Ecological Restoration Practices within a Semi-arid Natural Gas Field Improve Insect Abundance and Diversity during Early and Late Growing Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Sampling
2.3. Insect Sampling
2.4. Statistical Analysis
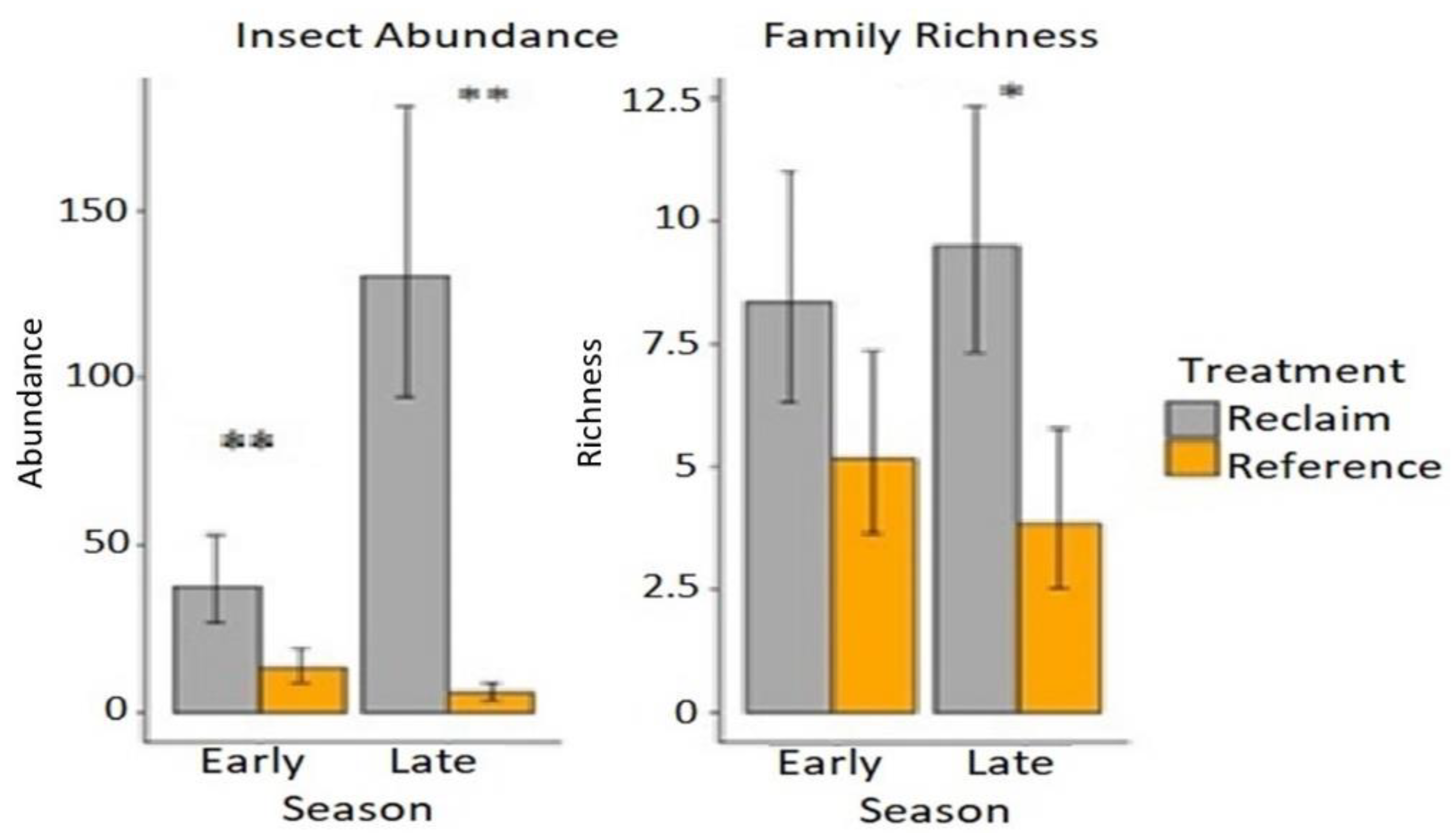
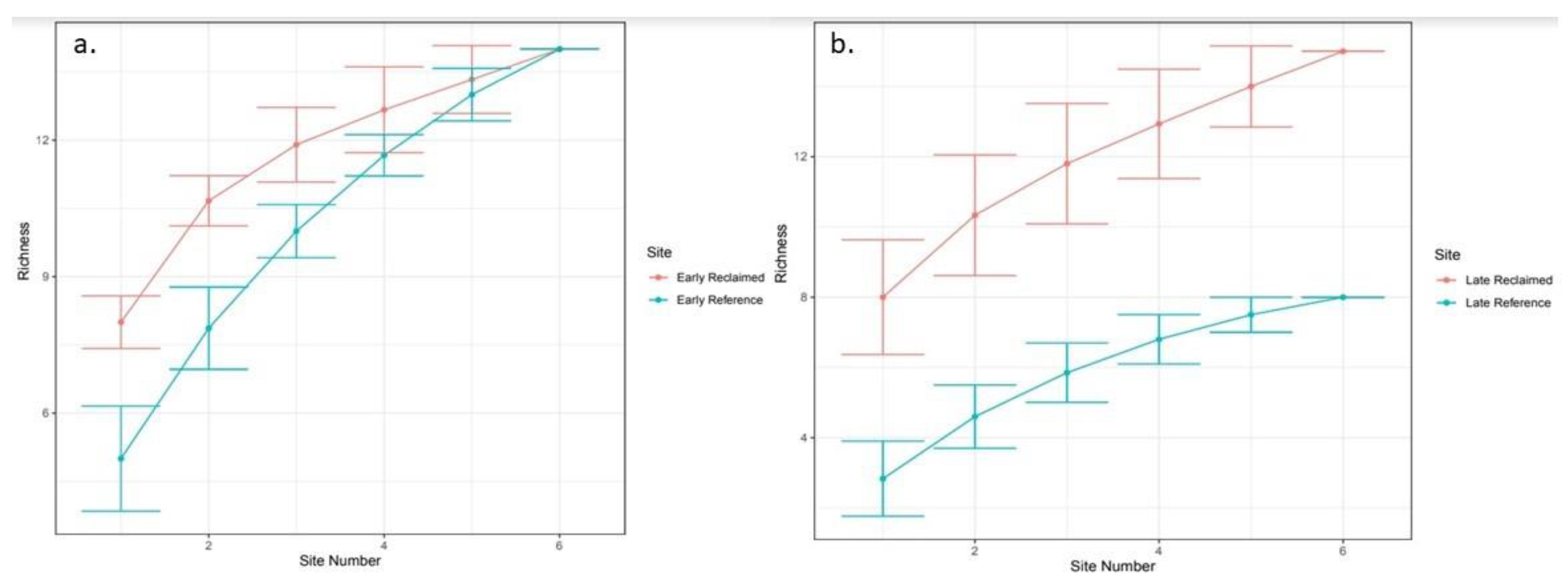
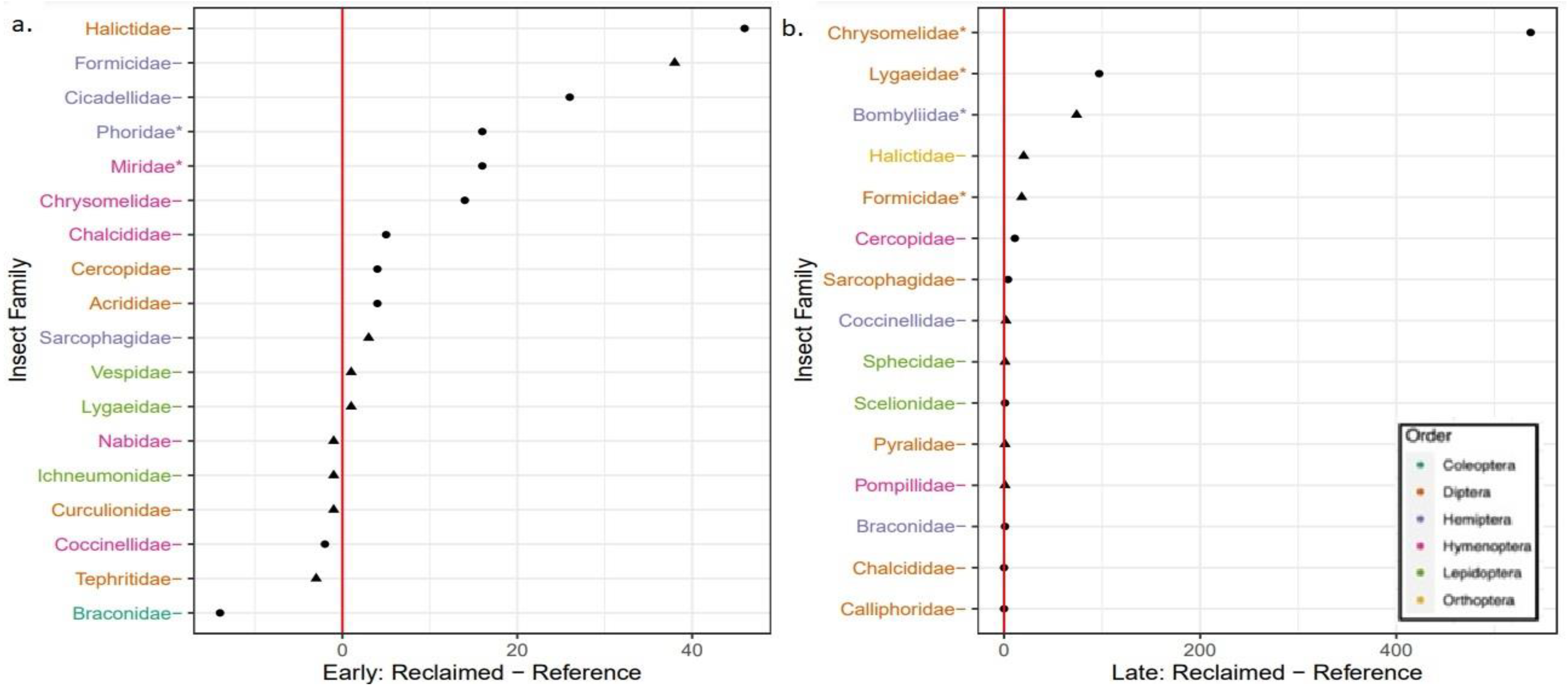
3. Results
3.1. Vegetation Sampling
3.2. Insect Sampling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Noreiga, J.A.; Hortal, J.; Azcarate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef]
- Lavelle, P. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- Belovsky, G.E.; Slade, J.B. Insect herbivory accelerates nutrient cycling and increases plant production. Proc. Natl. Acad. Sci. USA 2000, 97, 14412–14417. [Google Scholar] [CrossRef] [PubMed]
- Tallamy, D. Bringing Nature Home: How Native Plants Sustain Wildlife in Our Gardens; Timber Press: Portland, OR, USA, 2009. [Google Scholar]
- Archer, S.; Pyke, D.A. Plant-animal interactions affecting plant establishment and persistence on revegetated rangeland. Rangel. Ecol. Manag. 1991, 44, 558–565. [Google Scholar] [CrossRef]
- Dixon, K.W. Pollination and restoration. Science 2009, 325, 571–573. [Google Scholar] [CrossRef]
- Cusser, S.; Goodell, K. Diversity and distribution of floral resources influence the restoration of plant-pollinator networks on a reclaimed strip mine. Restor. Ecol. 2013, 26, 713–721. [Google Scholar] [CrossRef]
- Kimberling, D.N.; Karr, J.R.; Fore, L.S. Measuring human disturbance using terrestrial invertebrates in the shrub-steppe of eastern Washington (USA). Ecol. Indic. 2001, 1, 63–81. [Google Scholar] [CrossRef]
- Longcore, T. Terrestrial arthropods as indicators of ecological restoration success in coastal sage scrub (California, USA). Restor. Ecol. 2003, 11, 397–409. [Google Scholar] [CrossRef]
- Rohde, A.T.; Pilliod, D.S.; Novak, S.J. Insect communities in big sagebrush habitat are altered by wildfire and post-fire restoration seeding. Insect Conserv. Divers. 2019, 12, 216–230. [Google Scholar] [CrossRef]
- Menz, M.M.H.; Phillips, R.D.; Winfree, R.; Kremen, C.; Aizen, M.A.; Johnson, S.D.; Dixon, K.W. Reconnecting plants and pollinators: Challenges in the restoration of pollination mutualisms. Trends Plant Sci. 2011, 16, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Tonietto, R.K.; Larkin, D.J. Habitat restoration benefits wild bees: A meta-analysis. J. Appl. Ecol. 2018, 55, 582–590. [Google Scholar] [CrossRef]
- Curran, M.F.; Robinson, T.J.; Guernsey, P.; Sorenson, J.; Crow, T.M.; Smith, D.I.; Stahl, P.D. Insect abundance and diversity respond favorably to vegetation communities on interim reclamation sites in a semi-arid natural gas field. Land 2022, 11, 527. [Google Scholar] [CrossRef]
- Curran, M.F.; Wolff, B.J.; Stahl, P.D. Demonstration Study: Approaching oil and gas pad reclamation with data management: A framework for the future. J. Am. Soc. Min. Reclam. 2013, 2, 195–204. [Google Scholar]
- Curran, M.F.; Cox, S.E.; Robinson, T.J.; Robertson, B.L.; Rogers, K.A.; Sherman, Z.A.; Adams, T.A.; Strom, C.F.; Stahl, P.D. Spatially balanced sampling and ground-level imagery for revegetation monitoring on reclaimed well pads. Restor. Ecol. 2019, 27, 947–980. [Google Scholar] [CrossRef]
- Curran, M.F.; Stahl, P.D. Database management for large scale reclamation projects in Wyoming: Developing better data acquisition, monitoring, and models for applications to future projects. J. Environ. Solut. Oil Gas Min. 2015, 1, 31–43. [Google Scholar] [CrossRef]
- Stahl, P.D.; Curran, M.F. Collaborative Efforts Towards Ecological Habitat Restoration of a Threatened Species, Greater Sage-Grouse in Wyoming, USA. In Land Reclamation in Ecological Fragile Areas; CRC Press: London, UK, 2017; pp. 251–254. [Google Scholar]
- Grodsky, S.M.; Iglay, R.B.; Sorenson, C.E.; Moorman, C.E. Should invertebrates receive greater attention in wildlife research journals? J. Wildl. Manag. 2018, 79, 529–536. [Google Scholar] [CrossRef]
- Sylvain, Z.A.; Espeland, E.K.; Rand, T.A.; West, N.M.; Branson, D.H. Oilfield reclamation recovers productivity but not composition of arthropod herbivores and predators. Environ. Entomol. 2019, 48, 299–308. [Google Scholar] [CrossRef]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering crops enhance pollinator densities at a landscape scales. Ecol. Lett. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Mass-flowering crops enhance wild bee abundance. Oecologia 2013, 172, 477–484. [Google Scholar] [CrossRef]
- Todd, K.J.; Gardiner, M.M.; Lindquist, E.D. Mass flowering crops as a conservation resource for wild pollinators (Hymenoptera: Apoidea). J. Kans. Entomol. Soc. 2016, 89, 158–167. [Google Scholar] [CrossRef]
- Samways, M.J.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Solutions for humanity on how to conserve insects. Biol. Conserv. 2020, 242, 108427. [Google Scholar] [CrossRef]
- Cane, J.H. Breeding biologies, seed production and species-rich bee guilds of Cleome lutea and Cleome serrulata (Cleomaceae). Plant Species Biol. 2008, 23, 152–158. [Google Scholar] [CrossRef]
- Curran, M.F.; Sorenson, J.; Stahl, P.D. Rocky mountain beeplant aids revegetation in an arid natural gas field. Environ. Connect. 2019, 14, 27–29. [Google Scholar]
- Sommers, J. Green River Drift: A History of the Upper Green River Cattle Association; Falcon Press Publishing Co., Inc.: Helena, MT, USA, 1994. [Google Scholar]
- Cagney, J.; Cox, S.E.; Booth, D.T. Comparison of point intercept and image analysis for monitoring rangeland composition and trend. Rangel. Ecol. Manag. 2011, 64, 309–315. [Google Scholar] [CrossRef]
- Curran, M.F.; Cox, S.E.; Robinson, T.J.; Strom, C.F.; Stahl, P.D. Combining spatially balanced sampling, route optimization and remote sensing to assess biodiversity response to reclamation practices on semi-arid well pads. Biodiversity 2020, 21, 171–181. [Google Scholar] [CrossRef]
- Curran, M.F.; Hodza, P.; Cox, S.E.; Lanning, S.G.; Robertson, B.L.; Robinson, T.J.; Stahl, P.D. Ground-level unmanned aerial system imagery coupled with spatially balanced sampling and route optimization to monitor rangeland vegetation. J. Vis. Exp. 2020, 160, e61052. [Google Scholar] [CrossRef]
- Booth, D.T.; Cox, S.E.; Berryman, R.D. Sampling digital imagery with ‘SamplePoint’. Environ. Monit. Assess. 2006, 123, 97–108. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Inouye, R.S. Insect community response to plant diversity and productivity in a sagebrush-steppe ecosystem. J. Arid Environ. 2008, 72, 24–33. [Google Scholar] [CrossRef]
- Milne, L.; Milne, M. National Audubon Society: Field Guide to North American Insects and Spiders; No. 595.7 M659; Toppan Printing Co., Ltd.: Tokyo, Japan, 2011. [Google Scholar]
- Balmford, A.; Jayasuriya, A.H.M.; Green, M.J.B. Using higher-taxon richness as a surrogate for species richness. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1996, 263, 1571–1575. [Google Scholar]
- Riggins, J.J.; Davis, C.A.; Hoback, W.W. Biodiversity of belowground invertebrates as an indicator of wet meadow restoration success (Platte River, Nebraska). Restor. Ecol. 2009, 17, 495–505. [Google Scholar] [CrossRef]
- Cane, J.H.; Love, B. Floral guilds of bees in sagebrush steppe: Comparing bee usage of wildflowers available for postfire restoration. Nat. Areas J. 2016, 36, 377–391. [Google Scholar] [CrossRef]
- Cane, J.H. Habitat fragmentation and native bees: A premature verdict? Conserv. Ecol. 2001, 5, 3. [Google Scholar] [CrossRef]
- Shackelford, N.; Paterno, G.B.; Winkler, D.E.; Erickson, T.E.; Leger, E.A.; Svejcar, L.N.; Breed, M.F.; Faist, A.M.; Harrison, P.A.; Curran, M.F.; et al. Drivers of seedling establishment success in dryland restoration efforts. Nat. Ecol. Evol. 2021, 5, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Monroe, A.P.; Aldridge, C.L.; O’Donnell, M.S.; Manier, D.J.; Homer, C.G.; Anderson, P.J. Using remote sensing products to predict recovery of vegetation across space and time following energy development. Ecol. Indic. 2020, 110, 105872. [Google Scholar] [CrossRef]
- Hagler, J.R.; Jackson, C.G. Methods for marking insects: Current techniques and future prospects. Annu. Rev. Entomol. 2001, 46, 511–543. [Google Scholar] [CrossRef]
- Curran, M.F.; Crow, T.M.; Hufford, K.M.; Stahl, P.D. Forbs and greater sage-grouse habitat restoration efforts: Suggestions for improving commercial seed availability and restoration practices. Rangelands 2015, 37, 211–216. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Luna, T.; Pinto, J.R.; Landis, T.D. Forbs: Foundation for restoration of monarch butterflies, other pollinators, and greater sage-grouse in the Western United States. Nat. Areas J. 2016, 36, 499–511. [Google Scholar] [CrossRef]
- Curran, M.F.; Summerfield, K.; Alexander, E.-J.; Lanning, S.G.; Schwyter, A.R.; Torres, M.L.; Schell, S.; Vaughan, K.; Robinson, T.J.; Smith, D.I. Use of 3-dimensional videography as a non-lethal way to improve visual insect sampling. Land 2020, 9, 340. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curran, M.F.; Sorenson, J.R.; Craft, Z.A.; Crow, T.M.; Robinson, T.J.; Stahl, P.D. Ecological Restoration Practices within a Semi-arid Natural Gas Field Improve Insect Abundance and Diversity during Early and Late Growing Season. Animals 2023, 13, 134. https://doi.org/10.3390/ani13010134
Curran MF, Sorenson JR, Craft ZA, Crow TM, Robinson TJ, Stahl PD. Ecological Restoration Practices within a Semi-arid Natural Gas Field Improve Insect Abundance and Diversity during Early and Late Growing Season. Animals. 2023; 13(1):134. https://doi.org/10.3390/ani13010134
Chicago/Turabian StyleCurran, Michael F., Joshua R. Sorenson, Zoe A. Craft, Taylor M. Crow, Timothy J. Robinson, and Peter D. Stahl. 2023. "Ecological Restoration Practices within a Semi-arid Natural Gas Field Improve Insect Abundance and Diversity during Early and Late Growing Season" Animals 13, no. 1: 134. https://doi.org/10.3390/ani13010134
APA StyleCurran, M. F., Sorenson, J. R., Craft, Z. A., Crow, T. M., Robinson, T. J., & Stahl, P. D. (2023). Ecological Restoration Practices within a Semi-arid Natural Gas Field Improve Insect Abundance and Diversity during Early and Late Growing Season. Animals, 13(1), 134. https://doi.org/10.3390/ani13010134





