Transcriptome Analysis Indicates Immune Responses against Vibrio harveyi in Chinese Tongue Sole (Cynoglossus semilaevis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish Treatment and Sample Collection
2.2. RNA Extraction and Library Sequencing
2.3. Data Filtering and Genome Mapping
2.4. Comprehensive Analysis of DEGs
2.5. Quantitative Real-Time PCR Validation
3. Results
3.1. Transcriptome Sequencing Data
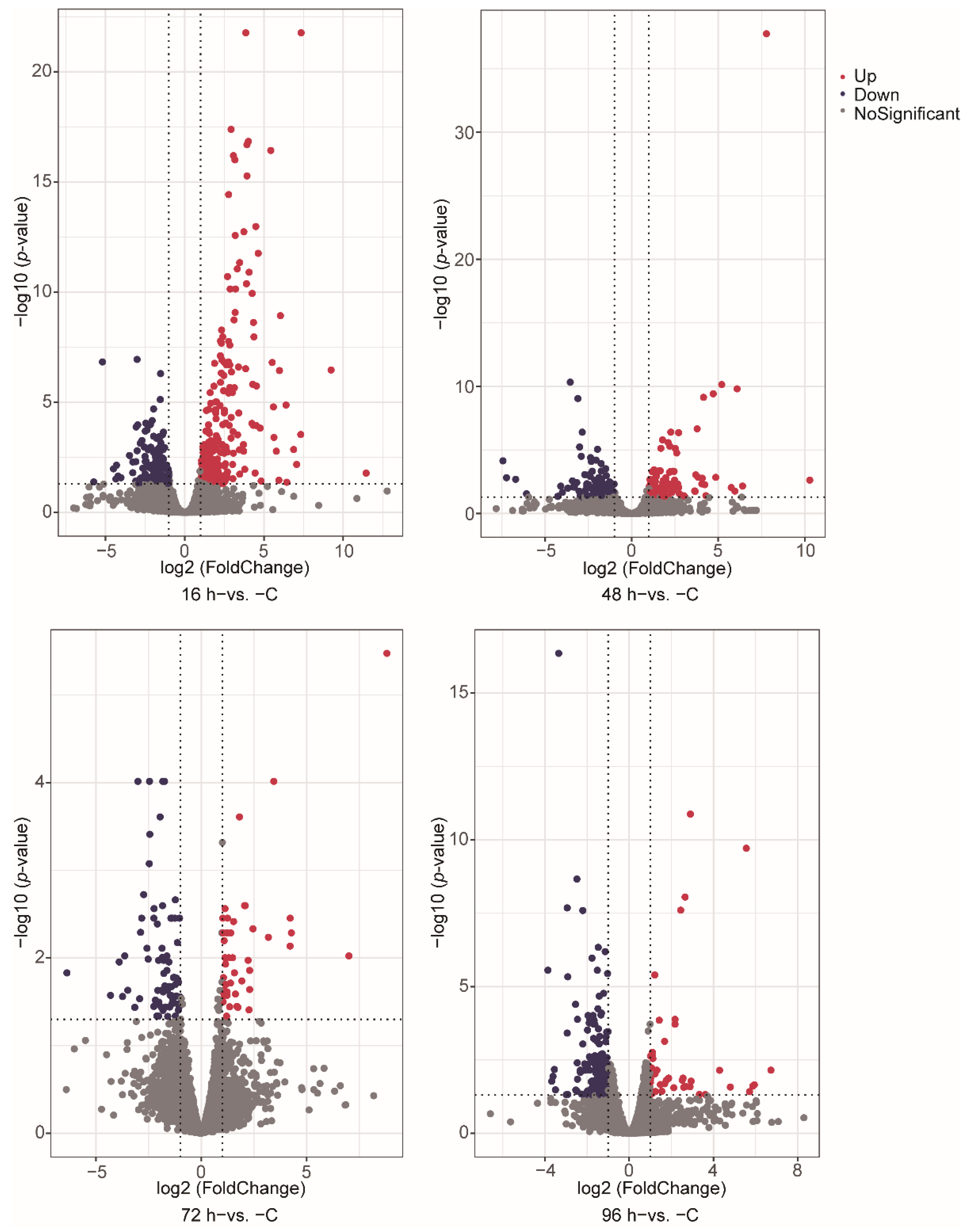
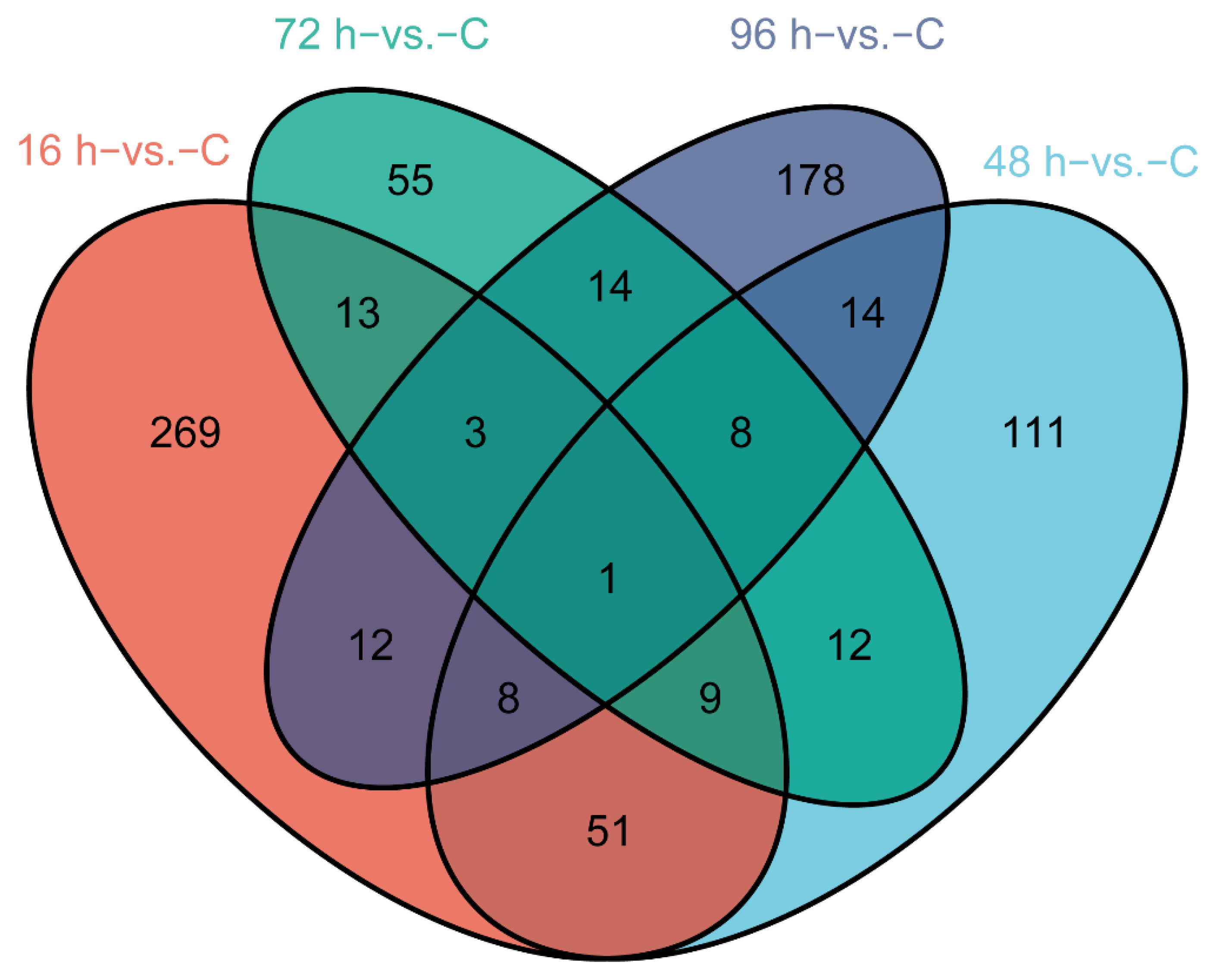
3.2. Gene Expression during V. harveyi Infection
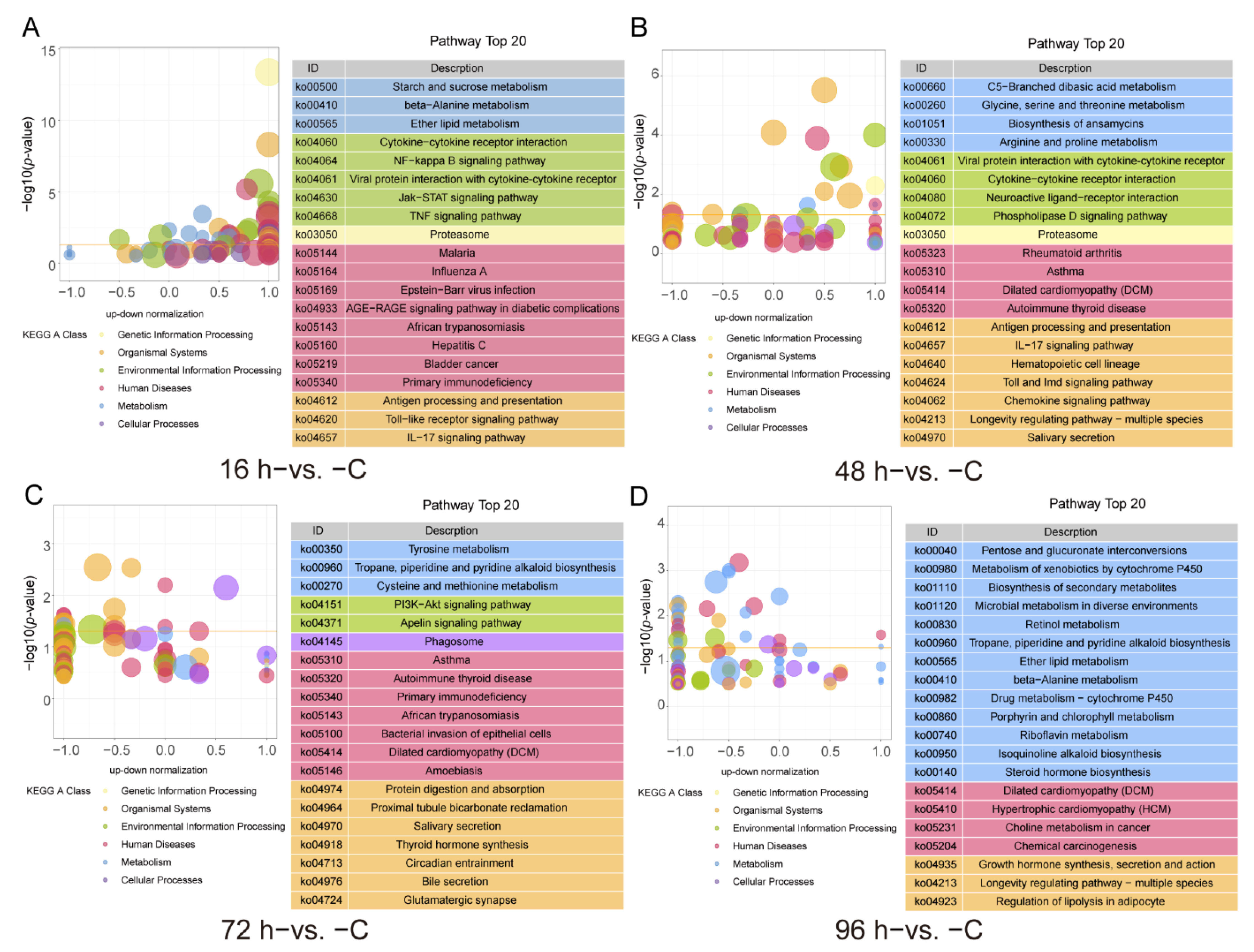
3.3. GO and KEGG Functional Enrichment of the DEGs
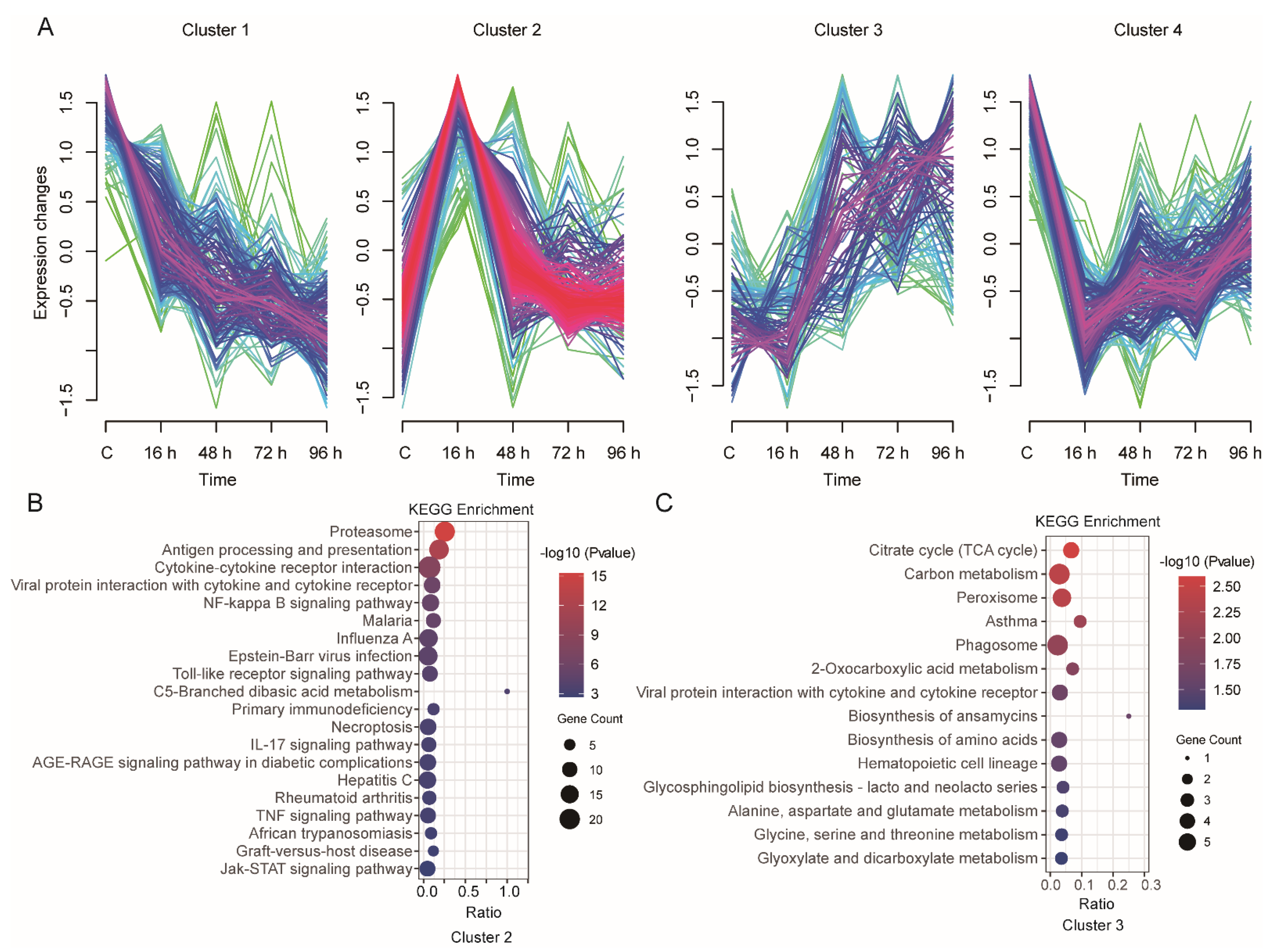
3.4. Time-Series Expression Profile of DEGs during V. harveyi Infection
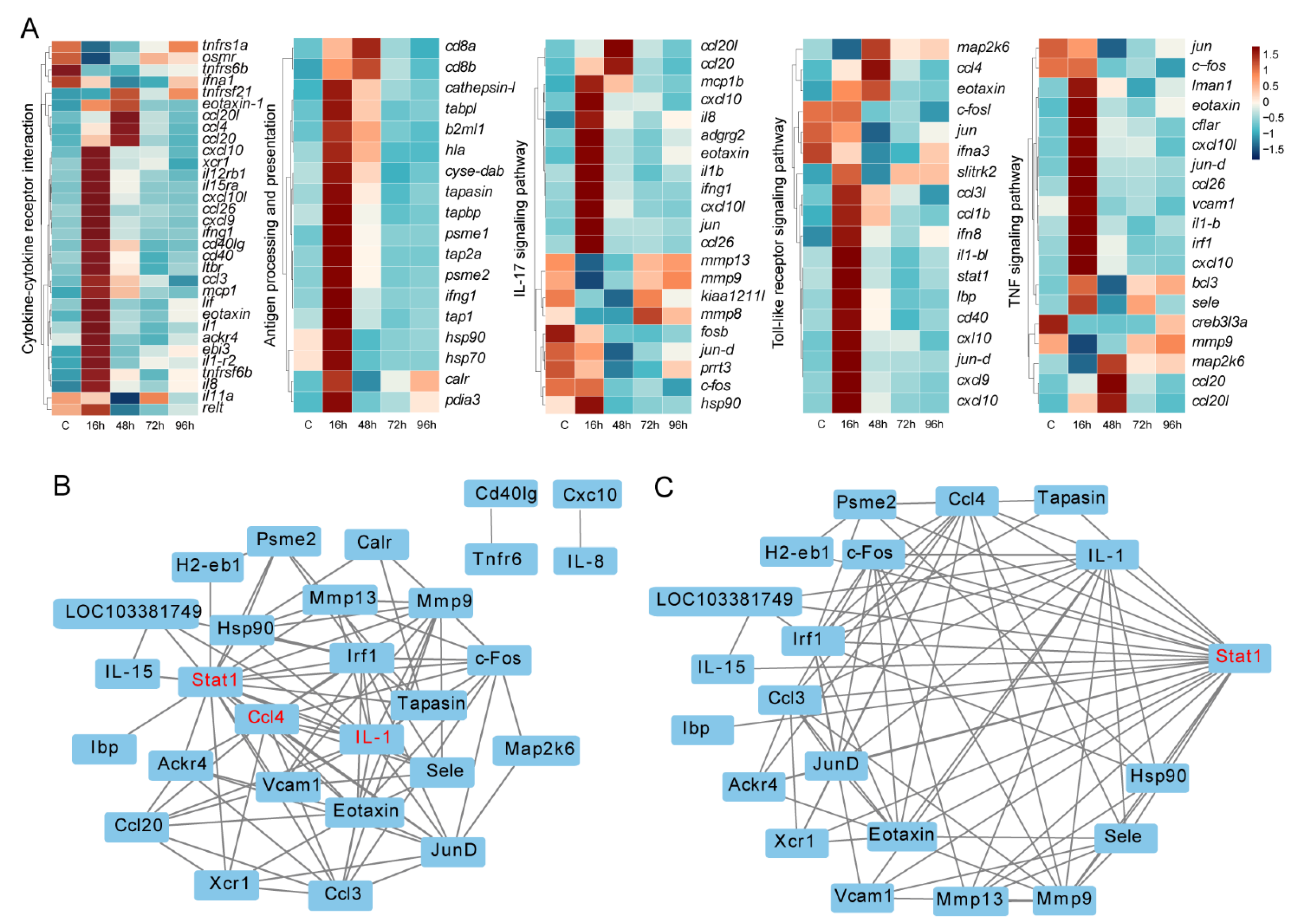
3.5. Key Immune-Related DEGs and Protein-Protein Interaction Networks
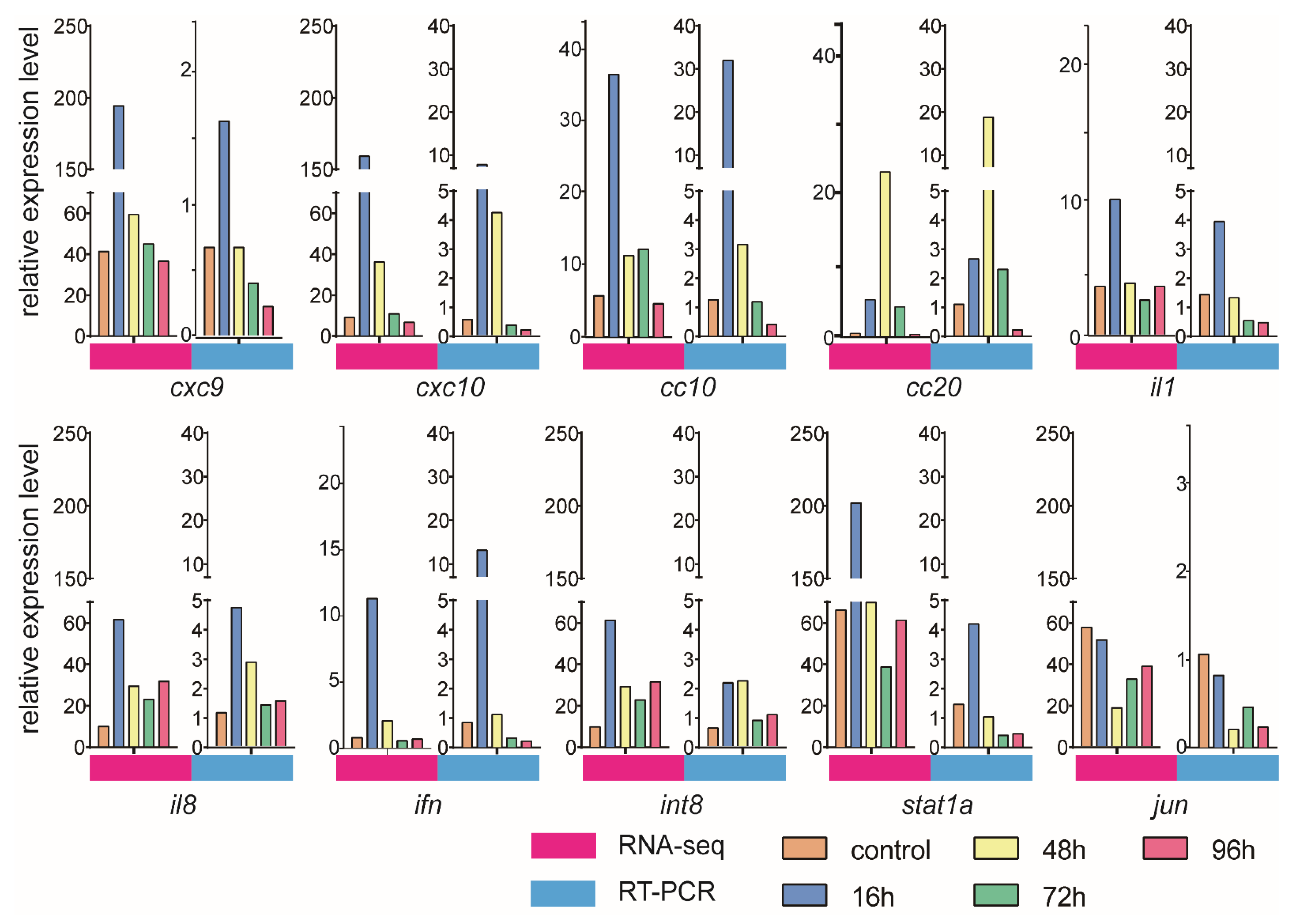
3.6. Validation of DEGs by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litman, G.W.; Cannon, J.P.; Dishaw, L.J. Reconstructing immune phylogeny: New perspectives. Nat. Rev. Immunol. 2005, 5, 866–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geven, E.J.; Klaren, P.H. The teleost head kidney: Integrating thyroid and immune signalling. Dev. Comp. Immunol. 2017, 66, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef] [PubMed]
- Zwollo, P.; Mott, K.; Barr, M. Comparative analyses of B cell populations in trout kidney and mouse bone marrow: Establishing “B cell signatures”. Dev. Comp. Immunol. 2010, 34, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Bromage, E.S.; Kaattari, I.M.; Zwollo, P.; Kaattari, S.L. Plasmablast and plasma cell production and distribution in trout immune tissues. J. Immunol. 2004, 173, 7317–7323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebl, A.; Goldammer, T. Under control: The innate immunity of fish from the inhibitors’ perspective. Fish Shellfish Immunol. 2018, 77, 328–349. [Google Scholar] [CrossRef]
- Kaisho, T.; Akira, S. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 2002, 1589, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Kwon, M.G.; Jung, S.-H.; Park, M.A.; Kim, D.-W.; Cho, W.S.; Park, J.W.; Son, M.-H. RNA-Seq transcriptome analysis of the olive flounder (Paralichthys olivaceus) kidney response to vaccination with heat-inactivated viral hemorrhagic septicemia virus. Fish Shellfish Immunol. 2017, 62, 221–226. [Google Scholar] [CrossRef]
- Gallo, V.P.; Civinini, A. Survey of the adrenal homolog in teleosts. Int. Rev. Cytol. 2003, 230, 89–187. [Google Scholar]
- Pettersen, E.F.; Bjerknes, R.; Wergeland, H.I. Studies of Atlantic salmon (Salmo salar L.) blood, spleen and head kidney leucocytes using specific monoclonal antibodies, immunohistochemistry and flow cytometry. Fish Shellfish Immunol. 2000, 10, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Grabowski, P.S.; Cunningham, C.; Secombes, C.J. Molecular cloning of interleukin 1beta from rainbow trout Oncorhynchus mykiss reveals no evidence of an ice cut site. Cytokine 1999, 11, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Secombes, C.J. Identification and expression analysis of two fish-specific IL-6 cytokine family members, the ciliary neurotrophic factor (CNTF)-like and M17 genes, in rainbow trout Oncorhynchus mykiss. Mol. Immunol. 2009, 46, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J.; Wang, T.; Bird, S. The interleukins of fish. Dev. Comp. Immunol. 2011, 35, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Peddie, S.; Campos-Pérez, J.J.; Zou, J.; Secombes, C.J. The effect of intraperitoneally administered recombinant IL-1beta on immune parameters and resistance to Aeromonas salmonicida in the rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2003, 27, 801–812. [Google Scholar] [CrossRef]
- Yin, Z.; Kwang, J. Carp interleukin-1β in the role of an immuno-adjuvant. Fish Shellfish Immunol. 2000, 10, 375–378. [Google Scholar] [CrossRef]
- Khalil, B.A.; Elemam, N.M.; Maghazachi, A.A. Chemokines and chemokine receptors during COVID-19 infection. Comput. Struct. Biotechnol. J. 2021, 19, 976–988. [Google Scholar] [CrossRef]
- Dunn, A.J. The HPA axis and the immune system: A perspective. NeuroImmune Biol. 2007, 7, 3–15. [Google Scholar]
- Kumar, V.; Sharma, A. Is neuroimmunomodulation a future therapeutic approach for sepsis? Int. Immunopharmacol. 2010, 10, 9–17. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.; MacKenzie, S. Fish health challenge after stress. Indicators of immunocompetence. Contrib. Sci. 2004, 2, 443–454. [Google Scholar]
- Baoprasertkul, P.; He, C.; Peatman, E.; Zhang, S.; Li, P.; Liu, Z. Constitutive expression of three novel catfish CXC chemokines: Homeostatic chemokines in teleost fish. Mol. Immunol. 2005, 42, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Pagniello, K.; Bols, N.; Lee, L. Effect of corticosteroids on viability and proliferation of the rainbow trout monocyte/macrophage cell line, RTS11. Fish Shellfish Immunol. 2002, 13, 199–214. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, S.; Iliev, D.; Liarte, C.; Koskinen, H.; Planas, J.; Goetz, F.; Mölsä, H.; Krasnov, A.; Tort, L. Transcriptional analysis of LPS-stimulated activation of trout (Oncorhynchus mykiss) monocyte/macrophage cells in primary culture treated with cortisol. Mol. Immunol. 2006, 43, 1340–1348. [Google Scholar] [CrossRef]
- Jawahar, A.; Manley, R.; Palaniappan, R.; Dhevendran, K. Pathogenicity and antibiotic sensitivity of luminous Vibrio harveyi isolated from diseased penaeid shrimp. J. Aquat. Trop. 1997, 12, 1–8. [Google Scholar]
- Pass, D.; Dybdahl, R.; Mannion, M. Investigations into the causes of mortality of the pearl oyster, Pinctada maxima (Jamson), in western Australia. Aquaculture 1987, 65, 149–169. [Google Scholar] [CrossRef]
- Thompson, F.L.; Hoste, B.; Vandemeulebroecke, K.; Engelbeen, K.; Denys, R.; Swings, J. Vibrio trachuri Iwamoto et al. 1995 is a junior synonym of Vibrio harveyi (Johnson and Shunk 1936) Baumann et al. 1981. Int. J. Syst. Evol. Microbiol. 2002, 52, 973–976. [Google Scholar]
- Zhang, X.H.; Austin, B. Pathogenicity of Vibrio harveyi to salmonids. J. Fish Dis. 2000, 23, 93–102. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, gix120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.-N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Yang, Y.; Li, X.; Dai, H.; Chen, S. Genetic analysis of disease resistance to Vibrio harveyi by challenge test in Chinese tongue sole (Cynoglossus semilaevis). Aquaculture 2019, 503, 430–435. [Google Scholar] [CrossRef]
- Zhang, X.-H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Nekouei, O.; Vanderstichel, R.; Kaukinen., K.H.; Thakur, K.; Ming, T.; Patterson, D.A.; Trudel, M.; Neville, C.; Miller, K.M. Comparison of infectious agents detected from hatchery and wild juvenile Coho salmon in British Columbia, 2008–2018. PloS ONE 2019, 14, e0221956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Majewska, M.; Szczepanik, M. The role of Toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Postepy Hig. Med. Dosw. (Online) 2006, 60, 52–63. [Google Scholar]
- Engelsma, M.Y.; Huising, M.O.; van Muiswinkel, W.B.; Flik, G.; Kwang, J.; Savelkoul, H.F.; Kemenade, B.V.-V. Neuroendocrine-immune interactions in fish: A role for interleukin-1. Vet. Immunol. Immunopathol. 2002, 87, 467–479. [Google Scholar] [CrossRef]
- Krupa, A.; Fol, M.; Dziadek, B.R.; Kepka, E.; Wojciechowska, D.; Brzostek, A.; Torzewska, A.; Dziadek, J.; Baughman, R.P.; Griffith, D.; et al. Binding of CXCL8/IL-8 to Mycobacterium tuberculosis Modulates the Innate Immune Response. Mediat. Inflamm. 2015, 2015, 124762. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Li, Y.; Zhao, S.; Cao, M.; Yang, N.; Huo, H.; Yan, X.; Cao, Z.; Zhang, P.; Li, C. CC chemokines and their receptors in black rockfish (Sebastes schlegelii): Characterization, evolutionary analysis, and expression patterns after Aeromonas salmonicida infection. Aquaculture 2022, 546, 737377. [Google Scholar] [CrossRef]
- Murray, P.J. The JAK-STAT signaling pathway: Input and output integration. J. Immunol. 2007, 178, 2623–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Ning, X.; Jiang, S.; Sun, L. Transcriptome analysis reveals seven key immune pathways of Japanese flounder (Paralichthys olivaceus) involved in megalocytivirus infection. Fish Shellfish Immunol. 2020, 103, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Bathige, S.; Umasuthan, N.; Godahewa, G.; Thulasitha, W.S.; Jayasinghe, J.; Wan, Q.; Lee, J. Molecular insights of two STAT1 variants from rock bream (Oplegnathus fasciatus) and their transcriptional regulation in response to pathogenic stress, interleukin-10, and tissue injury. Fish Shellfish Immunol. 2017, 69, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486. [Google Scholar] [CrossRef] [Green Version]
- Jara, L.J.; Navarro, C.; Medina, G.; Vera-Lastra, O.; Blanco, F. Immune-neuroendocrine interactions and autoimmune diseases. Clin. Dev. Immunol. 2006, 13, 109–123. [Google Scholar] [CrossRef]
- Baschant, U.; Tuckermann, J. The role of the glucocorticoid receptor in inflammation and immunity. J. Steroid Biochem. Mol. Biol. 2010, 120, 69–75. [Google Scholar] [CrossRef]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Tort, L.; Padrós, F.; Rotllant, J.; Crespo, S. Winter syndrome in the gilthead sea bream Sparus aurata. Immunological and histopathological features. Fish Shellfish Immunol. 1998, 8, 37–47. [Google Scholar] [CrossRef]
- Vardas, K.; Ilia, S.; Sertedaki, A.; Charmandari, E.; Briassouli, E.; Goukos, D.; Apostolou, K.; Psarra, K.; Botoula, E.; Tsagarakis, S.; et al. Increased glucocorticoid receptor expression in sepsis is related to heat shock proteins, cytokines, and cortisol and is associated with increased mortality. Intensive Care Med. Exp. 2017, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.C.; Chen, Q.; Ma, Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam. Horm. 2011, 86, 103–126. [Google Scholar] [PubMed] [Green Version]

| Prime | 5′–3′ |
|---|---|
| ifn-qF | CTTGTCAGGTCTTGACCCTG |
| ifn-qR | GTAACAGCAGGTTTTGGATGG |
| cxc-9-qF | CAGCAAAGTGACAGTGAT |
| cxc-9-qR | AGGCACACTTTCACATCA |
| cxc-10-qF | CAAGTCAGAAGGTGTCAG |
| cxc-10-qR | GGAAGTGACTGGAGTTGG |
| int-8-qF | TGAAGAACTGAAACTGCAACACT |
| int-8-qR | TGCTGATCGGTACTATTCCATTG |
| cc10-qF | CCAGAGTCACCACTTGGAAA |
| cc10-qR | GCTGAGGTTCCTGAGTTTGTT |
| cc20-qF | GTTTCAGGTGATCAAGGGCT |
| cc20-qR | TCGTCCCTCTTAGTCACACA |
| il8-qF | ACCGATCAGCAGGGACTTTA |
| il8-qR | CTTCTTCCCGTTCACCAGAC |
| jun-qF | TTTCTCCCAGCACGAAAACA |
| jun-qR | GGGATGTAAGGATGTCGCTC |
| il1-qF | GGACATCATCTGCACAACCA |
| il1-qR | TAGAGGCATACGACACCAGT |
| stat1-qF | TCACTAAACGGGGCCTAAAC |
| stat1-qR | CTTTCTCGTTAGCACTCTGCTT |
| β-actin-qF | GCTGTGCTGTCCCTGTA |
| β-actin-qR | GAGTAGCCACGCTCTGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hao, X.; Ma, W.; Zhu, T.; Zhang, Z.; Wang, Q.; Liu, K.; Shao, C.; Wang, H.-Y. Transcriptome Analysis Indicates Immune Responses against Vibrio harveyi in Chinese Tongue Sole (Cynoglossus semilaevis). Animals 2022, 12, 1144. https://doi.org/10.3390/ani12091144
Zhang X, Hao X, Ma W, Zhu T, Zhang Z, Wang Q, Liu K, Shao C, Wang H-Y. Transcriptome Analysis Indicates Immune Responses against Vibrio harveyi in Chinese Tongue Sole (Cynoglossus semilaevis). Animals. 2022; 12(9):1144. https://doi.org/10.3390/ani12091144
Chicago/Turabian StyleZhang, Xianghui, Xiancai Hao, Wenxiu Ma, Tengfei Zhu, Zhihua Zhang, Qian Wang, Kaiqiang Liu, Changwei Shao, and Hong-Yan Wang. 2022. "Transcriptome Analysis Indicates Immune Responses against Vibrio harveyi in Chinese Tongue Sole (Cynoglossus semilaevis)" Animals 12, no. 9: 1144. https://doi.org/10.3390/ani12091144
APA StyleZhang, X., Hao, X., Ma, W., Zhu, T., Zhang, Z., Wang, Q., Liu, K., Shao, C., & Wang, H.-Y. (2022). Transcriptome Analysis Indicates Immune Responses against Vibrio harveyi in Chinese Tongue Sole (Cynoglossus semilaevis). Animals, 12(9), 1144. https://doi.org/10.3390/ani12091144






