestinal Inflammation in Zebrafish (Danio rerio)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Husbandry
2.2. Experimental Design and Feeding Protocol
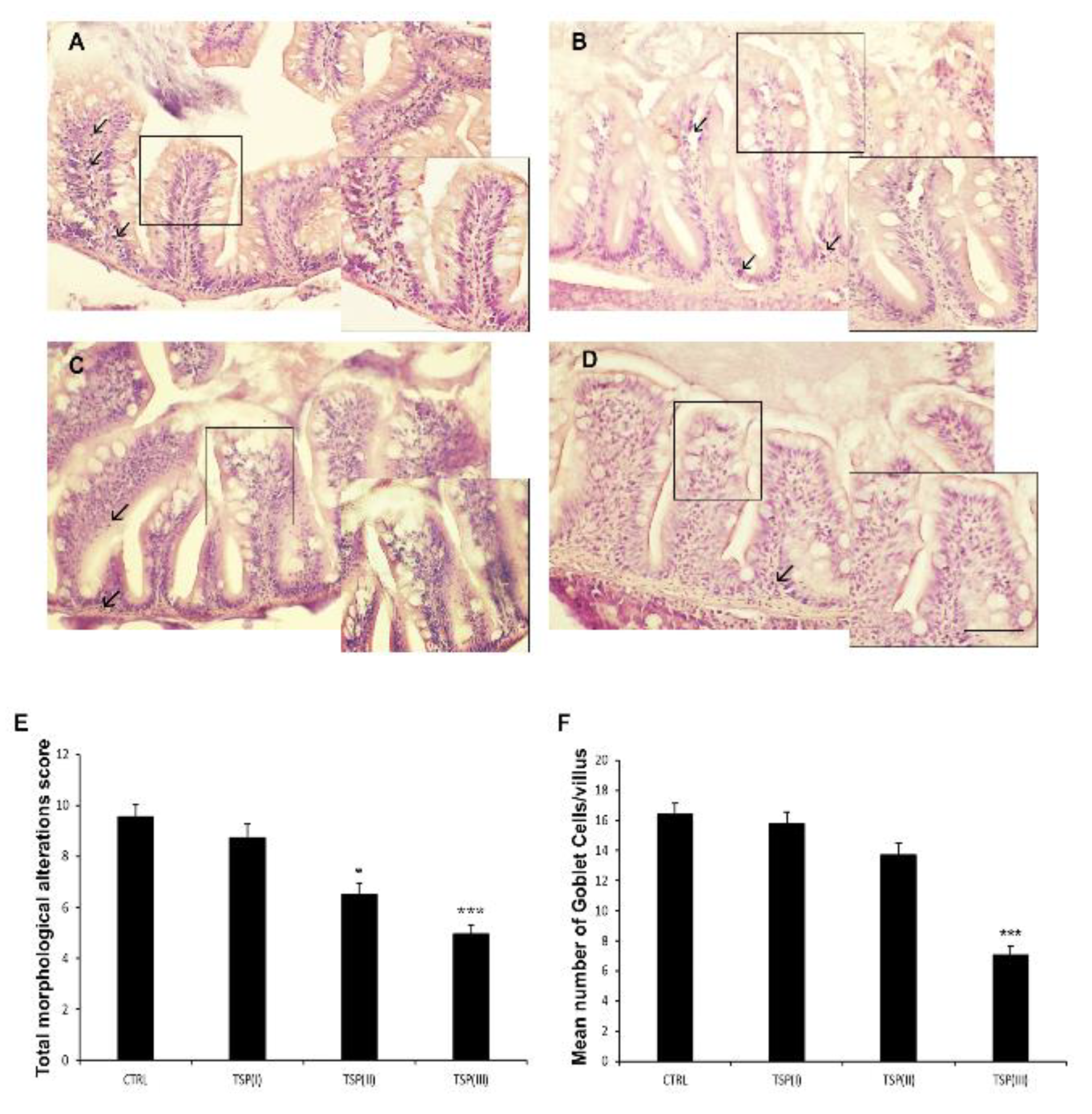
2.3. Histological Analysis
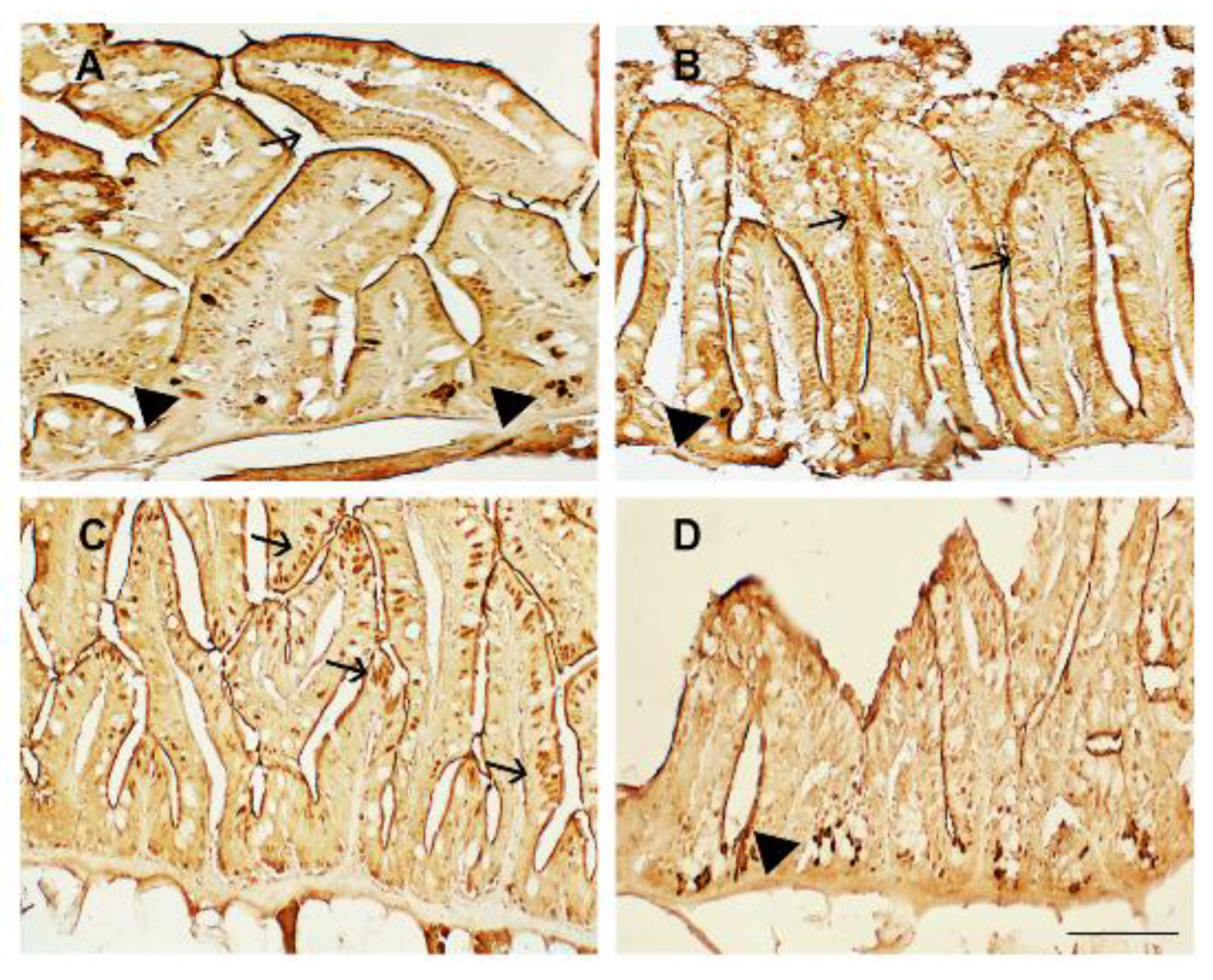
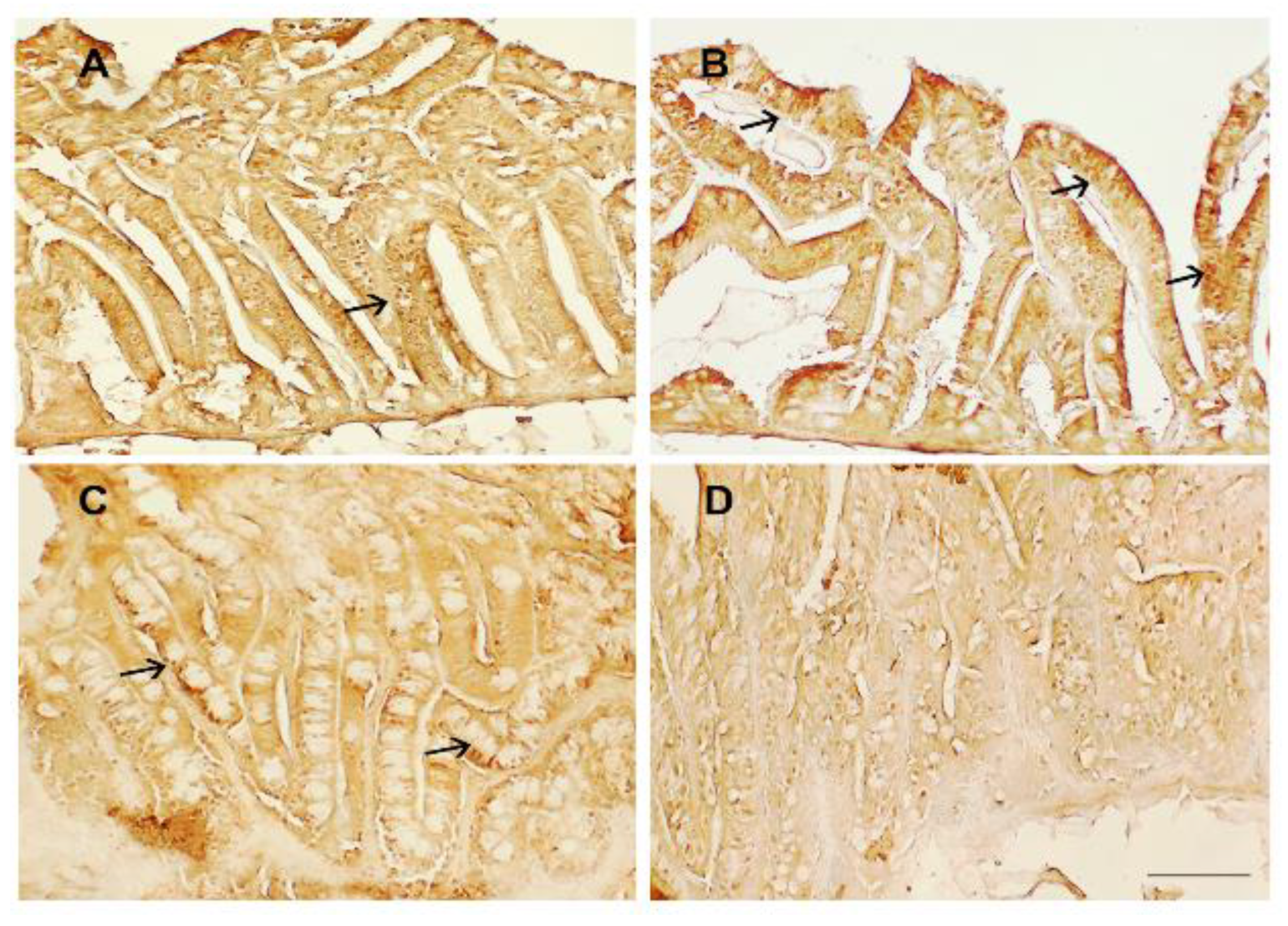
2.4. Immunohistochemical Analysis
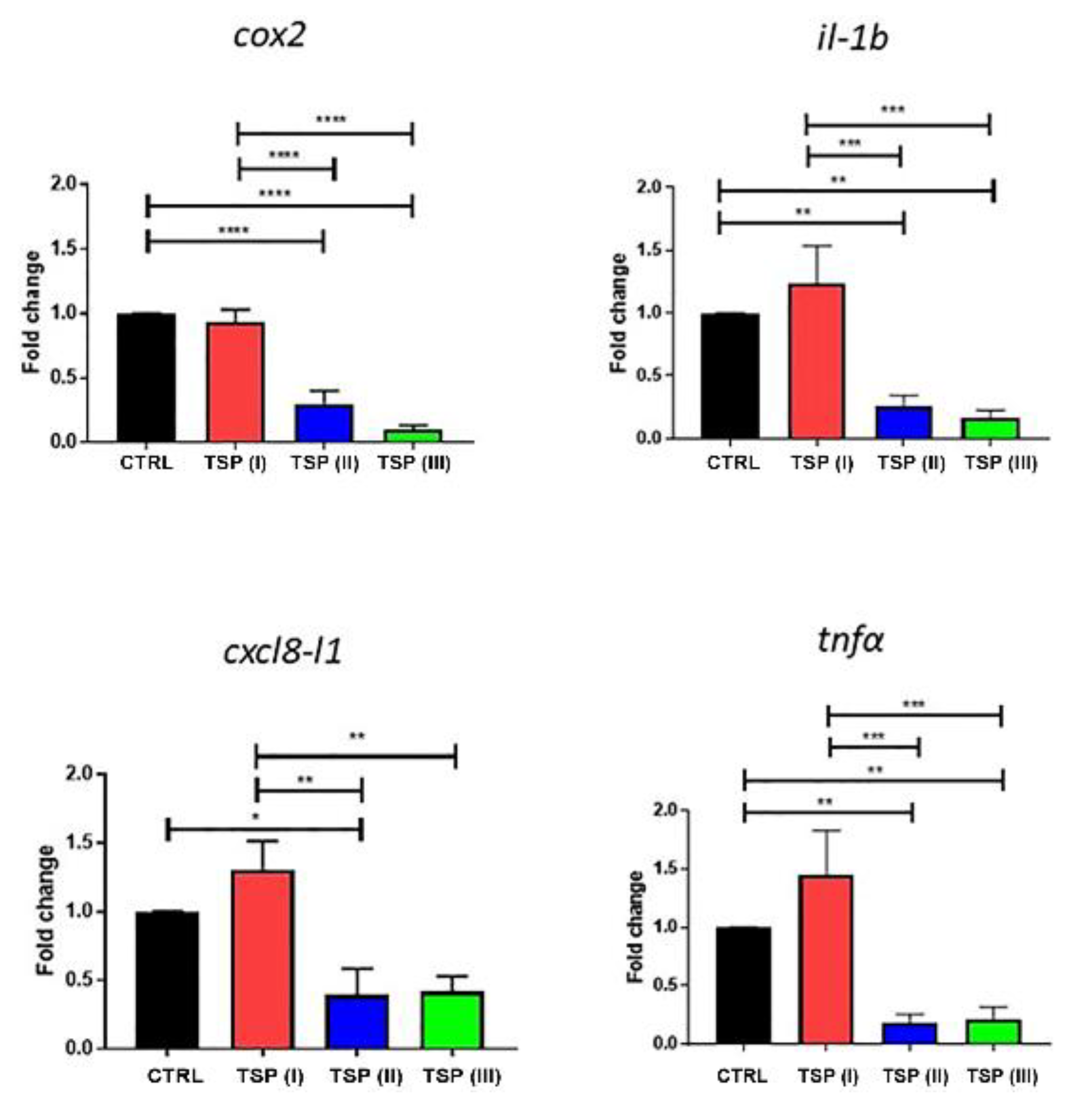
2.5. RNA Isolation, cDNA Synthesis, and Real-Time PCR
2.6. Microbiome Analysis
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performances
3.2. Intestinal Histology
3.3. Intestinal Immunohistochemistry
3.4. Inflammatory Factor Analysis
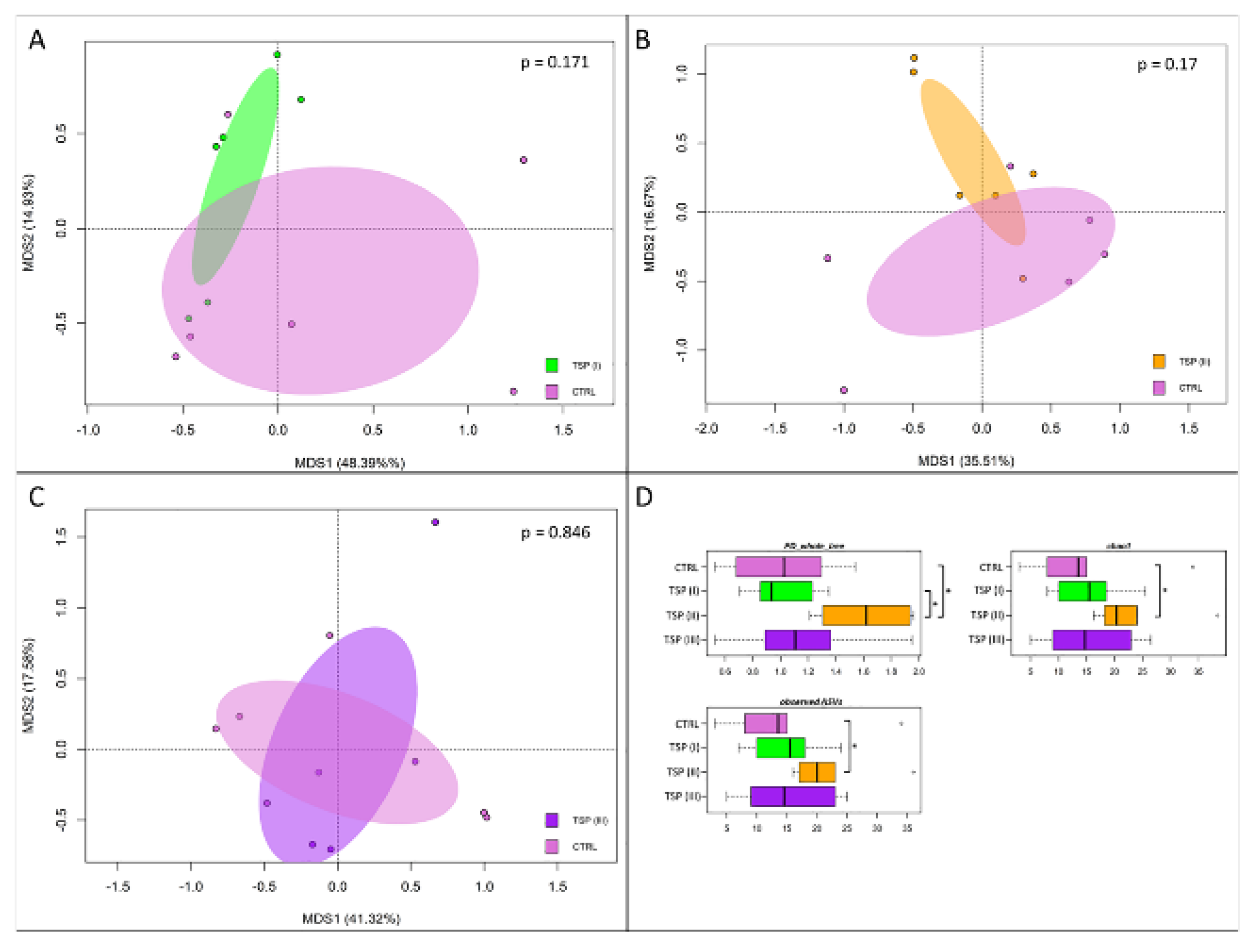
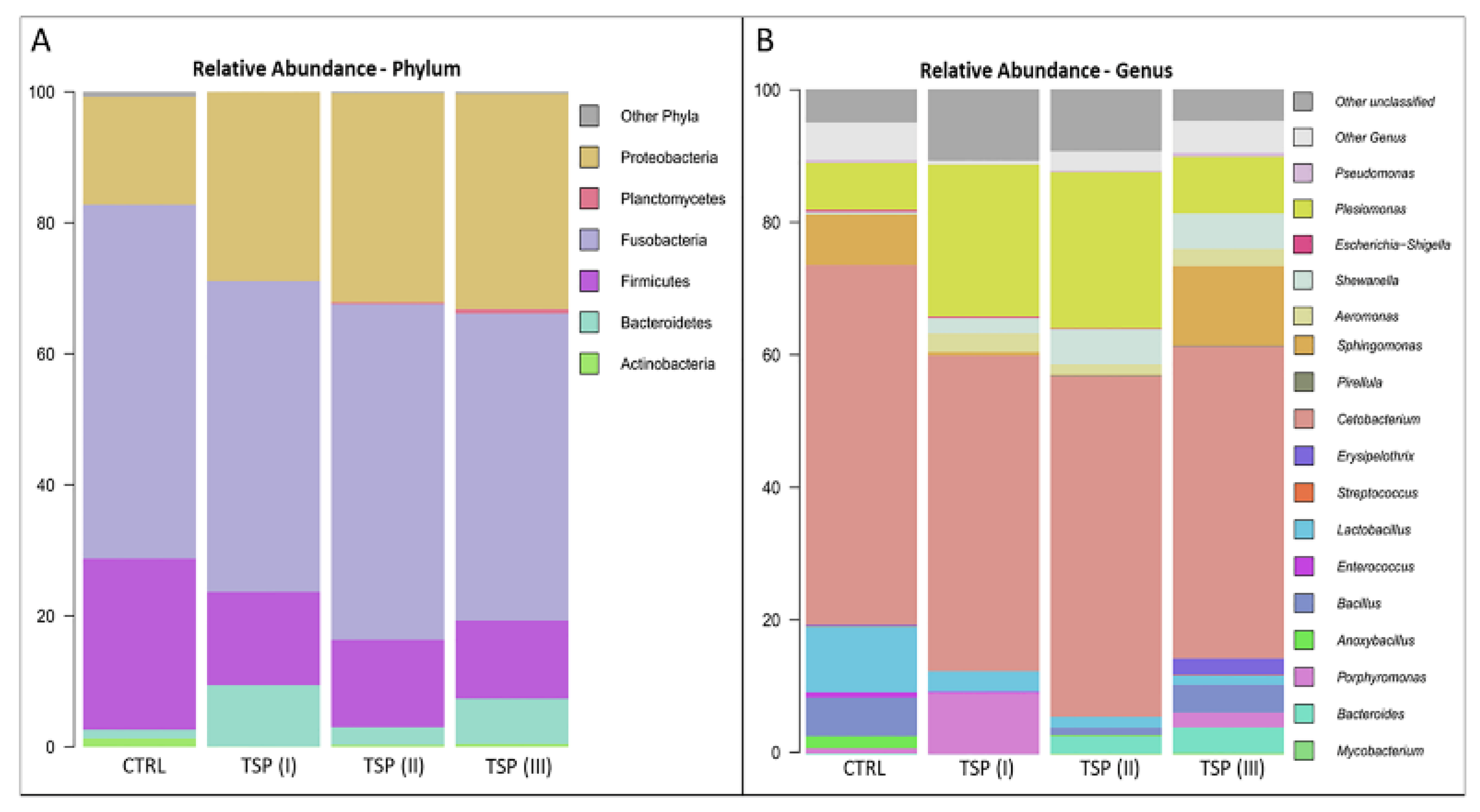
3.5. Intestinal Bacterial Community Profile
3.6. HPLC Profile of TSP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Liu, X.; Han, B.; Xu, J.; Zhu, J.; Hu, J.; Wan, W.; Miao, S. Replacement of fishmeal with soybean meal affects the growth performance, digestive enzymes, intestinal microbiota and immunity of Carassius auratus gibelio♀ × Cyprinus carpio♂. Aquac. Rep. 2020, 18, 100472. [Google Scholar] [CrossRef]
- Duan, X.D.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Tan, B.P.; Yang, Q.H.; Kuang, S.Y.; Tang, L.; Zhou, X.Q.; et al. Soybean β-conglycinin caused intestinal inflammation and oxidative damage in association with NF-κB, TOR and Nrf2 in juvenile grass carp (Ctenopharyngodon idella): Varying among different intestinal segments. Fish Shellfish Immunol. 2019, 95, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Tello, K.; Ehrenfeld, N.; Solís, C.J.; Ulloa, P.E.; Hedrera, M.; Pizarro-Guajardo, M.; Paredes-Sabja, D.; Feijóo, C.G. Effect of microalgae on intestinal inflammation triggered by soybean meal and bacterial infection in zebrafish. PLoS ONE 2017, 12, e0187696. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal. Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef]
- Shimizu, M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017, 25, 93–99. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef]
- Aleström, P.; Winther-Larsen, H.C. Zebrafish offer aquaculture research their services. In Genomics in Aquaculture, 1st ed.; MacKenzie, S., Jentoft, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 165–194. [Google Scholar]
- Licitra, R.; Marchese, M.; Brogi, L.; Fronte, B.; Pitto, L.; Santorelli, F.M. Nutraceutical screening in a zebrafish model of muscular dystrophy: Gingerol as a possible food aid. Nutrients 2021, 13, 998. [Google Scholar] [CrossRef]
- Ogi, A.; Licitra, R.; Naef, V.; Marchese, M.; Fronte, B.; Gazzano, A.; Santorelli, F.M. Social Preference Tests in Zebrafish: A Systematic Review. Front. Vet. Sci. 2021, 7, 590057. [Google Scholar] [CrossRef]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Medrano, J.F.; Feijo, C.G. Zebrafish as animal model for aquaculture nutrition research. Front. Genet. 2014, 5, 313. [Google Scholar] [CrossRef] [PubMed]
- Fronte, B.; Licitra, R.; Bibbiani, C.; Casini, L.; de Zoysa, M.; Miragliotta, V.; Sagona, S.; Coppola, F.; Brogi, L.; Abramo, F. Fishmeal replacement with hermetia illucens meal in aquafeeds: Effects on zebrafish growth performances, intestinal morphometry, and enzymology. Fishes 2021, 6, 28. [Google Scholar] [CrossRef]
- Watts, S.A.; Powell, M.; D’Abramo, L.R. Fundamental approaches to the study of zebrafish nutrition. ILAR J. 2012, 53, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Appelgren, P.; Opazo, R.; Barros, L.; Feijoó, C.G.; Urzúa, V.; Romero, J. Effect of the dietary inclusion of soybean components on the innate immune system in zebrafish. Zebrafish 2014, 11, 41–49. [Google Scholar] [CrossRef]
- Perera, E.; Yúfera, M. Soybean Meal and Soy Protein Concentrate in Early Diet Elicit Different Nutritional Programming Effects on Juvenile Zebrafish. Zebrafish 2016, 13, 61–69. [Google Scholar] [CrossRef]
- Molinari, G.S.; Wojno, M.; McCracken, V.J.; Kwasek, K. The use of dipeptide supplementation as a means of mitigating the negative effects of dietary soybean meal on Zebrafish Danio rerio. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 257, 110958. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Flores, M.V.; Okuda, K.S.; Hall, C.J.; Crosier, K.E.; Crosier, P.S. A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev. Dyn. 2011, 240, 288–298. [Google Scholar] [CrossRef]
- Hedrera, M.I.; Galdames, J.A.; Jimenez-Reyes, M.F.; Reyes, A.E.; Avendaño-Herrera, R.; Romero, J.; Feijóo, C.G. Soybean meal induces intestinal inflammation in zebrafish larvae. PLoS ONE 2013, 8, e69983. [Google Scholar] [CrossRef]
- Orso, G.; Solovyev, M.M.; Facchiano, S.; Tyrikova, E.; Sateriale, D.; Kashinskaya, E.; Pagliarulo, C.; Hoseinifar, H.S.; Simonov, E.; Varricchio, E.; et al. Chestnut shell tannins: Effects on intestinal inflammation and dysbiosis in Zebrafish. Animals 2021, 11, 1538. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [PubMed]
- Li, M.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Condensed tannins decreased the growth performance and impaired intestinal immune function in on-growing grass carp (Ctenopharyngodon idella). Br. J. Nutr. 2020, 123, 737–755. [Google Scholar] [CrossRef]
- Niaz, K.; Khan, F. Analysis of polyphenolics. In Recent Advances in Natural Products Analysis, 1st ed.; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 3, pp. 39–197. [Google Scholar]
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and Antiviral Activity of Hydrolysable Tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Wang, Y.; Majak, W.; McAllister, T.A. Frothy bloat in ruminants: Cause, occurrence, and mitigation strategies. Anim. Feed Sci. Technol. 2012, 172, 103–114. [Google Scholar] [CrossRef]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar] [CrossRef]
- Imperatore, R.; Orso, G.; Facchiano, S.; Scarano, P.P.; Hoseinifar, S.H.; Ashouri, G.; Guarino, C.; Paolucci, M. Anti-inflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture 2023, 563, 738878. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Imanpour, M.R.; Mazandarani, M.; Sanchouli, H.; Paolucci, M. Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture 2020, 528, 735494. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Dietary inclusion of chestnut (Castanea sativa) polyphenols to Nile tilapia reared in biofloc technology: Impacts on growth, immunity, and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Jahazi, M.A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; van Doan, H.; Paolucci, M. Dietary supplementation of polyphenols positively affects the innate immune response, oxidative status, and growth performance of common carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Jahazi, M.A.; Nikdehghan, N.; van Doan, H.; Volpe, M.G.; Paolucci, M. Effects of dietary polyphenols from agricultural by-products on mucosal and humoral immune and antioxidant responses of convict cichlid (Amatitlania nigrofasciata). Aquaculture 2020, 517, 734790. [Google Scholar] [CrossRef]
- Omnes, M.H.; le Goasduff, J.; le Delliou, H.; le Bayon, N.; Quazuguel, P.; Robin, J.H. Effects of dietary tannin on growth, feed utilization and digestibility, and carcass composition in juvenile European seabass (Dicentrarchus labrax L.). Aquac. Rep. 2017, 6, 21–27. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Royes, J.A.B.; Chapman, F.A. Preparing Your Own Fish Feeds; Document Circular 97; Department of Fisheries and Aquatic Science, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2003; Volume 6, pp. 1–9. [Google Scholar]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Imperatore, R.; Tunisi, L.; Mavaro, I.; D’angelo, L.; Attanasio, C.; Safari, O.; Motlagh, H.A.; de Girolamo, P.; Cristino, L.; Varricchio, E.; et al. Immunohistochemical analysis of intestinal and central nervous system morphology in an obese animal model (Danio rerio) treated with 3,5-t2: A possible farm management practice? Animals 2020, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, R.; Coccia, E.; D’Angelo, L.; Varricchio, E.; de Girolamo, P.; Paolucci, M. Evidence for leptin receptor immunoreactivity in the gastrointestinal tract and gastric leptin regulation in the rainbow trout (Oncorhynchus mykiss). Ann. Anat. 2018, 220, 70–78. [Google Scholar] [CrossRef]
- Licitra, R.; Martinelli, M.; Jasinski, L.P.; Marchese, M.; Kiferle, C.; Fronte, B. In vivo evaluation of cannabis sativa full extract on zebrafish larvae development, locomotion behavior and gene expression. Pharmaceuticals 2021, 14, 1224. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pelusio, N.F.; Scicchitano, D.; Parma, L.; Dondi, F.; Brini, E.; D’Amico, F.; Candela, M.; Yúfera, M.; Gilannejad, N.; Moyano, F.J.; et al. Interaction Between Dietary Lipid Level and Seasonal Temperature Changes in Gilthead Sea Bream Sparus aurata: Effects on Growth, Fat Deposition, Plasma Biochemistry, Digestive Enzyme Activity, and Gut Bacterial Community. Front. Mar. Sci. 2021, 8, 664701. [Google Scholar] [CrossRef]
- Brown, R.; Moore, L.; Mani, A.; Patel, S.; Salinas, I. Effects of ploidy and salmonid alphavirus infection on the skin and gill microbiome of Atlantic salmon (Salmo salar). PLoS ONE 2021, 16, e0243684. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. Peer J. 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Culhane, A.C.; Thioulouse, J.; Perrière, G.; Higgins, D.G. MADE4: An R package for multivariate analysis of gene expression data. Bioinformatics 2005, 21, 2789–2790. [Google Scholar] [CrossRef]
- Peng, Z.; Hayasaka, Y.; Iland, P.G.; Sefton, M.; Høj, P.; Waters, E.J. Quantitative Analysis of Polymeric Procyanidins (Tannins) from Grape Vitis vinifera Seeds by Reverse Phase High-Performance Liquid Chromatography. J. Agric. Food Chem. 2001, 49, 26–31. [Google Scholar] [CrossRef]
- Fronte, B.; Abramo, F.; Brambilla, F.; de Zoysa, M.; Miragliotta, V. Effect of hydrolysed fish protein and autolysed yeast as alternative nitrogen sources on gilthead sea bream (Sparus aurata) growth performances and gut morphology. Ital. J. Anim. Sci. 2019, 18, 799–808. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Robaina, L.; Real, F.; Sweetman, J.; Tort, L.; Izquierdo, M.S. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 2007, 23, 969–981. [Google Scholar] [CrossRef]
- Torrecillas, S.; Montero, D.; Izquierdo, M. Improved health and growth of fish fed mannan oligosaccharides: Potential mode of action. Fish Shellfish Immunol. 2014, 36, 525–544. [Google Scholar] [CrossRef]
- Arias-Jayo, N.; Abecia, L.; Alonso-Sáez, L.; Ramirez-Garcia, A.; Rodriguez, A.; Pardo, M.A. High-Fat Diet Consumption Induces Microbiota Dysbiosis and Intestinal Inflammation in Zebrafish. Microb. Ecol. 2018, 76, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Micheloni, G.; Carnovali, M.; Millefanti, G.; Rizzetto, M.; Moretti, V.; Montalbano, G.; Acquati, F.; Giaroni, C.; Valli, R.; Costantino, L.; et al. Soy diet induces intestinal inflammation in adult Zebrafish: Role of OTX and P53 family. Int. J. Exp. Path. 2022, 103, 13–22. [Google Scholar] [CrossRef]
- Urán, P.A.; Gonçalves, A.A.; Taverne-Thiele, J.J.; Schrama, J.W.; Verreth, J.A.J.; Rombout, J.H.W.M. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2008, 25, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, Å.; Bakke-McKellep, A.M.; Baeverfjord, G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo solar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Kortner, T.M.; Penn, M.H.; Björkhem, I.; Måsøval, K.; Krogdahl, Å. Bile components and lecithin supplemented to plant based diets do not diminish diet related intestinal inflammation in Atlantic salmon. BMC Vet. Res. 2016, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Estruch, G.; Collado, M.C.; Monge-Ortiz, R.; Tomás-Vidal, A.; Jover-Cerdá, M.; Peñaranda, D.S.; Pérez Martínez, G.; Martínez-Llorens, S. Long-term feeding with high plant protein based diets in gilthead seabream (Sparus aurata, L.) leads to changes in the inflammatory and immune related gene expression at intestinal level. BMC. Vet. Res. 2018, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, X.; Tan, B.; Du, T.; Zhang, S.; Yang, Y.; Chi, S.; Yang, Q.; Liu, H. Effects of fish meal replacement by low gossypol cottonseed meal on growth performance, digestive enzyme activity, intestine histology and inflammatory gene expression of silver sillago (Sillago sihama Forsskàl) (1775). Aquac. Nutr. 2020, 26, 1724–1735. [Google Scholar] [CrossRef]
- Yin, B.; Liu, H.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S. Preliminary study of mechanisms of intestinal inflammation induced by plant proteins in juvenile hybrid groupers (Epinephelus fuscoguttatus x E. lanceolatu). Fish Shellfish Immunol. 2020, 106, 341–356. [Google Scholar] [CrossRef]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 12, 525–539. [Google Scholar] [CrossRef]
- Wang, T.; Secombes, C.J. The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol. 2013, 35, 1703–1718. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.K. Dietary Application of Tannins as a Potential Mitigation Strategy for Current Challenges in Poultry Production: A Review. Animals 2020, 10, 2389. [Google Scholar] [CrossRef] [PubMed]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Wang, G.; Wang, Y.; Chen, B.; Sun, Y.; Mo, W.; Li, G.; Huang, Y. Condensed tannins enhanced antioxidant capacity and hypoxic stress survivability but not growth performance and fatty acid profile of juvenile Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2020, 269, 114671. [Google Scholar] [CrossRef]
- Peng, K.; Lv, X.; Zhao, H.; Chen, B.; Chen, X.; Huang, W. Antioxidant and intestinal recovery function of condensed tannins in Lateolabrax maculatus responded to in vivo and in vitro oxidative stress. Aquaculture 2022, 547, 737399. [Google Scholar] [CrossRef]
- Coccia, E.; Imperatore, R.; Orso, G.; Melck, D.; Varricchio, E.; Volpe, M.G.; Paolucci, M. Explants of Oncorhynchus mykiss intestine to detect bioactive molecules uptake and metabolic effects: Applications in aquaculture. Aquaculture 2019, 506, 193–204. [Google Scholar] [CrossRef]
- Moccia, F.; Piscitelli, A.; Giovando, S.; Giardina, P.; Panzella, L.; d’Ischia, M.; Napolitano, A. Hydrolyzable vs. Condensed Wood Tannins for Bio-based Antioxidant Coatings: Superior Properties of Quebracho Tannins. Antioxidants 2020, 9, 804. [Google Scholar] [CrossRef]
- Venter, P.B.; Sisa, M.; van der Merwe, M.J.; Bonnet, S.L.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 1: The chemical composition of quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 73, 95–105. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Krogdahl, Å. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- De Francesco, M.; Parisi, G.; Médale, F.; Lupi, P.; Kaushik, S.J.; Poli, B.M. Effect of long-term feeding with a plant protein mixture based diet on growth and body/fillet quality traits of large rainbow trout (Oncorhynchus mykiss). Aquaculture 2004, 236, 413–429. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, L.; Zhang, C.X.; Song, K. Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides). Aquacult. Rep. 2017, 5, 52–57. [Google Scholar] [CrossRef]
- Hansen, A.C.; Rosenlund, G.; Karlsen, Ø.; Koppe, W.; Hemre, G.I. Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I—Effects on growth and protein retention. Aquaculture 2007, 272, 599–611. [Google Scholar] [CrossRef]
- Marjara, I.S.; Chikwati, E.M.; Valen, E.C.; Krogdahl, Å.; Bakke, A.M. Transcriptional regulation of IL-17A and other inflammatory markers during the development of soybean meal-induced enteropathy in the distal intestine of Atlantic salmon (Salmo salar L.). Cytokine 2012, 60, 186–196. [Google Scholar] [CrossRef]
- Montero, D.; Benitez-Dorta, V.; Caballero, M.J.; Ponce, M.; Torrecillas, S.; Izquierdo, M.; Zamorano, M.J.; Manchado, M. Dietary vegetable oils: Effects on the expression of immune-related genes in Senegalese sole (Solea senegalensis) intestine. Fish Shellfish Immunol. 2015, 44, 100–108. [Google Scholar] [CrossRef]
- Bruneton, J. Principles of herbal pharmacology. In Principles and Practice of Phytotherapy, 2nd ed.; Bone, K., Mills, S., Eds.; Churchill Livingstone: London, UK, 2013; pp. 17–82. [Google Scholar]
- Goel, G.; Puniya, A.K.; Aguilar, C.N.; Singh, K. Interaction of gut microflora with tannins in feeds. Naturwissenschaften 2005, 92, 497–503. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 21, 370. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 13, 2216. [Google Scholar] [CrossRef]
- López Nadal, A.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Lu, H.; Li, P.; Huang, X.; Wang, C.H.; Li, M.; Xu, Z.Z. Zebrafish model for human gut microbiome-related studies: Advantages and limitations. Med. Microecol. 2021, 8, 100042. [Google Scholar] [CrossRef]
- Jin, C.; Luo, T.; Zhu, Z.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z.; Jin, Y. Imazalil exposure induces gut microbiota dysbiosis and hepatic metabolism disorder in zebrafish. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2017, 202, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Ruiz, M.; Balebona, M.C.; Esteban, M.Á.; Moriñigo, M.Á. Probiotic Shewanella putrefaciens (SPPDP11) as a fish health modulator: A review. Microorganisms 2020, 14, 1990. [Google Scholar] [CrossRef]
- Thitichayaphong, N.; Viriyarampa, S.; Moonjit, P.; Rukkwamsuk, T. Efficacy of Condensed Tannin on Antibacterial Activities against Pathogenic Bacteria of Aquatic Animals. J. Kasetsart Vet. 2015, 25, 135–145. [Google Scholar]
- Yao, J.; Liu, N.; Li, N.; Li, X.; Hua, X. Different metabolomic responses of grass carp (Ctenopharyngodon idellus) to dietary tannin and rapeseed meal. Aquac. Fish. 2022, 7, 40–51. [Google Scholar] [CrossRef]
- Ke, W.; Zhang, H.; Li, S.; Xue, Y.; Wang, Y.; Dong, W.; Cai, Y.; Zhang, G. Influence of Condensed and Hydrolysable Tannins on the Bacterial Community, Protein Degradation, and Fermentation Quality of Alfalfa Silage. Animals 2022, 25, 831. [Google Scholar] [CrossRef]

| Control | TSP I | TSP II | TSP III | ||
|---|---|---|---|---|---|
| Ingredient | % | % | % | % | |
| Soybean meal 48 | 43.00 | 43.00 | 43.00 | 43.00 | |
| Corn meal | 22.00 | 22.00 | 22.00 | 22.00 | |
| Corn gluten | 15.00 | 15.00 | 15.00 | 15.00 | |
| Wheat gluten | 5.20 | 5.11 | 5.03 | 4.86 | |
| Rapeseed meal | 5.00 | 5.00 | 5.00 | 5.00 | |
| Dicalcium phosphate | 3.40 | 3.40 | 3.40 | 3.40 | |
| Binder (guar gum) | 2.20 | 2.20 | 2.20 | 2.20 | |
| Soybean oil | 2.00 | 2.00 | 2.00 | 2.00 | |
| Choline chloride | 1.30 | 1.30 | 1.30 | 1.30 | |
| Vitamin and mineral premix a | 0.50 | 0.50 | 0.50 | 0.50 | |
| Sodium propionate | 0.10 | 0.10 | 0.10 | 0.10 | |
| L-Lysine | 0.10 | 0.10 | 0.10 | 0.10 | |
| L-Threonine | 0.10 | 0.10 | 0.10 | 0.10 | |
| DL-Methionine | 0.10 | 0.10 | 0.10 | 0.10 | |
| TSP | 0.00 | 0.09 | 0.17 | 0.34 | |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | |
| Proximate Composition | As Fed | ||||
| Dry matter | % | 96.11 | 96.1 | 96.08 | 96.06 |
| Crude protein | % | 36.95 | 36.88 | 36.81 | 36.67 |
| Crude fat | % | 4.53 | 4.53 | 4.53 | 4.53 |
| Fiber | % | 3.59 | 3.59 | 3.59 | 3.59 |
| Starch | % | 18.65 | 18.64 | 18.64 | 18.62 |
| Ash | % | 4.99 | 4.98 | 4.98 | 4.98 |
| Gross energy | MJ/kg | 17.05 | 17.03 | 17.01 | 16.97 |
| Parameters | Initial BW (mg) | Final BW (mg) | BW Increment | ||
|---|---|---|---|---|---|
| Treatments | Mean | SD | Mean | SD | |
| Control | 291.0 | 116.93 | 350.1 | 117.63 | 20.3% |
| TSP I | 316.9 | 108.85 | 375.1 | 140.84 | 18.4% |
| TSP II | 307.2 | 126.57 | 372.3 | 120.54 | 21.2% |
| TSP III | 318.6 | 121.84 | 382.9 | 157.84 | 20.2% |
| SEM * | 14.617 | 16.427 | |||
| p-value | 0.9021 | 0.9031 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imperatore, R.; Fronte, B.; Scicchitano, D.; Orso, G.; Marchese, M.; Mero, S.; Licitra, R.; Coccia, E.; Candela, M.; Paolucci, M. Dietary Supplementation with a Blend of Hydrolyzable and Condensed Tannins Ameliorates Diet-Induced Intestinal Inflammation in Zebrafish (Danio rerio). Animals 2023, 13, 167. https://doi.org/10.3390/ani13010167
Imperatore R, Fronte B, Scicchitano D, Orso G, Marchese M, Mero S, Licitra R, Coccia E, Candela M, Paolucci M. Dietary Supplementation with a Blend of Hydrolyzable and Condensed Tannins Ameliorates Diet-Induced Intestinal Inflammation in Zebrafish (Danio rerio). Animals. 2023; 13(1):167. https://doi.org/10.3390/ani13010167
Chicago/Turabian StyleImperatore, Roberta, Baldassare Fronte, Daniel Scicchitano, Graziella Orso, Maria Marchese, Serena Mero, Rosario Licitra, Elena Coccia, Marco Candela, and Marina Paolucci. 2023. "Dietary Supplementation with a Blend of Hydrolyzable and Condensed Tannins Ameliorates Diet-Induced Intestinal Inflammation in Zebrafish (Danio rerio)" Animals 13, no. 1: 167. https://doi.org/10.3390/ani13010167
APA StyleImperatore, R., Fronte, B., Scicchitano, D., Orso, G., Marchese, M., Mero, S., Licitra, R., Coccia, E., Candela, M., & Paolucci, M. (2023). Dietary Supplementation with a Blend of Hydrolyzable and Condensed Tannins Ameliorates Diet-Induced Intestinal Inflammation in Zebrafish (Danio rerio). Animals, 13(1), 167. https://doi.org/10.3390/ani13010167







