Feeding Habits of Sarda chiliensis chiliensis (Cuvier, 1832) in Northern Chile and Southern Perú
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Capture and Study Area
2.2. Stomach Extraction
2.3. Treatment of Stomach Contents
2.4. Food Dynamics Analysis
- (a)
- Vacuity Index (VI)
- (b)
- Weight of stomach contents (Wsc)
- (c)
- Mean Fullness Index (IR)
- (d)
- Average weight of the prey per stomach (Wp)
2.5. Study of the Trophic Composition of the Diet
- (a)
- Numerical composition (NC%)
- (b)
- Frequency of Occurrence (F%)
- (c)
- Gravimetric method (G%)
- (d)
- Relative Importance Index (RII)
- (e)
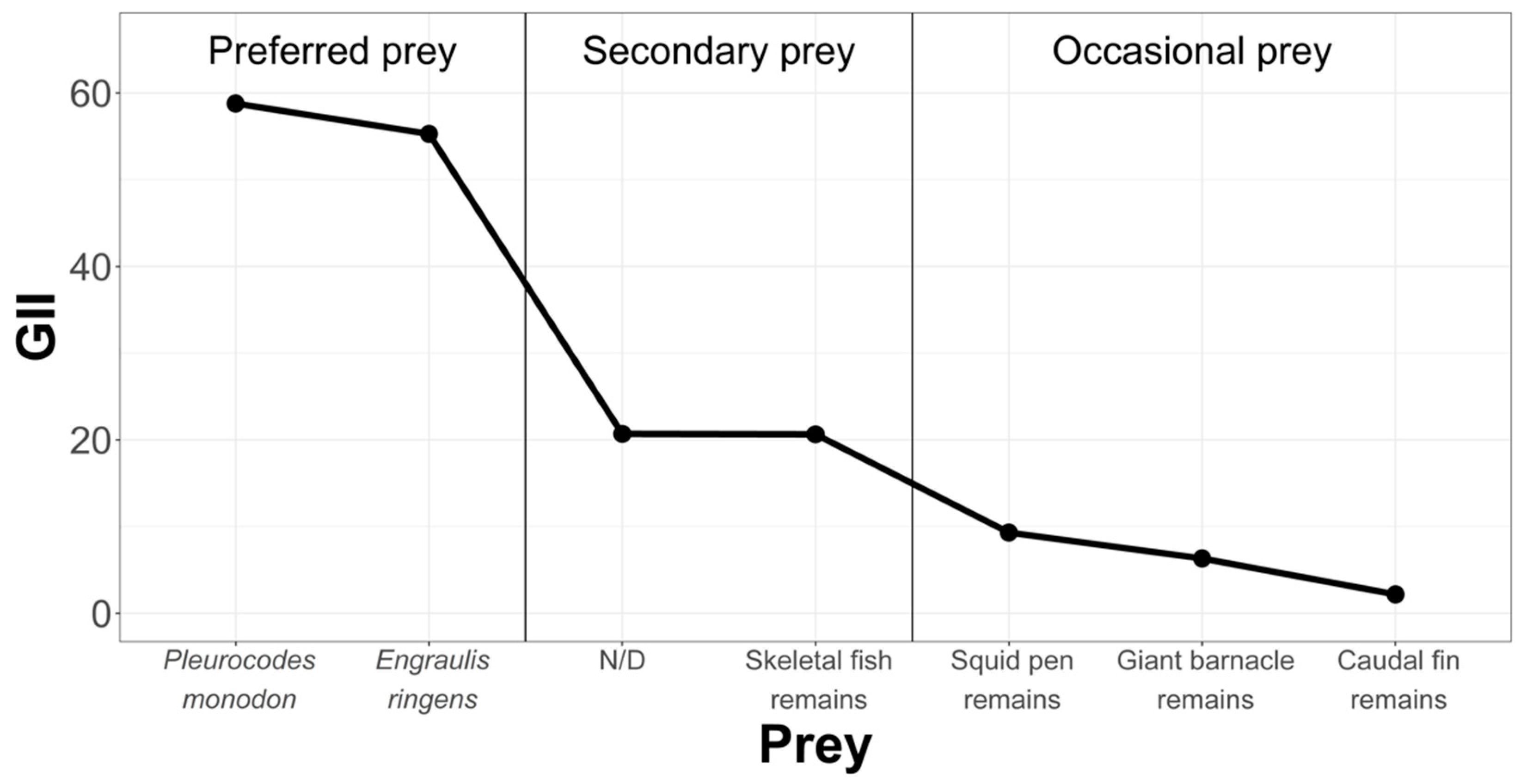
- Geometric Importance Index (GII)
- Vi: represents the value of each measurement of the relative quantity i of prey i, i.e., the value of each applied index (numerical, frequency and gravimetric).
- n: number of indexes applied.
2.6. Evaluation of Feeding Strategy
2.7. Study of Trophic Relationships
- (a)
- Shannon’s Diversity Index (H′)
- i: type of trophic resource;
- pi: is the number of species i, expressed as the proportion of the sum of Pj of prey species;
- N: number of prey or trophic resources.
- (b)
- Homogeneity Index (J′)
2.8. Statistical Analysis
3. Results
3.1. Analysis of Feeding Dynamics
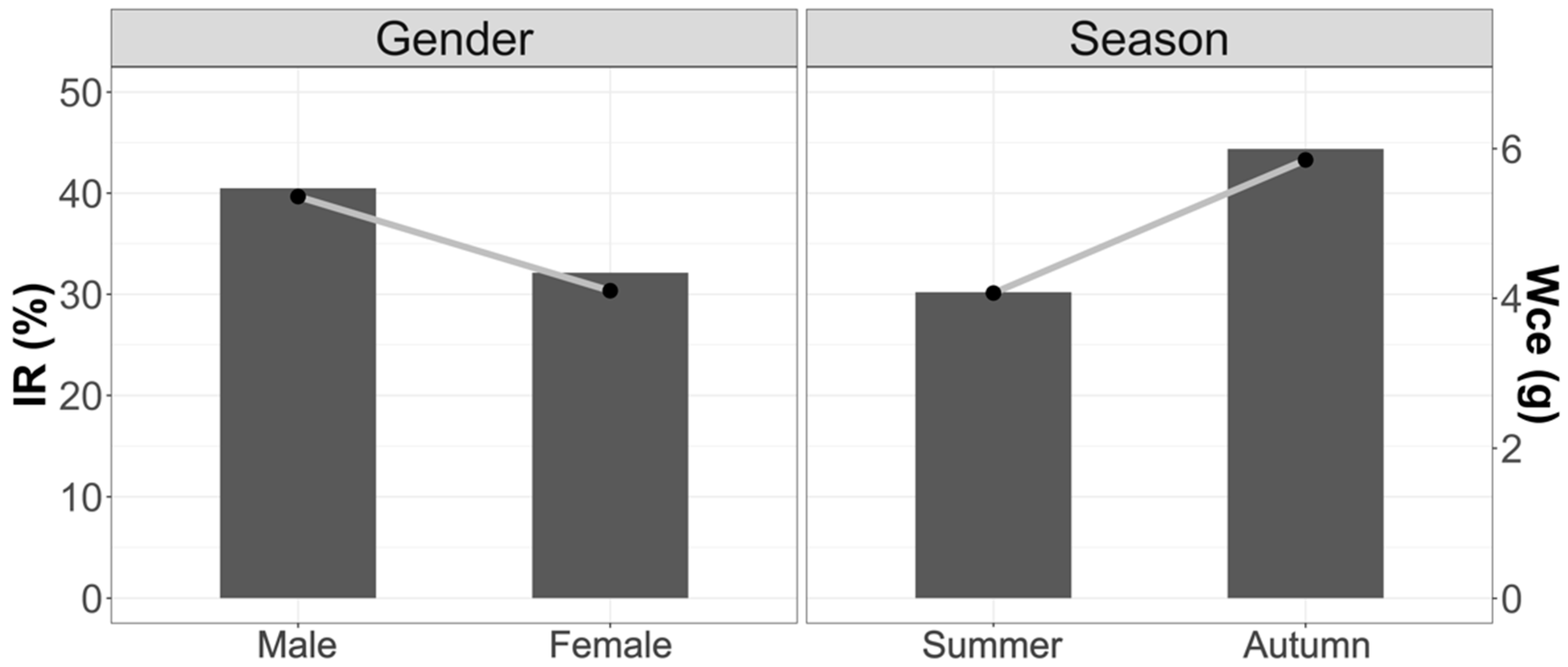
3.1.1. Vacuity Index (VI) and Mean Fullness Index (IR)
3.1.2. Weight of Stomach Contents (Wce)
3.1.3. Average Weight of Prey per Stomach (Wp)
3.2. Study of the Trophic Composition of the Diet
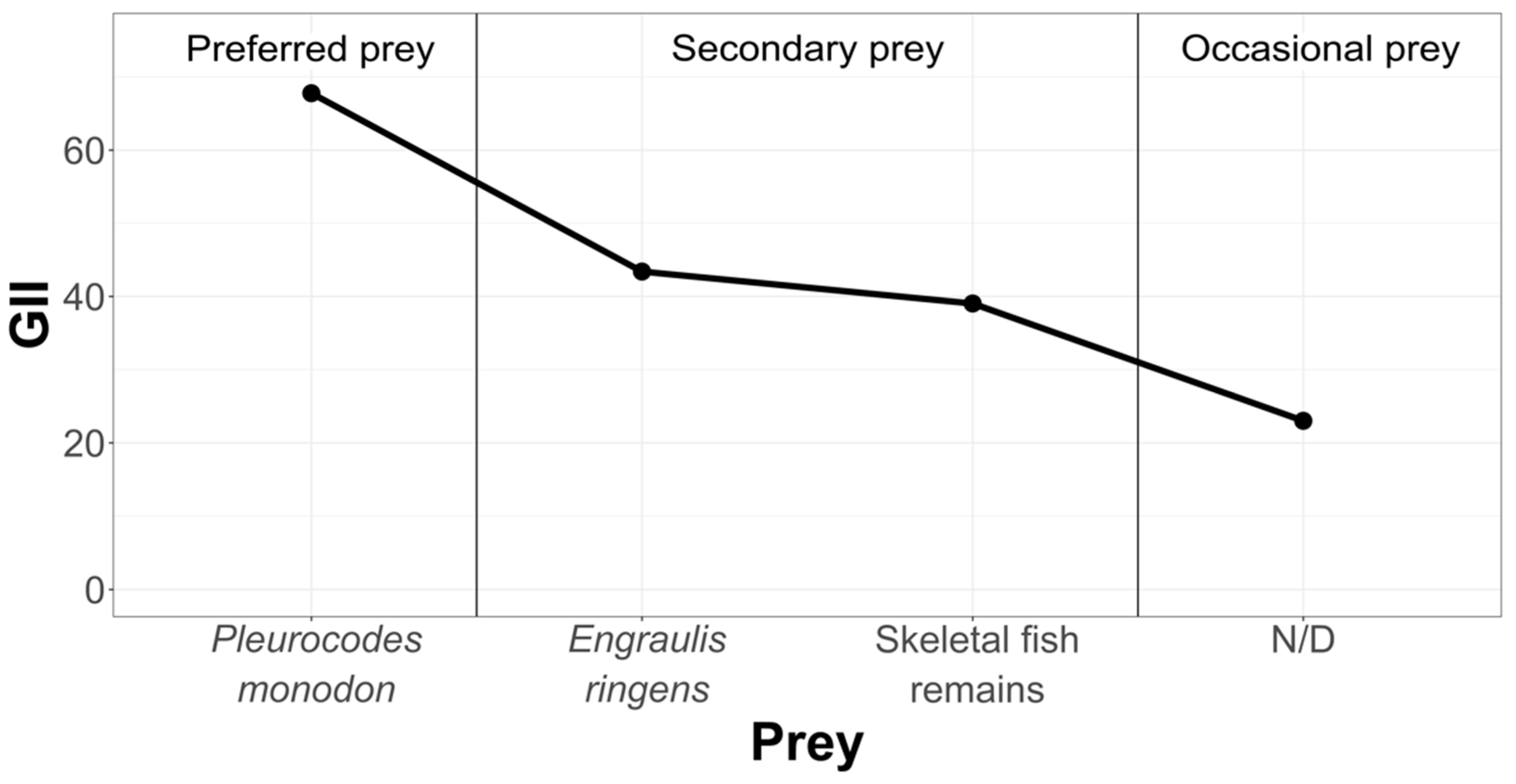
3.2.1. Pozo de Lisas Study Area
- Geometric Importance Index (GII)
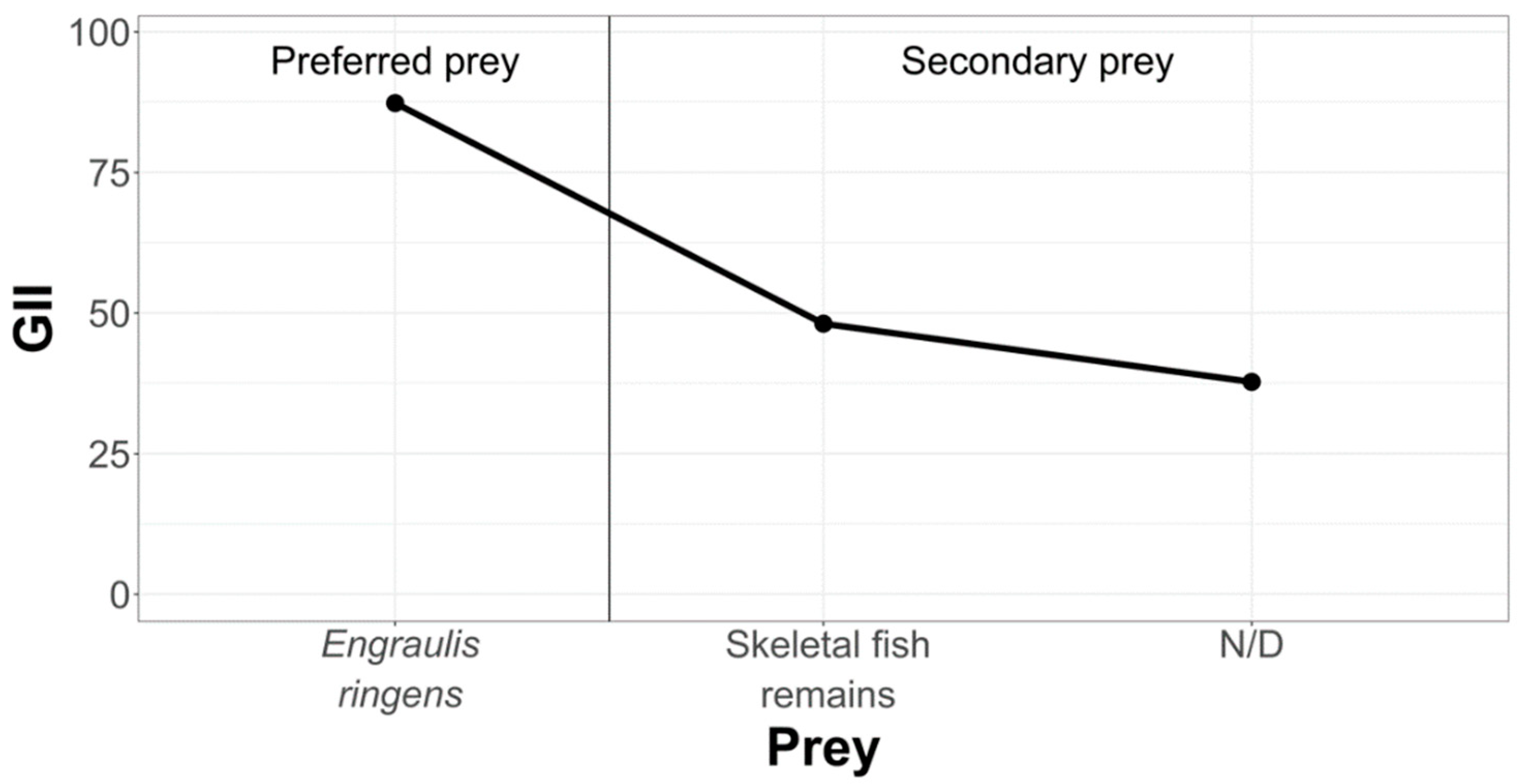
3.2.2. La Capilla Study Area
- Geometric Importance Index (GII)
3.2.3. Chanavayita Study Area
- Frequency of Occurrence (F%)
- Geometric Importance Index (GII)
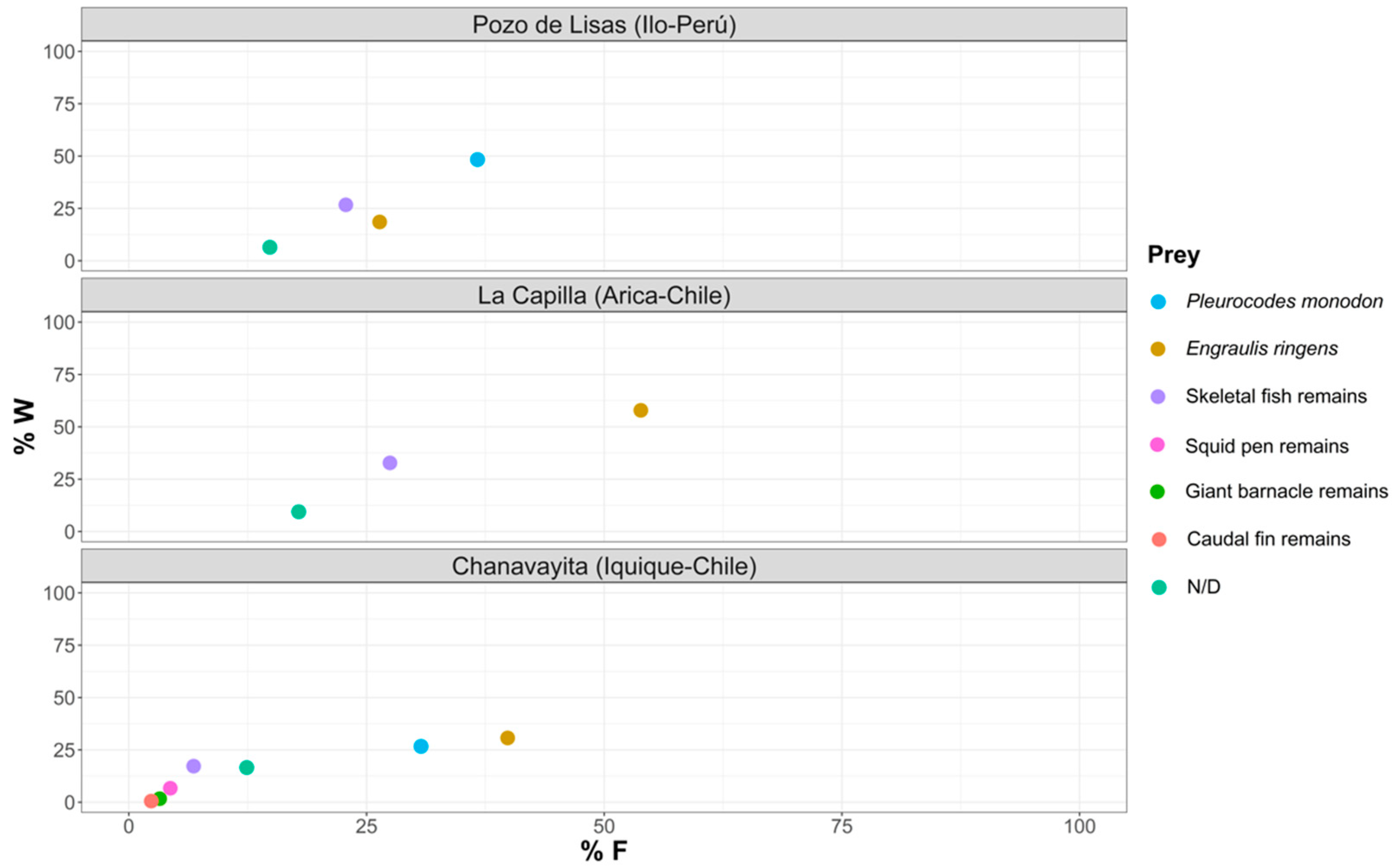
3.3. Evaluation of the Food Strategy
3.4. Study of Trophic Relationships
- Shannon’s Diversity Index (H’) and homogeneity
4. Discussion
4.1. Analysis of Feeding Dynamics
4.2. Study of the Trophic Composition of the Diet
4.3. Evaluation of the Food Strategy
4.4. Study of Trophic Relationships
4.5. Statistical Analysis
5. Conclusions
- Fish feed more during the autumn season.
- The hierarchy of prey items in the diet of Sarda chiliensis chiliensis in the three study areas reveals Pleuroncodes monodon and Engraulis ringens as the main food prey.
- On the northern coasts of Chile and the southern coasts of Peru, Sarda chiliensis chiliensis would be an ichthyophagous and stenophagous fish, i.e., its diet is not diverse, consisting mainly of Engraulis ringens (anchoveta) and Pleuroncodes monodon (dwarf shrimp).
- Regarding the importance of prey in the diet of Sarda chiliensis chiliensis in the three study areas, this would be a generalist species.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibarra, M.G.; Guadalupe, A.S. Alimentación de Characidium rachovii (Characiformes: Crenuchidae) y Pyrrhulina australis (Characiformes: Lebiasinidae) en lagunas someras de la provincia de Corrientes, Argentina. Rev. Biol. Trop. 2016, 64, 603. [Google Scholar] [CrossRef]
- Vázquez-Ozuna, M.I.; Cerdenares-Ladrón de Guevara, G.; Rojas-Herrera, A.A.; Violante-González, J.; García-Ibañez, S.; Rosas-Guerrero, V.M. Hábitos alimenticios de Caranx vinctus (Perciformes: Carangidae), especie de importancia comercial en la bahía de Acapulco, Guerrero, México. Rev. Biol. Trop. 2020, 68, 752–764. [Google Scholar] [CrossRef]
- Amezaga, R. Analisis de Contenidos Estomacales en Peces. Revisión Bibliográfica de los Objetivos y la Metodología, 68th ed.; Ministerio de Agricultura, Pesca y Alimentaciόn: Madrid, Spain, 1988; Volume 63.
- Granados, C.L. Ecología de Peces; Universidad de Sevilla: Sevilla, Spain, 1997; ISBN 9788447202423. [Google Scholar]
- Woehler, O.; Sanchez, F. Feeding ecology of castaneta (Cheilodactylus bergi; Pisces: Cheilodactylidae) in the south-western Atlantic (34–47° S). Mar. Freshw. Res. 1994, 45, 507–520. [Google Scholar] [CrossRef]
- Heupel, M.R.; Bennett, M.B. Observations on the diet and feeding habits of the epaulette shark, Hemiscyllium ocellatum (Bonnaterre), on Heron Island Reef, Great Barrier Reef, Australia. Mar. Freshw. Res. 1998, 49, 753. [Google Scholar] [CrossRef]
- Wolff, M. On assessing a fish species role in its community by stomach and gut content analyses: A critical note with suggestions. Medio Ambiente 1989, 10, 80–83. [Google Scholar]
- Berg, J. Discussion of methods of investigating the food of fishes, with reference to a preliminary study of the prey of Gobiusculus flavescens (Gobiidae). Mar. Biol. 1979, 50, 263–273. [Google Scholar] [CrossRef]
- Medina, M.; Arancibia, H. Dinámica trófica del jurel (Trachurus symmetricus murphyi) en el norte de Chile. Investig. Mar. 2002, 30, 45–55. [Google Scholar] [CrossRef]
- García, M.C.; Chong, J. Composicion de la dieta de Brama Australis Valenciennes 1837. En La Zona Centro-Sur De Chile (Viii Región) En Otoño 2000 Y Verano 2001. Gayana 2002, 66, 225–230. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, S.; Sánchez-Lizaso, J.L.; Ramos Esplá, A.A. Cambios estacionales en la dieta de Diplodus annularis (L., 1758) en el sudeste ibérico. Bol. Inst. Esp. Oceanogr. 2001, 17, 87–95. [Google Scholar]
- Vidal, R.; Acuña, E.; Rey Méndez, M. Dieta de la merluza Merluccius gayi (Guichenot, 1848) del norte de Chile. Bol. Inst. Esp. Oceanogr. 1997, 13, 35–45. [Google Scholar]
- Aron, A.; Flores, H.; Wolff, M.; Rodrigo, R. Análisis del contenido estomacal de Seriola peruana Steindachner, 1891 (Perciformes, Carangidae), en el área de Coquimbo, Norte de Chile. Rev. Biol. Mar. Oceanogr. 1992, 27, 147–156. [Google Scholar]
- Flores, H.; Rojas, P. Contenido gástrico de Dissostichus eleginoides Smith 1898 capturado frente a Valparaíso (Perciformes: Nototheniidae). Investig. Mar. 1987, 15, 33–40. [Google Scholar]
- Silva, M.; Stuardo, J. Alimentación y relaciones troficas generales entre algunos peces demersales y el bentos de bahía Coliumo (provincia de Concepción, Chile). Gayana Zool. 1985, 49, 77–102. [Google Scholar]
- Letelier, S.; Vega, M.A.; María, A.; Carreño, E. Base de datos del Museo Nacional de Historia Natural: Moluscos de Chile. Rev. Biol. Trop. 2003, 51, 33–137. [Google Scholar]
- Nikolsky, G.V. The Ecology of Fishes; Academic Press: London, UK, 1963. [Google Scholar]
- Wootton, R.J. Ecology of Teleost Fishes, 2nd ed.; Springer: Dordrecht, The Netherlands, 1999; Volume 24. [Google Scholar]
- Sernapesca. Anuario Estadístico de Pesca año 2019; Sernapesca: Santiagio, Chile, 2020. [Google Scholar]
- PRODUCE. 2020. Anuario Estadístico Pesquero y Acuícola. Available online: https://ogeiee.produce.gob.pe/index.php/en/shortcode/oee-documentos-publicaciones/publicaciones-anuales/item/1001-anuario-estadisticoo-pesquero-y-acuicola-2020 (accessed on 3 January 2022).
- Collette, B.B.; Nauen, C.E. FAO Species Catalogue Vol. 2 Scombrids of the world an annotated and illustrated catalogue of Tunas, Mackerels, Bonitos and related species know to date. FAO Fish. Synop. 1983, 2, 137. [Google Scholar]
- Serra, J.; Rojas, O.; Hinostroza, F.; Cañon, J. Bonito Sarda chiliensis (Cuvier) Teleostomi Perciforme Scombridae. In Estado Actual de las Principales Pesquerías Nacionales; Instituto de fomento Pesquero: Santiago, Chile, 1979; p. 59. [Google Scholar]
- Mann, G. Vida de Los Peces en Aguas Chilenas, 1st ed.; Ministerio de Agricultura, Universidad de Chile: Santiago, Chile, 1954. [Google Scholar]
- Medina, M.; Araya, M.C.; Vega, C. Guía de Peces Marinos de la Zona Norte de Chile. Proyectos en Docencia Universitaria “Medios de Apoyo a la Docencia”, 1st ed.; Universidad Arturo Prat, Departamento de Ciencias del Mar: Iquique, Chile, 2004. [Google Scholar]
- Pepe-Victoriano, R.; Miranda, L.; Ortega, A.; Merino, G.E. Descriptive morphology and allometric growth of the larval development of Sarda chiliensis chiliensis (Cuvier, 1832) in a hatchery in northern Chile. Aquac. Rep. 2021, 19, 100576. [Google Scholar] [CrossRef]
- Pepe-Victoriano, R.; Miranda, L.; Ortega, A.; Merino, G. First natural spawning of wild-caught premature south pacific bonito (Sarda chiliensis chiliensis, cuvier 1832) conditioned in recirculating aquaculture system and a descriptive characterization of their eggs embryonic development. Aquac. Rep. 2021, 19, 100563. [Google Scholar] [CrossRef]
- Chirichigno, N. Clave Para Identificar Los Peces Marinos del Perú; Informe IMARPE: Lima, Perú, 1974; Volume 44. [Google Scholar]
- Morte, M.S.; Sanz, A. Estudio de las Relaciones Trόficas de Algunos Peces Bentόnicos de Interés Comercial del Golfo de Valencia (Mediterraneo Occidental); Universitat de Valѐncia: Valencia, Spain, 2004. [Google Scholar]
- Granadeiro, J.P.; Silva, M.A. The use of otoliths and vertebrae in the identification and size-estimation of fish in predator-prey studies. Cybium 2000, 24, 383–393. [Google Scholar]
- Jobling, M.; Breiby, A. The use and abuse of fish otoliths in studies of feeding habits of marine piscivores. Sarsia 1986, 71, 265–274. [Google Scholar] [CrossRef]
- Ramos, J. Fisiología de la Reproducciόn y Biología del Lenguado (Solea solea L.); Universidad Complutense deMadrid, Servicio de Reprografía: Madrid, Spain, 1981. [Google Scholar]
- Vicent, J.J.; Aparici, V. Ecologia trofica primaveral de Aidablennius sphynx (Valenciennes, 1836), Parabennius incognitus (Bath, 1968) y Lipophrys dalmatinus (Steindachner y Kolombatovic, 1883) (Pisces, Blenniidae) en las escolleras de las playas de Borriana y Nules (golfo de Vale). Inst. Esp. Oceanog. 1998, 23, 161–170. [Google Scholar]
- Aguirre, H. Aspectos Biolόgicos y Ecolόgicos del Salmonente de Fango Mullus barbatus L. 1758 y del Salmonete de Roca Mullus sumuletus L.; 1758 del Mediterráneo noroccidental; Universitat Politѐcnica de Catalunya: Barcelona, Spain, 2000. [Google Scholar]
- Cabral, H.N. Utilização do Estuário do Tejo Como área de Viveiro Pelos Linguados, Solea solea (L, 1758) e Solea Senegalensis Kaup, 1858, e robalo, Dicentrarchus labrax (L, 1758); Universidad de Lisboa: Lisbon, Portugal, 1998. [Google Scholar]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Cabral, H.; Catarino, A.I.; Figueiredo, J.; Garcia, J.; Henriques, M. Feeding ecology, age, growth and sexual cycle of the Portuguese sole, Synaptura lusitanica. J. Mar. Biol. Assoc. UK 2003, 83, 613–618. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L. Food Habits of Albacore, Bluefin Tuna, and Bonito In California Waters. Scripps Inst. Oceanogr. 1970, 152, 1–105. [Google Scholar]
- Assis, C.A. A generalised index for stomach contents analysis in fish. Sci. Mar. 1996, 60, 385–389. [Google Scholar]
- Costello, M.J. Predator feeding strategy and prey importance: A new graphical analysis. J. Fish Biol. 1990, 36, 261–263. [Google Scholar] [CrossRef]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems, 4th ed.; John Wiley and Sons Ltd.: Oxford, UK, 2005. [Google Scholar]
- Pauly, D.; Vildoso, A.C.; Mejia, J.; Samamé, M.; Palomares, M.L. Population dynamics and estimated Tsukayama. In The Peruvian Anchoveta and Its Upwelling Ecosystem: Three Decades of Changes; Pauly, D., Tsukayama, I., Eds.; Instituto del Mar del Perú (IMARPE): Lima, Perú, 1987; Volume 3, pp. 248–267. [Google Scholar]
- SERNAPESCA. Anuario Estadístico en Pesca y Acuicultura 2020. 26 de marzo 2022. Available online: http://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura (accessed on 3 January 2022).
- Amundsen, P.A. Feeding strategy of Arctic charr (Salvelinus alpinus): General opportunist, but individual specialist. Nord. J. Freshw. Res. 1995, 71, 150–156. [Google Scholar]
- Stoner, A.W. Effects of environmental variables on fish feeding ecology: Implications for the performance of baited fishing gear and stock assessment. J. Fish Biol. 2004, 65, 1445–1471. [Google Scholar] [CrossRef]
- Gibson, R.N. Flatfishes: Biology and Exploitation, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2005; ISBN 9781405143387. [Google Scholar]
- Flores-Ortega, J.R.; Godínez-Domínguez, E.; Rojo-Vázquez, J.A.; Corgos, A.; Galván-Piña, V.H.; González-Sansón, G. Interacciones tróficas de las seis especies de peces más abundantes en la pesquería artesanal en dos bahías del Pacífico Central Mexicano. Rev. Biol. Trop. 2009, 58, 383–397. [Google Scholar] [CrossRef][Green Version]
- Chirinos, A. Estudio preliminar sobre el Bonito Sarda chilensis (Cuvier Valenciennes) de la costa del Perú. Pesca Caza 1955, 6, 1–38. [Google Scholar]
- Ancieta, F. Sinopsis sobre la biología y pesquería del “Bonito” Sarda chilensis (Cuvier y Valenciennes) frente a la costa del Perú (1964). REBIOL 1964, 1, 17–19. [Google Scholar]
- Canal, E.R. Niveles Tróficos del Bonito Sarda sarda chiliensis, Cuvier en el Área del Callao; Universidad Nacional San Antonio Abad del Cusco: Lima, Peru, 1974. [Google Scholar]
- Mayo, R. Algunos aspectos alimentarios del bonito (Sarda chiliensis) Callao, 1976. In Proceedings of the Libro de Resúmenes; VI Congreso Nacional de Biología: Chiclayo, Peru, 1979; pp. 142–143. [Google Scholar]
- Luquet, P.; Watanabe, T. Interaction nutrition-reproduction in fish. In Fish Physiology and Biochemistry; Springer: Berlin/Heidelberg, Germany, 1986; Volume 2, pp. 121–129. [Google Scholar]
- Pepe-Victoriano, R.; Araya, M.; Faúndez, V. Efecto de la Temperatura en la Supervivencia Embrionaria y Primeros Estadios Larvales de Psetta maxima. Int. J. Morphol. 2012, 30, 1551–1557. [Google Scholar] [CrossRef]
- Ohshimo, S.; Hiraoka, Y.; Sato, T.; Nakatsuka, S. Feeding habits of bigeye tuna (Thunnus obesus) in the North Pacific from 2011 to 2013. Mar. Freshw. Res. 2018, 69, 585–606. [Google Scholar] [CrossRef]
- Cailliet, G.M. Several approaches to the feeding ecology of fishes. In Fish Food Habits Studies: Proceedings of the 1st Pacific Northwest Technical Workshop; Simenstad, C.A., Lipovsky, S.J., Eds.; Washington Sea Grant Publication. Washington Sea Grant Program, University of Washington: Seattle, WA, USA, 1977; pp. 1–13. [Google Scholar]
- Carrassón, M.; Stefanescu, C.; Cartes, J.E. Diets and bathymetric distributions of two bathyal sharks of the Catalan deep sea (western Mediterranean). Mar. Ecol. Prog. Ser. 1992, 82, 21–30. [Google Scholar] [CrossRef]
- Ebert, D.A.; Cowley, P.D.; Compagno, L.J. V A preliminary investigation of the feeding ecology of catsharks (Scyliorhinidae) off the west coast of southern Africa. S. Afr. J. Mar. Sci. 1996, 17, 233–240. [Google Scholar] [CrossRef]
- Cross, J.N. Aspects of the biology of two scyliorhinid sharks, Apristurus brunneus and Parmaturus xaniurus, from the upper continental slope off southern California. Fish. Bull. 1988, 86, 691–702. [Google Scholar]
- Medina, M.; Araya, M.C. Hábitos tróficos del bonito Sarda chiliensis (Cuvier 1832) (Pisces: Scombridae) en el norte de Chile. In Proceedings of the Congreso de Ciencias del Mar, Libro de resúmenes Iquique, Chile, 27–31 May 2019; p. 478. [Google Scholar]
- Sánchez de Benites, G.; Alamo, A.; Fuentes, M. Alteraciones en la dieta alimentaria de algunos peces comerciales por efecto del fenómeno. In Proceedings of the Conferencias del Symposium El fenómeno El Niño y su impacto en la fauna marina, Arequipa, Peru, 9–15 October 1983; pp. 135–142. [Google Scholar]
- George, E.L.; Hadley, W.F. Food and Habitat Partitioning Between Rock Bass (Ambloplites rupestris) and Smallmouth Bass (Micropterus dolomieui) Young of the Year. Trans. Am. Fish. Soc. 1979, 108, 253–261. [Google Scholar] [CrossRef]
- Hacunda, J. Trophic relationships among demersal fishes in a coastal area of the Gulf of Maine. Fish. Bull. 1981, 79, 775–788. [Google Scholar]
- Moreno, X.; Perez, P.; Irigoyen, M.; Marin, E.; Abitia, L.; Escobar, O. Feeding habits of the leopard grouper, Mycteroperca rosacea (Actinopterygii: Perciformes: Epinephelidae), in the central Gulf of California, BCS, Mexico. Acta Ichthyol. Piscat. 2019, 49, 9–22. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Heitz, J.G. Gill raker apparatus and food selectivity among mackerels, tunas, and dolphins. Fish. Bull. 1971, 69, 361–370. [Google Scholar]
- Collette, B.; Chao, N. Systematics and morphology of the bonitos (Sarda) and their relatives (Scombridae, Sardini). Fish. Bull. 1975, 73, 516–625. [Google Scholar]
- Blasković, V.; Fernández, C.; Navarro, I.; Alegre, A. Seguimiento de las Relaciones Tróficas de los Principales Recursos Pelágicos en el Litoral Peruano, 2007; Instituto del Mar del Perú: Lima, Peru, 2008. [Google Scholar]
- Labropoulou, M.; Machias, A.; Tsimenides, N.; Eleftheriou, A. Feeding habits and ontogenetic diet shift of the striped red mullet, Mullus surmuletus Linnaeus, 1758. Fish. Res. 1997, 31, 257–267. [Google Scholar] [CrossRef]
- Arceo-Carranza, D.; Dorantes Acereto, E.A.; Hernández-Mendoza, L.C.; Chiappa Carrara, X. Temporal shifts in the abundance and feeding of a marine fish community in a coastal lagoon in southeastern mexico. Cienc. Mar. 2021, 47, 17–32. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Harper & Row: New York, NY, USA, 1999. [Google Scholar]
- Marshall, S.; Elliott, M. A comparison of univariate and multivariate numerical and graphical techniques for determining inter- and intraspecific feeding relationships in estuarine fish. J. Fish Biol. 1997, 51, 526–545. [Google Scholar] [CrossRef]

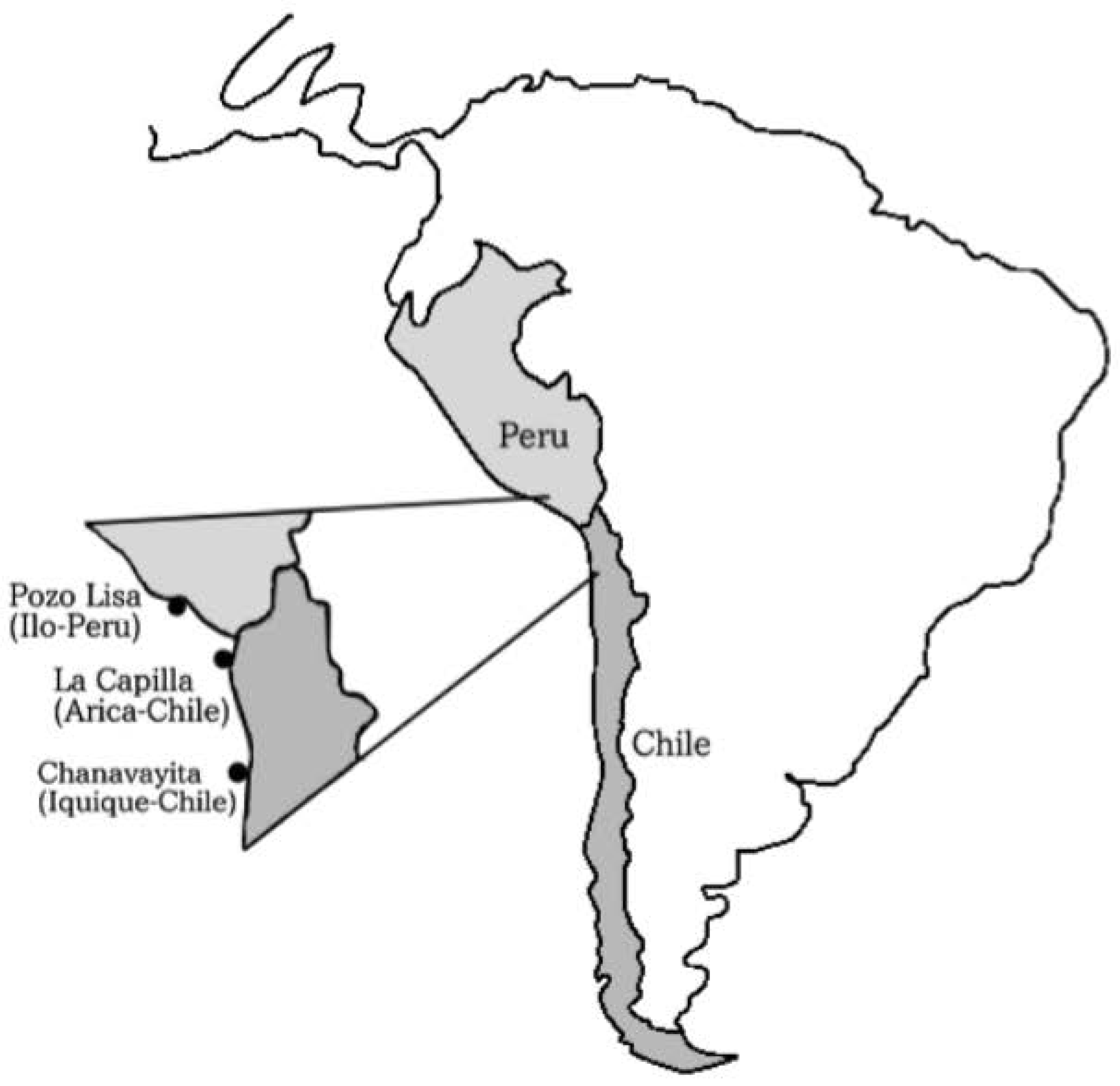


| Capture Area | Number of Specimens Captured without Stomach Contents | Number of Specimens Captured with Stomach Contents | Total Average Weight (g) | SD (Standard Deviation) | Average Standard Length (cm) | SD (Standard Deviation) | Sex Ratio (Male/ Female) |
|---|---|---|---|---|---|---|---|
| Pozo de Lisas (Ilo, Perú) | 345 | 145 | 905 | 173.49 | 39.4 | 1.81 | 99/46 |
| La Capilla (Arica, Chile) | 301 | 132 | 698 | 186.59 | 36.4 | 1.73 | 91/41 |
| Chanavayita (Iquique, Chile) | 352 | 129 | 1124 | 182.85 | 45.4 | 1.72 | 73/56 |
| Numeric Composition Index | Frequency of Occurrence Index | Gravimetric Method (g) | Relative Importance Index | ||||
|---|---|---|---|---|---|---|---|
| Item-Prey | N | %N | F | %F | W | %W | |
| Skeletal fish remains | 23 | 18.40 | 18 | 22.50 | 32.34 | 26.70 | 10.15 |
| Pleurocodes monodon | 41 | 32.80 | 29 | 36.25 | 58.56 | 48.34 | 29.41 |
| Engraulis ringens | 38 | 30.40 | 21 | 26.25 | 22.43 | 18.50 | 12.84 |
| N/D | 23 | 18.40 | 12 | 15.00 | 7.8 | 6.44 | 3.73 |
| Total | 125 | 100.00 | 80 | 100.00 | 121,13 | 100.00 | |
| Numeric Composition Index | Frequency of Occurrence Index | Gravimetric Method (g) | Relative Importance Index | ||||
|---|---|---|---|---|---|---|---|
| Item-Prey | N | %N | F | %F | W | %W | |
| Skeletal fish remains | 35 | 22.88 | 23 | 27.71 | 33.29 | 32,76 | 15.42 |
| Engraulis ringens | 60 | 39.22 | 45 | 54.22 | 58.78 | 57.84 | 52.62 |
| N/D | 58 | 37.91 | 15 | 18.07 | 9.56 | 9.41 | 8.55 |
| Total | 153 | 100.00 | 83 | 100.00 | 101.63 | 100.00 | |
| Numeric Composition Index | Frequency of Occurrence Index | Gravimetric Method (g) | Relative Importance Index | ||||
|---|---|---|---|---|---|---|---|
| Item-Prey | N | %N | F | %F | W | %W | |
| Skeletal fish remains | 12 | 11.76 | 12 | 6.78 | 16.43 | 17.20 | 1.96 |
| Pleurocodes monodon | 45 | 44.12 | 55 | 31.07 | 25.45 | 26.64 | 21.99 |
| Squid pen remains | 5 | 4.90 | 8 | 4.52 | 6.40 | 6.70 | 0.52 |
| Giant barnacle remains | 6 | 5.88 | 6 | 3.39 | 1.60 | 1.68 | 0.26 |
| Caudal fin remains | 1 | 0.98 | 4 | 2.26 | 0.50 | 0.52 | 0.03 |
| Engraulis ringens | 26 | 25.49 | 70 | 39.55 | 29.34 | 30.72 | 22.23 |
| N/D | 7 | 6.86 | 22 | 12.43 | 15.80 | 16.54 | 2.91 |
| Total | 102 | 100.00 | 177 | 100.00 | 95.52 | 100.00 | |
| No. of Fish | H′ | Hmax | J′ | |
|---|---|---|---|---|
| Pozo de Lisas | 125 | 1.35 | 1.39 | 0.97 |
| La Capilla | 153 | 1.07 | 1.10 | 0.97 |
| Chanavayita | 102 | 1.50 | 1.95 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe-Victoriano, R.; Aravena-Ambrosetti, H.; Huanacuni, J.I.; Méndez-Abarca, F.; Godoy, K.; Álvarez, N. Feeding Habits of Sarda chiliensis chiliensis (Cuvier, 1832) in Northern Chile and Southern Perú. Animals 2022, 12, 930. https://doi.org/10.3390/ani12070930
Pepe-Victoriano R, Aravena-Ambrosetti H, Huanacuni JI, Méndez-Abarca F, Godoy K, Álvarez N. Feeding Habits of Sarda chiliensis chiliensis (Cuvier, 1832) in Northern Chile and Southern Perú. Animals. 2022; 12(7):930. https://doi.org/10.3390/ani12070930
Chicago/Turabian StylePepe-Victoriano, Renzo, Héctor Aravena-Ambrosetti, Jordan I. Huanacuni, Felipe Méndez-Abarca, Karina Godoy, and Nathalia Álvarez. 2022. "Feeding Habits of Sarda chiliensis chiliensis (Cuvier, 1832) in Northern Chile and Southern Perú" Animals 12, no. 7: 930. https://doi.org/10.3390/ani12070930
APA StylePepe-Victoriano, R., Aravena-Ambrosetti, H., Huanacuni, J. I., Méndez-Abarca, F., Godoy, K., & Álvarez, N. (2022). Feeding Habits of Sarda chiliensis chiliensis (Cuvier, 1832) in Northern Chile and Southern Perú. Animals, 12(7), 930. https://doi.org/10.3390/ani12070930






