Novel Quantitative PCR for Rhodococcus equi and Macrolide Resistance Detection in Equine Respiratory Samples
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Genomes
2.2. Oligo and Probe Design and In-Silico Validation
2.3. Bacterial Strains and Culture Conditions
2.4. DNA Extraction and Real-Time qPCR Assay
2.5. Standard Curve Construction
2.6. Limit of Detection (LOD) Calculation
3. Results
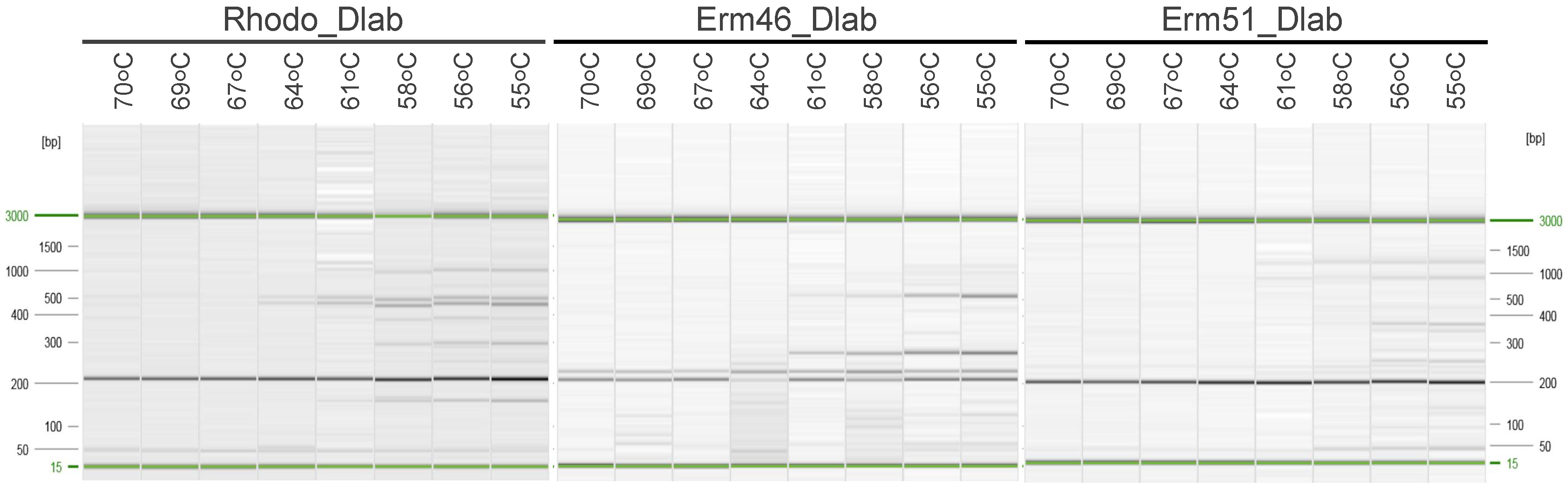
3.1. Oligo Set Design and In-silico Validation
3.2. Testing Oligos and Probes in Singleplex and Multiplex qPCR Assays
3.3. Testing the Analytic Sensitivity and Specificity in Mocking Equine Respiratory Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giguère, S.; Cohen, N.; Chaffin, M.K.; Hines, S.; Hondalus, M.; Prescott, J.; Slovis, N. Rhodococcus equi: Clinical Manifestations, Virulence, and Immunity. J. Veter. Intern. Med. 2011, 25, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Boland, J.A.; Giguère, S.; Hapeshi, A.; MacArthur, I.; Anastasi, E.; Valero-Rello, A. Rhodococcus equi: The many facets of a pathogenic actinomycete. Veter Microbiol. 2013, 167, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F. Rhodococcus equi: An animal and human pathogen. Clin. Microbiol. Rev. 1991, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Narváez, S.; Giguère, S.; Cohen, N.; Slovis, N.; Vázquez-Boland, J.A. Spread of Emerging Multidrug-Resistant Rhodococcus equi in USA. Emerg. Infect. Dis. 2021, 27, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Narváez, S.; Huber, L.; Giguère, S.; Hart, K.A.; Berghaus, R.D.; Sanchez, S.; Cohen, N.D. Epidemiology and Molecular Basis of Multidrug Resistance in Rhodococcus equi. Microbiol. Mol. Biol. Rev. 2021, 85, e00011-21. [Google Scholar] [CrossRef]
- Huber, L.; Giguère, S.; Slovis, N.M.; Álvarez-Narváez, S.; Hart, K.A.; Greiter, M.; Morris, E.R.A.; Cohen, N.D. The novel and transferable erm (51) gene confers macrolides, lincosamides and streptogramins B (MLS B) resistance to clonal Rhodococcus equi in the environment. Environ. Microbiol. 2020, 22, 2858–2869. [Google Scholar] [CrossRef]
- Álvarez-Narváez, S.; Giguère, S.; Anastasi, E.; Hearn, J.; Scortti, M.; Vázquez-Boland, J.A. Clonal Confinement of a Highly Mobile Resistance Element Driven by Combination Therapy in Rhodococcus equi. mBio 2019, 10, e02260-19. [Google Scholar] [CrossRef] [Green Version]
- Anastasi, E.; MacArthur, I.; Scortti, M.; Alvarez, S.; Giguère, S.; Vázquez-Boland, J.A. Pangenome and Phylogenomic Analysis of the Pathogenic Actinobacterium Rhodococcus equi. Genome Biol. Evol. 2016, 8, 3140–3148. [Google Scholar] [CrossRef] [Green Version]
- Arriaga, J.M.; Cohen, N.; Derr, J.N.; Chaffin, M.K.; Martens, R.J. Detection of Rhodococcus equi by Polymerase Chain Reaction Using Species-Specific Nonproprietary Primers. J. Veter. Diagn. Investig. 2002, 14, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Halbert, N.D.; Reitzel, R.A.; Martens, R.J.; Cohen, N.D. Evaluation of a multiplex polymerase chain reaction assay for simultaneous detection of Rhodococcus equi and the vapA gene. Am. J. Veter. Res. 2005, 66, 1380–1385. [Google Scholar]
- Rutherford, K.; Parkhill, J.; Crook, J.; Horsnell, T.; Rice, P.; Rajandream, M.-A.; Barrell, B. Artemis: Sequence visualization and annotation. Bioinformatics 2000, 16, 944–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.W.; Jorgensen, E.M. ApE, A Plasmid Editor: A Freely Available DNA Manipulation and Visualization Program. Front. Bioinform. 2022, 2, 818619. [Google Scholar] [CrossRef]
- Kearse, M.D.; Sturrock, S.S.; Meintjes, P.L. The Geneious 6.0.3 Read Mapper. 2012. Available online: http://assets.geneious.com/documentation/geneious/GeneiousReadMapper.pdf (accessed on 17 March 2022).
- Tripathi, V.N.; Harding, W.C.; Willingham-Lane, J.M.; Hondalus, M.K. Conjugal Transfer of a Virulence Plasmid in the Opportunistic Intracellular Actinomycete Rhodococcus equi. J. Bacteriol. 2012, 194, 6790–6801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giguère, S.; Cohen, N.; Chaffin, M.K.; Slovis, N.; Hondalus, M.; Hines, S.; Prescott, J. Diagnosis, Treatment, Control, and Prevention of Infections Caused by Rhodococcus equi in Foals. J. Veter. Intern. Med. 2011, 25, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Giguère, S.; Slovis, N.M.; Carter, C.N.; Barr, B.S.; Cohen, N.D.; Elam, J.; Erol, E.; Locke, S.J.; Phillips, E.D.; et al. Emergence of Resistance to Macrolides and Rifampin in Clinical Isolates of Rhodococcus equi from Foals in Central Kentucky, 1995 to 2017. Antimicrob. Agents Chemother. 2019, 63, e01714-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, A.J.; Giguère, S.; Sturgill, T.L.; Berghaus, L.J.; Slovis, N.M.; Whitman, J.L.; Levering, C.; Kuskie, K.R.; Cohen, N.D. Macrolide- and Rifampin-Resistant Rhodococcus equion a Horse Breeding Farm, Kentucky, USA. Emerg. Infect. Dis. 2013, 19, 282–285. [Google Scholar] [CrossRef]
- Huber, L.; Giguère, S.; Cohen, N.D.; Slovis, N.M.; Hanafi, A.; Schuckert, A.; Berghaus, L.; Greiter, M.; Hart, K.A. Prevalence and risk factors associated with emergence of Rhodococcus equi resistance to macrolides and rifampicin in horse-breeding farms in Kentucky, USA. Veter. Microbiol. 2019, 235, 243–247. [Google Scholar] [CrossRef]
- Giguère, S. Treatment of Infections Caused by Rhodococcus equi. Veter. Clin. N. Am. Equine Pract. 2017, 33, 67–85. [Google Scholar] [CrossRef]
- Giguère, S.; Berghaus, L.J.; Willingham-Lane, J.M. Antimicrobial Resistance in Rhodococcus equi. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Giguère, S.; Lee, E.; Williams, E.; Cohen, N.D.; Chaffin, M.K.; Halbert, N.; Martens, R.J.; Franklin, R.P.; Clark, C.C.; Slovis, N.M. Determination of the prevalence of antimicrobial resistance to macrolide antimicrobials or rifampin in Rhodococcus equi isolates and treatment outcome in foals infected with antimicrobial-resistant isolates of R equi. J. Am. Veter. Med. Assoc. 2010, 237, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Giguère, S.; Berghaus, L.J.; Hondalus, M.K.; Willingham-Lane, J.M.; MacArthur, I.; Cohen, N.D.; Roberts, M.C.; Vazquez-Boland, J.A. Novel transferableerm (46) determinant responsible for emerging macrolide resistance in Rhodococcus equi. J. Antimicrob. Chemother. 2015, 70, 3184–3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Narváez, S.; Giguère, S.; Berghaus, L.J.; Dailey, C.; Vázquez-Boland, J.A. Horizontal Spread of Rhodococcus equi Macrolide Resistance Plasmid pRErm46 across Environmental Actinobacteria. Appl. Environ. Microbiol. 2020, 86, e00108-20. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lázaro, D.; Lewis, D.A.; Ocampo-Sosa, A.A.; Fogarty, U.; Makrai, L.; Navas, J.; Scortti, M.; Hernández, M.; Vázquez-Boland, J.A. Internally Controlled Real-Time PCR Method for Quantitative Species-Specific Detection and vapA Genotyping of Rhodococcus equi. Appl. Environ. Microbiol. 2006, 72, 4256–4263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladrón, N.; Fernández, M.; Agüero, J.; Zörn, B.G.; Vázquez-Boland, J.A.; Navas, J. Rapid Identification of Rhodococcus equi by a PCR Assay Targeting the choE Gene. J. Clin. Microbiol. 2003, 41, 3241–3245. [Google Scholar] [CrossRef] [Green Version]
- Ocampo-Sosa, A.A.; Lewis, D.A.; Navas, J.; Quigley, F.; Callejo, R.; Scortti, M.; Leadon, D.P.; Fogarty, U.; Vázquez-Boland, J.A. Molecular Epidemiology of Rhodococcus equi Based on traA, vapA, and vapB Virulence Plasmid Markers. J. Infect. Dis. 2007, 196, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Madrigal, R.; Shaw, S.; Witkowski, L.; Sisson, B.; Blodgett, G.; Chaffin, M.; Cohen, N. Use of Serial Quantitative PCR of the vapA Gene of Rhodococcus equi in Feces for Early Detection of R. equi Pneumonia in Foals. J. Veter. Intern. Med. 2016, 30, 664–670. [Google Scholar] [CrossRef]
- Pusterla, N.; Wilson, W.D.; Mapes, S.; Leutenegger, C.M. Diagnostic evaluation of real-time pcr in the detection of Rhodococcus equi in faeces and nasopharyngeal swabs from foals with pneumonia. Veter. Rec. 2007, 161, 272–274. [Google Scholar] [CrossRef] [Green Version]
- Harrington, J.R.; Golding, M.C.; Martens, R.J.; Halbert, N.D.; Cohen, N.D. Evaluation of a real-time quantitative polymerase chain reaction assay for detection and quantitation of virulent Rhodococcus equi. Am. J. Veter. Res. 2005, 66, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Narvaez, S. Conjugal Transfer of Host-Adaptive Determinants in the Pathogenic Actinobacterium Rhodococcus equi. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2017. [Google Scholar]
- Flannery, J.; Rajko-Nenow, P.; Arnold, H.; Van Weezep, E.; van Rijn, P.A.; Ngeleja, C.; Batten, C. Improved PCR diagnostics using up-to-date in-silico validation: An F-gene RT-qPCR assay for the detection of all four lineages of peste des petits ruminants virus. J. Virol. Methods 2019, 274, 113735. [Google Scholar] [CrossRef] [PubMed]
- Davi, M.J.P.; Jeronimo, S.M.B.; Lima, J.P.M.S.; Lanza, D.C.F. Design and in-silico validation of polymerase chain reaction primers to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Sci. Rep. 2021, 11, 12565. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Jiřinec, T.; Jiřincová, H.; Černíková, L.; Havlíčková, M. In-silico re-assessment of a diagnostic RT-qPCR assay for universal detection of Influenza A viruses. Sci. Rep. 2019, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Lefever, S.; Pattyn, F.; Hellemans, J.; Vandesompele, J. Single-Nucleotide Polymorphisms and Other Mismatches Reduce Performance of Quantitative PCR Assays. Clin. Chem. 2013, 59, 1470–1480. [Google Scholar] [CrossRef] [Green Version]
- Letowski, J.; Brousseau, R.; Masson, L. Designing better probes: Effect of probe size, mismatch position and number on hybridization in DNA oligonucleotide microarrays. J. Microbiol. Methods 2004, 57, 269–278. [Google Scholar] [CrossRef]


| Name | SEQUENCE 5’-3’ | Product Size | Purpose |
|---|---|---|---|
| Rhodo_Dlab_F | TGTCAACAACATCGACCAGGC | 200 bp | Amplifies choE gene, chromosomal marker in R. equi |
| Rhodo_Dlab_R | GCGTTGTTGCCGTAGATGAC | ||
| Rhodo_Dlab_P | /56-FAM/CCGCCCAAC/ZEN/GTTCGGGTTTCACAACCGCTT/3IABkFQ/ * | ||
| Erm46_Dlab_F | GTGGCGCAACGATGATGACT | 192 bp | Amplifies macrolide resistance gene erm46 |
| Erm46_Dlab_R | TGAAGACGGTGTGGACGAAG | ||
| Erm46_Dlab_P | /5HEX/CCGCATCGG/ZEN/CGTTCACACCACGGC/3IABkFQ/ * | ||
| Erm51_Dlab_F | CTGCCGTTTCACCTGACCAC | 198 bp | Amplifies macrolide resistance gene erm51 |
| Erm51_Dlab_R | GGGACGGAAATGTGTGGATG | ||
| Erm51_Dlab_P | /5Cy5/GCCGGCGTC/TAO/GGTGGTGCCACGATGATGA/3IAbRQSp/ * |
| Match with Rhodo_Dlab Set | Match with Erm46_Dlab Set | Match with Erm51_Dlab Set | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FW | RV | Probe | PCR Products | FW | RV | Probe | PCR Products | FW | RV | Probe | PCR Products | |
| Macrolide Susceptible [n = 88] | 88 | 88 | 88 | 87 | 1 | 88 | 1 | 0 | 1 | 0 | 88 | 0 |
| Macrolide Resistant erm(46)-positive [n = 85] | 85 | 85 | 85 | 83 | 85 | 85 | 85 | 85 | 2 | 1 | 85 | 0 |
| Macrolide Resistant erm(51)-positive [n = 29] | 29 | 29 | 29 | 29 | 0 | 29 | 14 | 0 | 29 | 29 | 29 | 29 |
| Non- R. equi species [n = 24] | 2 | 6 | 2 | 0 | 4 | 6 | 3 | 0 | 1 | 0 | 10 | 0 |
| Efficiency (%) | R2 | LOD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Singleplex | Multiplex | Multiplex Mocking * | Singleplex | Multiplex | Multiplex Mocking * | Singleplex | Multiplex | Multiplex Mocking * | |
| Rhodo_Dlab | 121.1 | 112.8 | 115.5 | 0.9976 | 0.9987 | 0.9904 | 10.7 | 11 | 10.7 |
| Erm46_Dlab | 104.7 | 105.5 | 102.8 | 0.9999 | 0.9994 | 0.9976 | 1.18 | 11.8 | 10.7 |
| Erm51_Dlab | 120.5 | 105.4 | 90.2 | 0.9993 | 0.9971 | 0.9917 | 1.07 | 10.7 | 106.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narváez, S.Á.; Fernández, I.; Patel, N.V.; Sánchez, S. Novel Quantitative PCR for Rhodococcus equi and Macrolide Resistance Detection in Equine Respiratory Samples. Animals 2022, 12, 1172. https://doi.org/10.3390/ani12091172
Narváez SÁ, Fernández I, Patel NV, Sánchez S. Novel Quantitative PCR for Rhodococcus equi and Macrolide Resistance Detection in Equine Respiratory Samples. Animals. 2022; 12(9):1172. https://doi.org/10.3390/ani12091172
Chicago/Turabian StyleNarváez, Sonsiray Álvarez, Ingrid Fernández, Nikita V. Patel, and Susan Sánchez. 2022. "Novel Quantitative PCR for Rhodococcus equi and Macrolide Resistance Detection in Equine Respiratory Samples" Animals 12, no. 9: 1172. https://doi.org/10.3390/ani12091172
APA StyleNarváez, S. Á., Fernández, I., Patel, N. V., & Sánchez, S. (2022). Novel Quantitative PCR for Rhodococcus equi and Macrolide Resistance Detection in Equine Respiratory Samples. Animals, 12(9), 1172. https://doi.org/10.3390/ani12091172






