De Novo Assembly, Characterization and Comparative Transcriptome Analysis of the Mature Gonads in Spinibarbus hollandi
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA Extraction and Library Construction
2.3. Library Sequencing, De Novo Assembly and Annotation
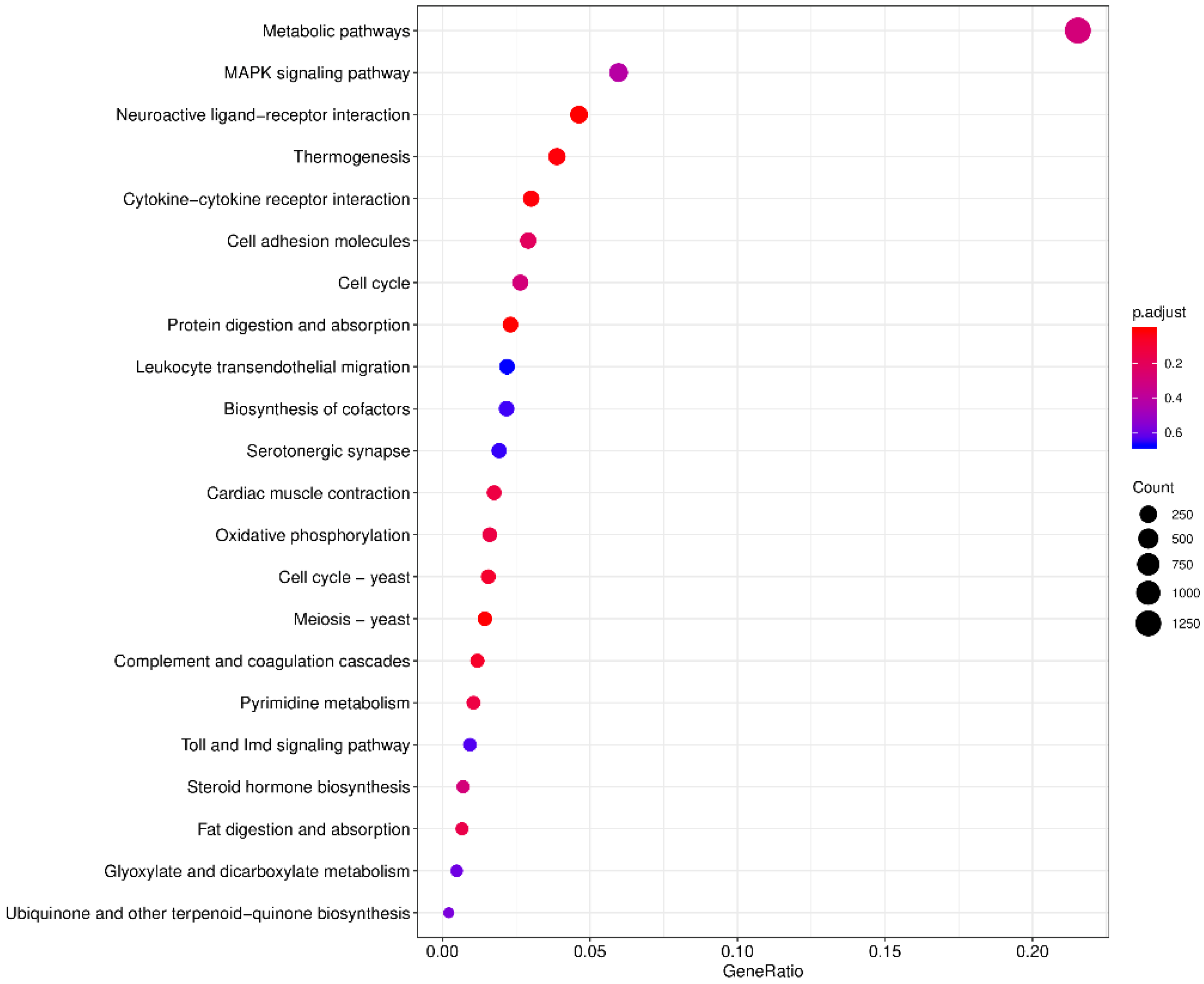
2.4. Identification of Differentially Expressed Genes and Enrichment Analysis
2.5. Validation of DEGs Using Quantitative Real-Time PCR
3. Results
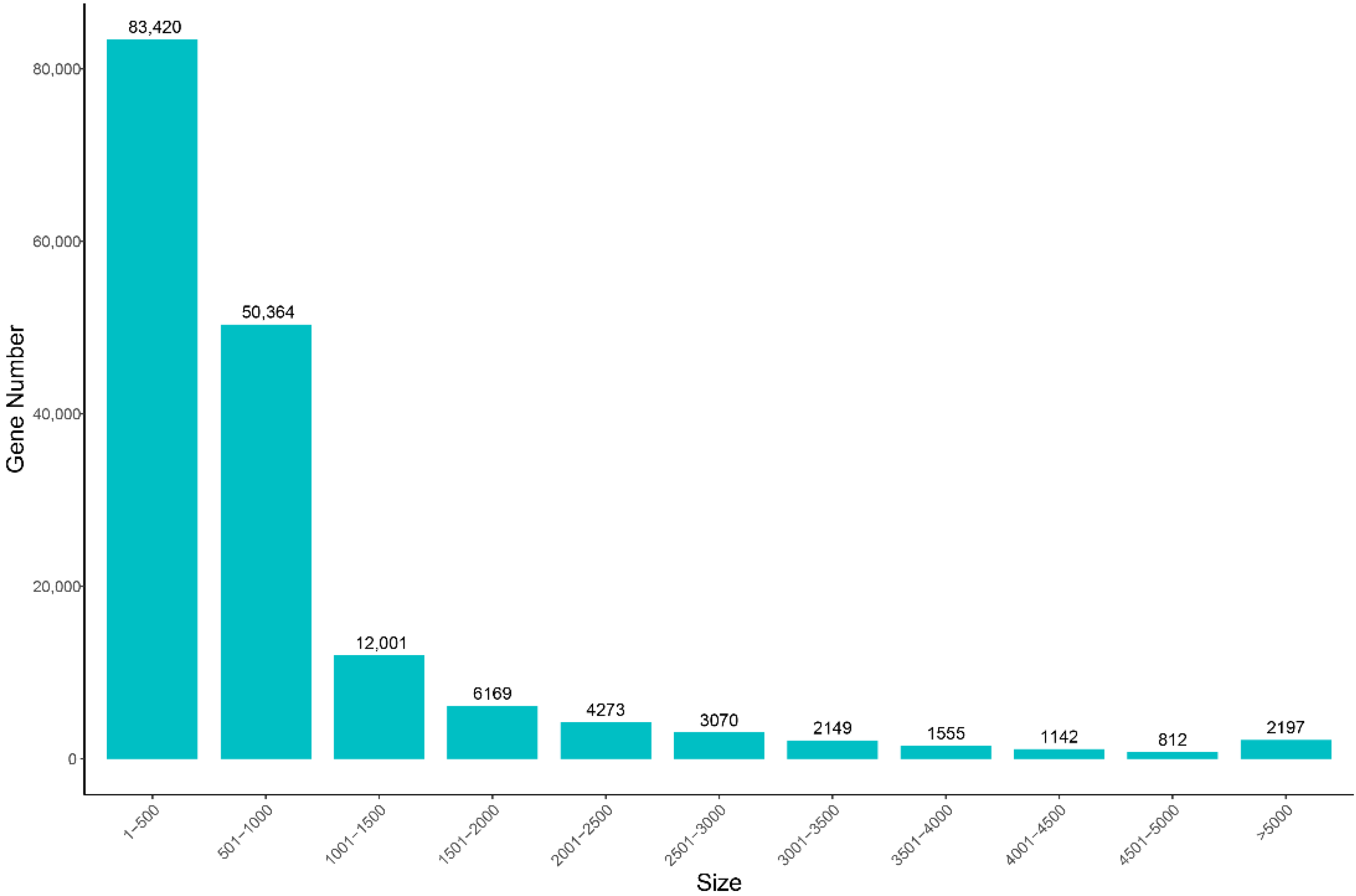
3.1. Overview of Transcriptome Assembly Quality
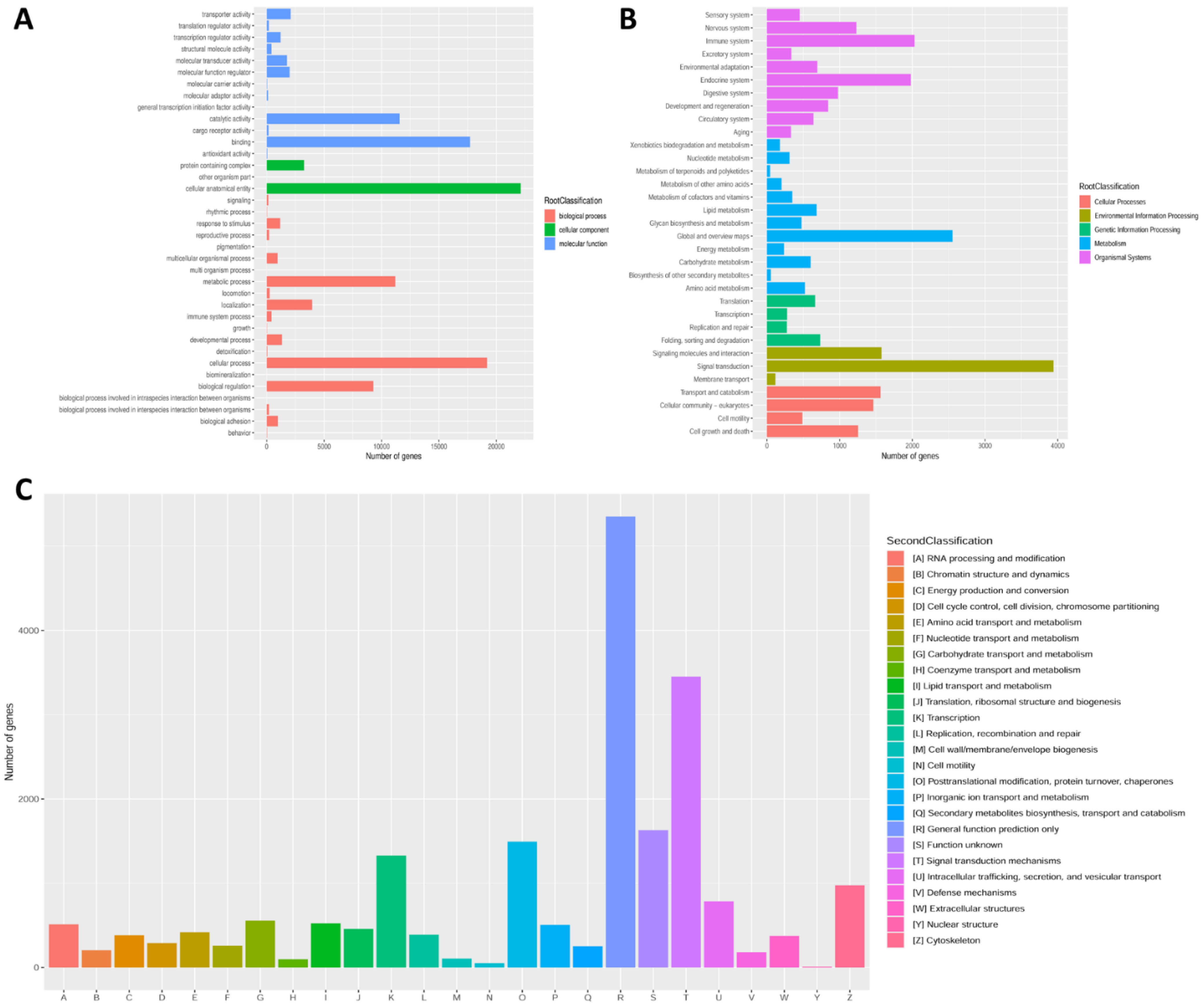
3.2. Unigene Annotation
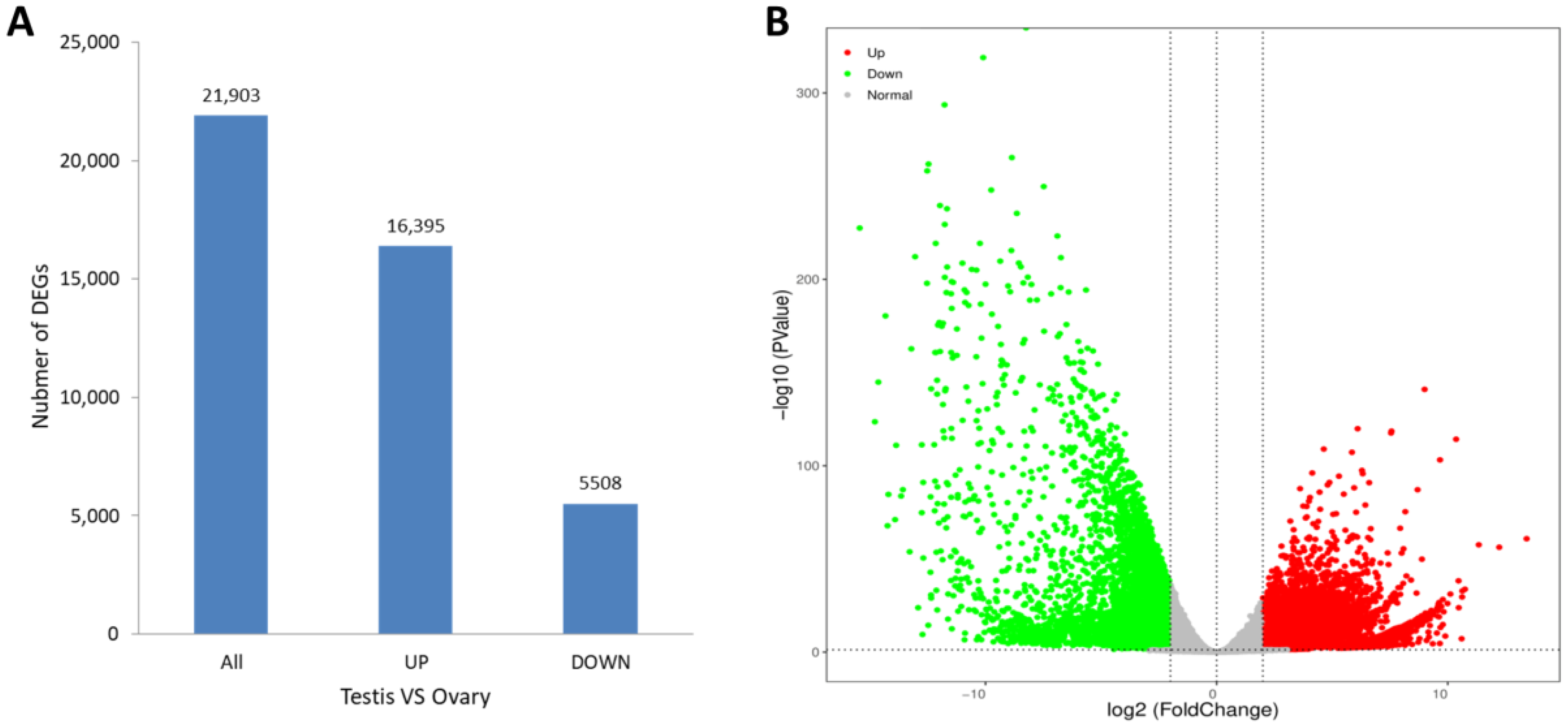
3.3. Differential Expression Analysis
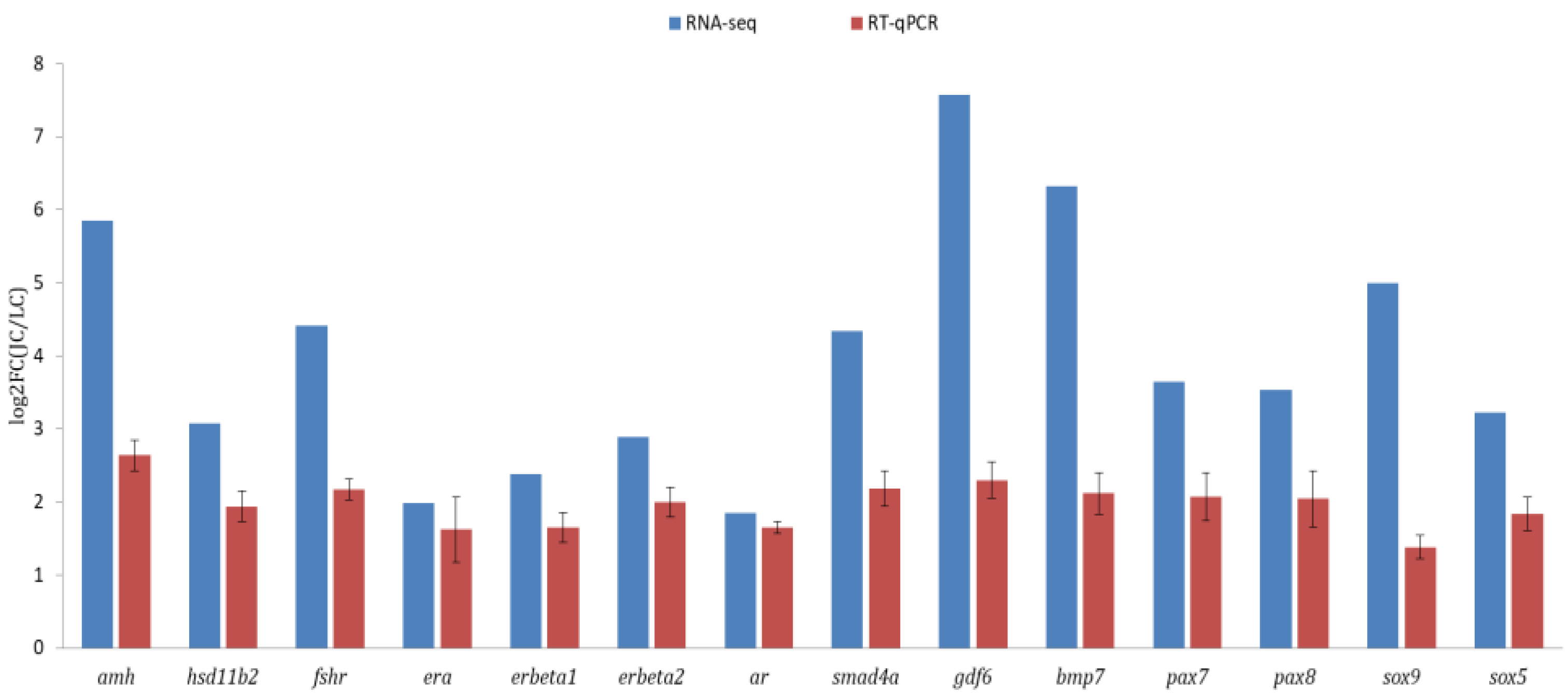
3.4. Validation of Transcriptomic Data
4. Discussion
4.1. DEGs Involved in Steroid Synthetic Pathway
4.2. DEGs Involved in Gonad Differentiation and Development
4.3. DEGs Involved in Gametogenesis and Gamete Maturation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Z.D.; Lin, G.; Ni, J.Y.; Ye, Z.; Tang, Z.B.; Zhang, S. Study on the reproductive biology of Spinibabus calddwellis. Guangxi Agric. Sci. 2007, 2, 24–28, (In Chinese with English Abstract). [Google Scholar]
- Yang, S.D.; Lin, T.S.; Liou, C.H.; Peng, H.K. Influence of dietary protein levels on growth performance, carcass composition and liver lipid classes of juvenile Spinibarbus hollandi (Oshima). Aquat. Res. 2003, 34, 661–666. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, Z.; Shu, H.; Zhou, H.; Jiang, X.; Hou, L.; Gu, P. Association between expression levels and growth trait-related SNPs located in promoters of the MC4R and MSTN genes in Spinibarbus hollandi. Genes Genom. 2018, 40, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Peizhen, Z.; Caiyan, W.; Jianrong, C.; Liangming, Z.; Qinhong, L.; Yuxuan, W. The gonadal development and the annual change of ovary in Spinibarbus hollamdi. Chin. J. Zool. 2004, 39, 7–10. [Google Scholar]
- Heule, C.; Salzburger, W.; Böhne, A. Genetics of sexual development: An evolutionary playground for fish. Genetics 2014, 196, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, H.; Hou, L.; Xing, K.; Shu, H. Transcriptional profiling of skeletal muscle reveals starvation response and compensatory growth in Spinibarbus hollandi. BMC Genom. 2019, 20, 938. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, H.; Shu, H.; Zhong, D.; Zhang, M.; Xia, J.H. Effect of fasting and subsequent refeeding on the transcriptional profiles of brain in juvenile Spinibarbus hollandi. PLoS ONE 2019, 14, e0214589. [Google Scholar] [CrossRef]
- Garber, M.; Grabherr, M.G.; Guttman, M.; Trapnell, C. Computational methods for transcriptome annotation and quantification using RNA-seq. Nat. Methods 2011, 8, 469–477. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.; Wang, Y.; Huang, H.; Yang, X.; Li, S.; Yang, W.; Li, G. Comparison of gonadal transcriptomes uncovers reproduction-related genes with sexually dimorphic expression patterns in Diodon hystrix. Animals 2021, 11, 1042. [Google Scholar] [CrossRef]
- Mustapha, U.F.; Peng, Y.X.; Huang, Y.Q.; Assan, D.; Zhi, F.; Shi, G.; Huang, Y.; Li, G.L.; Jiang, D.N. Comparative transcriptome analysis of the differentiating gonads in Scatophagus argus. Front. Mar. Sci. 2022, 9, 962534. [Google Scholar] [CrossRef]
- Du, X.; Wang, B.; Liu, X.; Liu, X.; He, Y.; Zhang, Q.; Wang, X. Comparative transcriptome analysis of ovary and testis reveals potential sex-related genes and pathways in spotted knifejaw Oplegnathus punctatus. Gene 2017, 637, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luan, P.; Zhang, X.; Xue, S.; Peng, L.; Mahbooband, S.; Sun, X. Gonadal transcriptomic analysis of yellow catfish (Pelteobagrus fulvidraco): Identification of sex-related genes and genetic markers. Physiol. Genom. 2014, 46, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Han, C.; Liu, S.; Ouyang, H.; Liu, D.; Zhang, Z.; Huang, J.J.; Han, L.Q.; Li, S.S.; Li, G.F.; et al. Development and gene expression analysis of gonad during 17α-methyltestosterone-induced sex reversal in mandarin fish (Siniperca chuatsi). Aquat. Rep. 2022, 23, 101049. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; McCarthy, D.J.; Chen, Y.; Okoniewski, M.; Smyth, G.K.; Huber, W.; Robinson, M.D. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat. Protoc. 2013, 8, 1765–1786. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. Cluster profiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, S.; Kaneko, H.; Kobayashi, T.; Wang, D.S.; Sakai, F.; Paul-Prasanth, B.; Nagahama, Y. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol. Reprod. 2008, 78, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Ma, H.; Liu, X.; Shi, H.; Li, M.; Wang, D. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile tilapia. Endocrinology 2017, 158, 2634–2647. [Google Scholar] [CrossRef]
- Lau, E.S.W.; Zhang, Z.; Qin, M.; Ge, W. Knockout of zebrafish ovarian aromatase gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Sci. Rep. 2016, 6, 37357. [Google Scholar] [CrossRef] [PubMed]
- Theodoridi, A.; Dinarello, A.; Badenetti, L.; Pavlidis, M.; Dalla Valle, L.; Tsalafouta, A. Knockout of the hsd11b2 Gene Extends the Cortisol Stress Response in Both Zebrafish Larvae and Adults. Int. J. Mol. Sci. 2021, 22, 12525. [Google Scholar] [CrossRef]
- Viñas, J.; Piferrer, F. Stage-specific gene expression during fish spermatogenesis as determined by laser-capture microdissection and quantitative-PCR in sea bass (Dicentrarchus labrax) gonads. Biol. Reprod. 2008, 79, 738–747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Q.; Han, C.; Peng, C.; Zhou, X.; Wang, C.; Han, L.; Li, S.S.; Li, G.F.; Lin, H.R.; Zhang, Y. Identification of potential sex-related genes in Siniperca chuatsi. J. Oceanol. Limnol. 2021, 39, 1500–1512. [Google Scholar] [CrossRef]
- Lin, Q.; Mei, J.; Li, Z.H.; Zhang, X.; Zhou, L.; Gui, J.F. Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics 2017, 207, 1007–1022. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A Tandem Duplicate of Anti-Mullerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Liu, X.; Dai, S.; Wu, J.; Wei, X.; Zhou, X.; Chen, M.; Tan, D.; Pu, D.; Li, M.; Wang, D. Roles of Anti-Müllerian hormone (Amh) and its duplicates in sex determination and germ cell proliferation of Nile tilapia. Genetics 2021, 220, iyab237. [Google Scholar] [CrossRef]
- Dranow, D.B.; Hu, K.; Bird, A.M.; Lawry, S.T.; Adams, M.T.; Sanchez, A.; Amatruda, G.F.; Draper, B.W. Bmp15 is an oocyte-produced signal required for maintenance of the adult female sexual phenotype in zebrafish. PLoS Genet. 2016, 12, e1006323. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yang, C.; Tao, W.; Wang, D. Genome-wide identification and transcriptome-based expression profiling of the Sox gene family in the Nile Tilapia (Oreochromis niloticus). Int. J. Mol. Sci. 2016, 17, 270. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M.; Schories, S.; Wakamatsu, Y.; Nagao, Y.; Hashimoto, H.; Bertin, C.; Mourot, B.; Schmidt, C.; Wilhelm, D.; Centanin, L.; et al. Sox5 is involved in germ-cell regulation and sex determination in medaka following co-option of nested transposable elements. BMC Biol. 2018, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, F.; Georg, I.; Scherthan, H.; Lécureuil, C.; Guillou, F.; Wegner, M.; Scherer, G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev. Biol. 2009, 327, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, M.; Lau, Y.F.C. The sex-determining factors SRY and SOX9 regulate similar target genes and promote testis cord formation during testicular differentiation. Cell Rep. 2014, 8, 723–733. [Google Scholar] [CrossRef]
- Fujihara, Y.; Oji, A.; Larasati, T.; Kojima-Kita, K.; Ikawa, M. Human globozoospermia-related gene Spata16 is required for sperm formation revealed by CRISPR/Cas9-mediated mouse models. Int. J. Mol. Sci. 2017, 18, 2208. [Google Scholar] [CrossRef] [PubMed]
- Dam, A.H.; Koscinski, I.; Kremer, J.A.; Moutou, C.; Jaeger, A.S.; Oudakker, A.R.; Tournaye, H.; Charlet, N.; Largier-Tourenne, C.; Bokhoven, H. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am. J. Hum. Genet. 2007, 81, 813–820. [Google Scholar] [CrossRef]
- Yang, D.; Yin, C.; Chang, Y.; Dou, Y.; Hao, Z.; Ding, J. Transcriptome analysis of male and female mature gonads of Japanese scallop Patinopecten yessonsis. Genes Genom. 2016, 38, 1041–1052. [Google Scholar] [CrossRef]

| Primer ID | Primer Sequences |
|---|---|
| GDCB-amh-QP1F | TCCACAGAGTCCCTGCTATCA |
| GDCB-amh-QP1R | CAATCACCTTTTGCCCCAC |
| GDCB-hsd11b2-QP1F | AGACAGGCTAAAGGCCGGA |
| GDCB-hsd11b2-QP1R | TGACGAAGTGTGTTGGTAAGAAGAT |
| GDCB-fshr-QP1F | CGCCTTCTTCAACCTCAC |
| GDCB-fshr-QP1R | GTTACTTGGAATCCTTTCTATG |
| GDCB-era-QP1F | GCATTTTCATTCTGCTCCAGTC |
| GDCB-era-QP1R | CATTCCTTTGTTGCTCATGTGTCT |
| GDCB-erbeta1-QP1F | CCGCTGGTGAAGAGGGTAGT |
| GDCB-erbeta1-QP1R | GCAGATGTAGTCATTGTGTCCTTGTAT |
| GDCB-erbeta2-QP1F | TGGAGAGCCGTGGGAAGGTT |
| GDCB-erbeta2-QP1R | TGTTGCTGAGGTGTCGAATATG |
| GDCB-ar-QP1F | CCCCCAGCAAGGGAATAATATG |
| GDCB-ar-QP1R | TCAGCGCCCCGAAAGAAA |
| GDCB-foxk2-QP1F | TGAAGCTGCCGAGAGTGTGC |
| GDCB-foxk2-QP1R | GGGATGTTGATGGTTAAAGGGG |
| GDCB-smad4a-QP1F | CCAGGGAGGAGAGAATGAGAGC |
| GDCB-smad4a-QP1R | CGAGCGTATATGACATGAGGGAA |
| GDCB-gdf6-QP1F | AGTGTCCAAGTCTGTTCCTGCT |
| GDCB-gdf6-QP1R | CTCGTTATGGTGTTTGCTGC |
| GDCB-bmp7-QP1F | CACCGCTCTAAAAACCCCA |
| GDCB-bmp7-QP1R | ATTCTCCCTCACAGTAATACGCA |
| GDCB-pax7-QP1F | AGGCCAGAGTGCAGGTTTGG |
| GDCB-pax7-QP1R | TGTGGTAATGTTGTTGAAGGATAGG |
| GDCB-pax8-QP1F | AAACCAGGAGTCATCGGGGG |
| GDCB-pax8-QP1R | CCTTTGCTGTCCAGAGGAAGG |
| GDCB-sox9-QP1F | AGGTCAGAGCTCCGGCTTGTACT |
| GDCB-sox9-QP1R | TGTGATTGGGTTGGGGAATGG |
| GDCB-sox5-QP1F | ACCAAAGCCCAAGGACGAGG |
| GDCB-sox5-QP1R | GCCGGAGGTGATGGATGACA |
| GDCB-beta-actin-QP1F | GTGTTGGCATACAGGTCCTTACG |
| GDCB-beta-actin-QP1R | ACGGACAGGTCATCACCATTG |
| Sample | Reads Number | Base Number | Total Base | Q20 | Q30 | GC |
|---|---|---|---|---|---|---|
| JC-K5 | 23,015,034 | 3,452,255,100 | 6,904,510,200 | 98.33% | 95.25% | 46.53% |
| 23,015,034 | 3,452,255,100 | 97.96% | 94.12% | 46.51% | ||
| JC-K8 | 24,277,601 | 3,641,640,150 | 7,283,280,300 | 98.29% | 95.16% | 46.56% |
| 24,277,601 | 3,641,640,150 | 97.86% | 93.88% | 46.54% | ||
| JC-K9 | 19,990,173 | 2,998,525,950 | 5,997,051,900 | 98.32% | 95.21% | 46.21% |
| 19,990,173 | 2,998,525,950 | 98.08% | 94.42% | 46.18% | ||
| LC-K1 | 23,109,316 | 3,466,397,400 | 6,932,794,800 | 98.11% | 94.96% | 48.05% |
| 23,109,316 | 3,466,397,400 | 97.85% | 94.20% | 48.03% | ||
| LC-K3 | 21,181,082 | 3,177,162,300 | 6,354,324,600 | 98.29% | 95.23% | 48.17% |
| 21,181,082 | 3,177,162,300 | 97.83% | 94.01% | 48.15% | ||
| LC-K9 | 20,827,911 | 3,124,186,650 | 6,248,373,300 | 98.42% | 95.45% | 48.03% |
| 20,827,911 | 3,124,186,650 | 98.12% | 94.58% | 48.00% |
| Number | |
|---|---|
| Gene Number (#): | 167,152 |
| Total length (nt): | 145,709,933 |
| Average length (nt): | 871 |
| Max length (nt): | 18,177 |
| Min length (nt): | 280 |
| N50 | 1198 |
| Unigene ID | log2FC (JC_vs_LC) | p Value (JC_vs_LC) | FDR (JC_vs_LC) | Nr Annotation | Gene Name |
|---|---|---|---|---|---|
| unigene074455 | 7.572 | 4.92 × 10−9 | 1.83 × 10−08 | Growth differentiation factor 6 | gdf6 |
| unigene027408 | 7.318 | 2.71 × 10−9 | 1.04 × 10−08 | Neuropeptide Y receptor type 2 | npy2r |
| unigene016644 | 7.159 | 7.36 × 10−14 | 4.50 × 10−13 | Cytochrome P450 11B | cyp11b1 |
| unigene058283 | 6.803 | 4.63 × 10−08 | 1.52 × 10−07 | Forkhead box protein K2 | foxk2 |
| unigene065602 | 6.718 | 3.09 × 10−04 | 5.54 × 10−04 | Paired box protein Pax-6 | pax6 |
| unigene047253 | 6.481 | 4.79 × 10−06 | 1.16 × 10−05 | Fibroblast growth factor 20 | fgf20 |
| unigene082603 | 6.325 | 2.40 × 10−05 | 5.23 × 10−05 | Bone morphogenetic protein 7 | Bmp7 |
| unigene100115 | 6.112 | 1.68 × 10−04 | 3.17 × 10−04 | Forkhead box G1-like protein | foxg1 |
| unigene034505 | 6.052 | 1.43 × 10−05 | 3.24 × 10−05 | Catenin delta-2 | ctnnd2 |
| unigene022426 | 5.941 | 1.44 × 10−19 | 1.38 × 10−18 | Paired box protein Pax-2a | pax2 |
| unigene141021 | 5.878 | 2.57 × 10−12 | 1.37 × 10−11 | Nanos homolog 2 | nanos2 |
| unigene021432 | 5.874 | 5.49 × 10−05 | 1.12 × 10−04 | Paired box protein Pax-7 | pax7 |
| unigene008910 | 5.867 | 3.93 × 10−05 | 8.24 × 10−05 | Forkhead box protein L1 | foxl1 |
| unigene106729 | 5.856 | 5.75 × 10−108 | 1.08 × 10−105 | Anti-Mullerian hormone | amh |
| unigene035421 | 5.798 | 7.51 × 10−13 | 4.21 × 10−12 | Insulin-like growth factor-I | igf1 |
| unigene086594 | 5.661 | 1.96 × 10−10 | 8.53 × 10−10 | Protein Wnt-6 | wnt6 |
| unigene130369 | 5.617 | 2.78 × 10−03 | 4.27 × 10−03 | Transforming growth factor beta receptor type 3 | tgfbr3 |
| unigene123135 | 4.990 | 7.83 × 10−33 | 1.54 × 10−31 | Transcription factor SOX9 | sox9 |
| unigene089170 | 4.878 | 1.56 × 10−13 | 9.30 × 10−13 | Protein Wnt-5a | wnt5 |
| unigene025970 | 4.539 | 6.90 × 10−11 | 3.16 × 10−10 | Protein Wnt-2b | wnt2 |
| unigene009697 | 4.487 | 1.83 × 10−10 | 8.00 × 10−10 | Gonadotropin-releasing hormone II receptor | gnrhr |
| unigene125833 | 4.461 | 1.52 × 10−19 | 1.45 × 10−18 | Sperm-associated antigen 1 | spag1 |
| unigene159834 | 4.418 | 1.43 × 10−06 | 3.77 × 10−06 | Follicle stimulating hormone receptor | fshr |
| unigene018630 | 4.344 | 6.47 × 10−23 | 7.59 × 10−22 | Mothers against decapentaplegic homolog 4a | smad4a |
| unigene143320 | 4.016 | 5.73 × 10−06 | 1.38 × 10−05 | Forkhead box D2-like protein | foxd2 |
| unigene036991 | 3.766 | 1.74 × 10−04 | 3.27 × 10−04 | Protein Wnt-3a | wnt3 |
| unigene131983 | 3.725 | 1.54 × 10−09 | 6.08 × 10−09 | Bone morphogenetic protein 6 isoform X1 | bmp6 |
| unigene072066 | 3.692 | 1.06 × 10−04 | 2.07 × 10−04 | Forkhead box P1-B-like isoform X1 | foxp1 |
| unigene021595 | 3.626 | 2.58 × 10−06 | 6.54 × 10−06 | Bone morphogenetic protein receptor type-2 | bmpr2 |
| unigene094351 | 3.575 | 1.65 × 10−09 | 6.47 × 10−09 | Paired box protein Pax-3 | pax3 |
| unigene077500 | 3.532 | 5.58 × 10−08 | 1.81 × 10−07 | Paired box protein Pax-8 | pax8 |
| unigene114563 | 3.461 | 6.23 × 10−06 | 1.49 × 10−05 | Forkhead box protein O6 | foxo6 |
| unigene020954 | 3.447 | 2.05 × 10−07 | 6.11 × 10−07 | Transcription factor Sox-1a | sox1 |
| unigene151019 | 3.352 | 6.41 × 10−08 | 2.06 × 10−07 | Protein Wnt-11 | wnt11 |
| unigene157484 | 3.304 | 7.84 × 10−33 | 1.54 × 10−31 | Fibroblast growth factor receptor 2 | fgfr2 |
| unigene060599 | 3.220 | 2.39 × 10−18 | 2.10 × 10−17 | Transcription factor SOX-5 | sox5 |
| unigene060624 | 3.212 | 9.24 × 10−11 | 4.17 × 10−10 | Growth-hormone-releasing hormone receptor | ghrhr |
| unigene071307 | 3.210 | 6.50 × 10−17 | 5.12 × 10−16 | Transforming growth factor beta-2 | tgfb2 |
| unigene119381 | 3.181 | 1.92 × 10−10 | 8.37 × 10−10 | Insulin-like growth-factor-binding 3 | igfb3 |
| unigene002746 | 3.074 | 6.17 × 10−20 | 6.04 × 10−19 | Corticosteroid 11-beta-dehydrogenase isozyme 2 | hsd11b2 |
| unigene073844 | 2.890 | 4.90 × 10−13 | 2.79 × 10−12 | Estrogen receptor beta 2 | erb2 |
| unigene076208 | 2.764 | 1.34 × 10−06 | 3.55 × 10−06 | Follitropin subunit beta | fshb |
| unigene145623 | 2.763 | 7.92 × 10−09 | 2.87 × 10−08 | Insulin-like growth-factor-binding protein 1 | igfbp1 |
| unigene144860 | 2.759 | 2.51 × 10−07 | 7.39 × 10−07 | Forkhead box protein O3 | foxo3 |
| unigene052054 | 2.730 | 6.22 × 10−11 | 2.86 × 10−10 | Fibroblast growth factor receptor 4 | fgfr4 |
| unigene126929 | 2.604 | 6.48 × 10−06 | 1.55 × 10−05 | Bone morphogenetic protein 2 | bmp2 |
| unigene146529 | 2.383 | 7.29 × 10−07 | 2.01 × 10−06 | Transcription factor sox7 | sox7 |
| unigene156535 | 2.380 | 2.36 × 10−13 | 1.38 × 10−12 | Estrogen receptor beta 1 | erb1 |
| unigene057687 | 2.372 | 8.63 × 10−07 | 2.35 × 10−06 | stAR-related lipid transfer protein 13 | stard13 |
| unigene031051 | 2.351 | 1.86 × 10−05 | 4.13 × 10−05 | Forkhead box protein D1 | foxd1 |
| unigene013811 | 2.265 | 3.43 × 10−03 | 5.18 × 10−03 | Androgen receptor protein | ar |
| unigene101954 | 2.264 | 2.05 × 10−04 | 3.80 × 10−04 | Gonadotropin-releasing hormone | gnrh |
| unigene100104 | 2.236 | 3.13 × 10−06 | 7.84 × 10−06 | double sex- and mab-3-related transcription factor 2 | dmrt2 |
| unigene008405 | 2.207 | 1.52 × 10−03 | 2.43 × 10−03 | Spermatogenesis-associated protein 16 | sgap16 |
| unigene105851 | 2.174 | 3.57 × 10−03 | 5.37 × 10−03 | Paired box protein Pax-4 | pax4 |
| unigene040229 | 2.160 | 1.35 × 10−08 | 4.77 × 10−08 | Growth/differentiation factor 10 | gdf10 |
| unigene122511 | 2.117 | 1.60 × 10−06 | 4.18 × 10−06 | Transcription factor SOX-4 | sox4 |
| unigene054659 | −2.416 | 3.36 × 10−13 | 1.94 × 10−12 | Spermatid perinuclear RNA-binding protein isoform X3 | strbp |
| unigene147986 | −2.463 | 3.91 × 10−09 | 1.47 × 10−08 | Cytochrome P450 aromatase | cyp19a1a |
| unigene017060 | −2.563 | 1.13 × 10−31 | 2.10 × 10−30 | TGF-beta receptor type-2-like isoform X1 | tgfbr2 |
| unigene124450 | −2.947 | 2.27 × 10−38 | 5.69 × 10−37 | Catenin beta-1 | ctnnb1 |
| unigene062930 | −2.964 | 5.89 × 10−11 | 2.72 × 10−10 | Transcription factor Sox-3 | sox3 |
| unigene014765 | −3.027 | 4.13 × 10−10 | 1.73 × 10−09 | Spermatogenesis-associated protein 13 | sgap13 |
| unigene000100 | −3.070 | 8.27 × 10−07 | 2.26 × 10−06 | Sperm-associated antigen 5 | spag5 |
| unigene127688 | −3.105 | 3.71 × 10−44 | 1.16 × 10−42 | sTART domain-containing protein 10 | stard10 |
| unigene152354 | −3.674 | 8.97 × 10−10 | 3.63 × 10−09 | Paired box protein Pax-1 | pax1 |
| unigene051443 | −3.717 | 2.71 × 10−18 | 2.37 × 10−17 | Double sex- and mab-3-related transcription factor A1 | dmrta1 |
| unigene143561 | −3.915 | 1.79 × 10−71 | 1.39 × 10−69 | Growth/differentiation factor 9 | gdf9 |
| unigene125277 | −3.925 | 5.06 × 10−13 | 2.88 × 10−12 | Estradiol 17-beta-dehydrogenase 2 | hsd17b2 |
| unigene069571 | −3.965 | 1.60 × 10−88 | 1.96 × 10−86 | Bone morphogenetic protein 15 | bmp15 |
| unigene086306 | −4.644 | 2.71 × 10−18 | 2.37 × 10−17 | Nuclear receptor subfamily 5 group A member 2 | nr5a2 |
| unigene007641 | −5.956 | 5.52 × 10−41 | 1.55 × 10−39 | Forkhead box protein P3 | foxp3 |
| unigene060440 | −5.993 | 3.07 × 10−50 | 1.20 × 10−48 | Transcription factor Sox-19b | sox19b |
| unigene150909 | −6.870 | 4.59 × 10−170 | 3.12 × 10−167 | Transcription factor Sox-21-A | sox31a |
| unigene158966 | −7.139 | 8.15 × 10−47 | 2.82 × 10−45 | Protein Wnt-8a | wnt8 |
| unigene043515 | −11.722 | 3.97 × 10−142 | 1.52 × 10−139 | Zona pellucida sperm-binding protein 4 | zp4 |
| unigene055600 | −12.153 | 5.03 × 10−220 | 1.32 × 10−216 | Zona pellucida sperm-binding protein 3 | zp3 |
| unigene077416 | −14.328 | 3.82 × 10−181 | 3.10 × 10−178 | Growth/differentiation factor 3 | gdf3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Huang, W.; Peng, S.; Zhou, J.; Zhan, H.; Zhang, Y.; Li, W.; Gong, J.; Li, Q. De Novo Assembly, Characterization and Comparative Transcriptome Analysis of the Mature Gonads in Spinibarbus hollandi. Animals 2023, 13, 166. https://doi.org/10.3390/ani13010166
Han C, Huang W, Peng S, Zhou J, Zhan H, Zhang Y, Li W, Gong J, Li Q. De Novo Assembly, Characterization and Comparative Transcriptome Analysis of the Mature Gonads in Spinibarbus hollandi. Animals. 2023; 13(1):166. https://doi.org/10.3390/ani13010166
Chicago/Turabian StyleHan, Chong, Wenwei Huang, Suhan Peng, Jiangwei Zhou, Huawei Zhan, Yuying Zhang, Wenjun Li, Jian Gong, and Qiang Li. 2023. "De Novo Assembly, Characterization and Comparative Transcriptome Analysis of the Mature Gonads in Spinibarbus hollandi" Animals 13, no. 1: 166. https://doi.org/10.3390/ani13010166
APA StyleHan, C., Huang, W., Peng, S., Zhou, J., Zhan, H., Zhang, Y., Li, W., Gong, J., & Li, Q. (2023). De Novo Assembly, Characterization and Comparative Transcriptome Analysis of the Mature Gonads in Spinibarbus hollandi. Animals, 13(1), 166. https://doi.org/10.3390/ani13010166






