Effect of Immune Stress on Growth Performance and Immune Functions of Livestock: Mechanisms and Prevention
Abstract
:Simple Summary
Abstract
1. Introduction
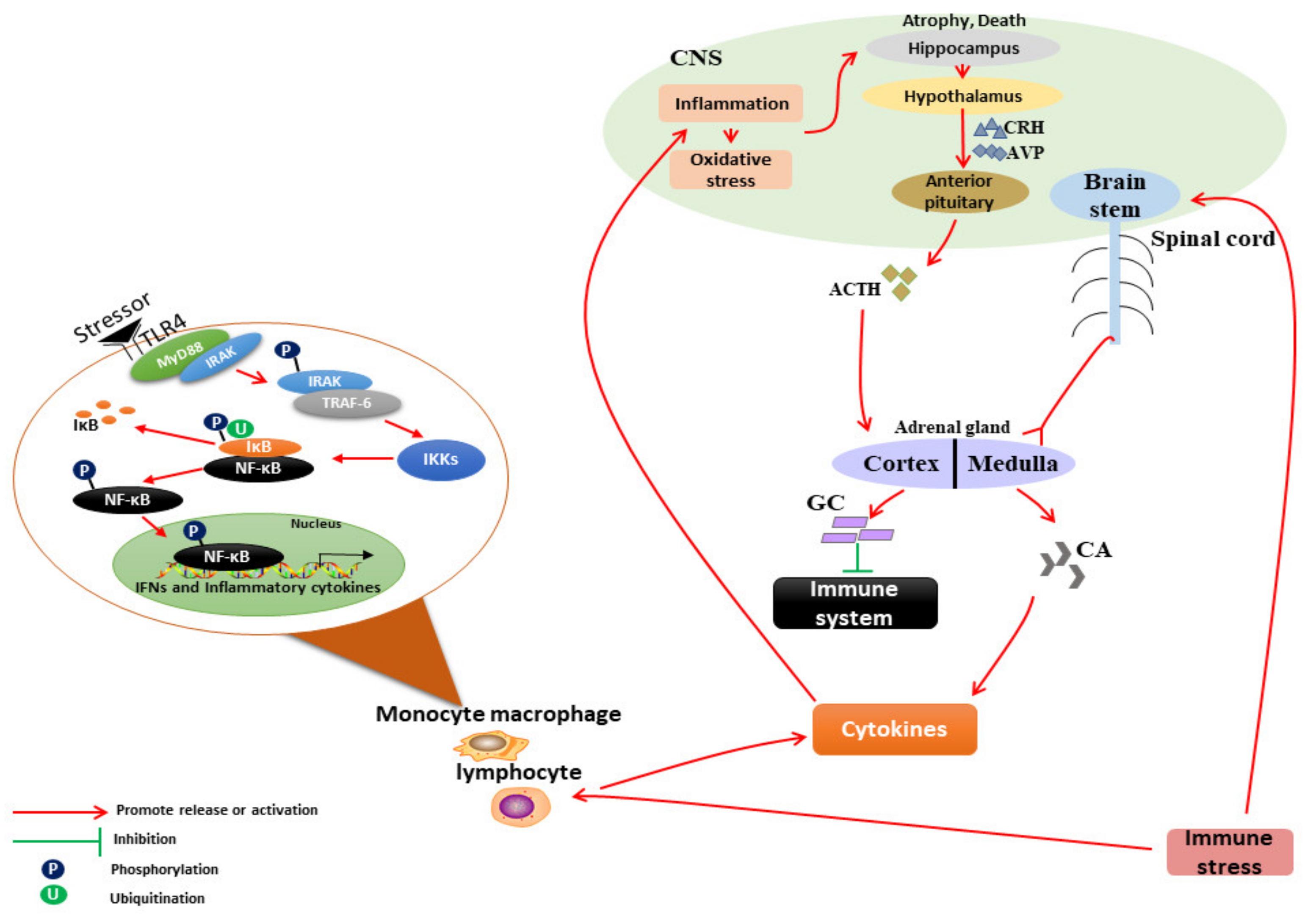
2. Mechanism of IS on the Neuroendocrine–Immune System
2.1. IS Leads to Immune Dysfunction of Livestock
2.1.1. Acute IS Causes Enhanced Immune Function of Livestock
2.1.2. Chronic IS Causes Immunosuppression in Livestock
3. Mechanism of IS on Growth Performance of Livestock and Poultry
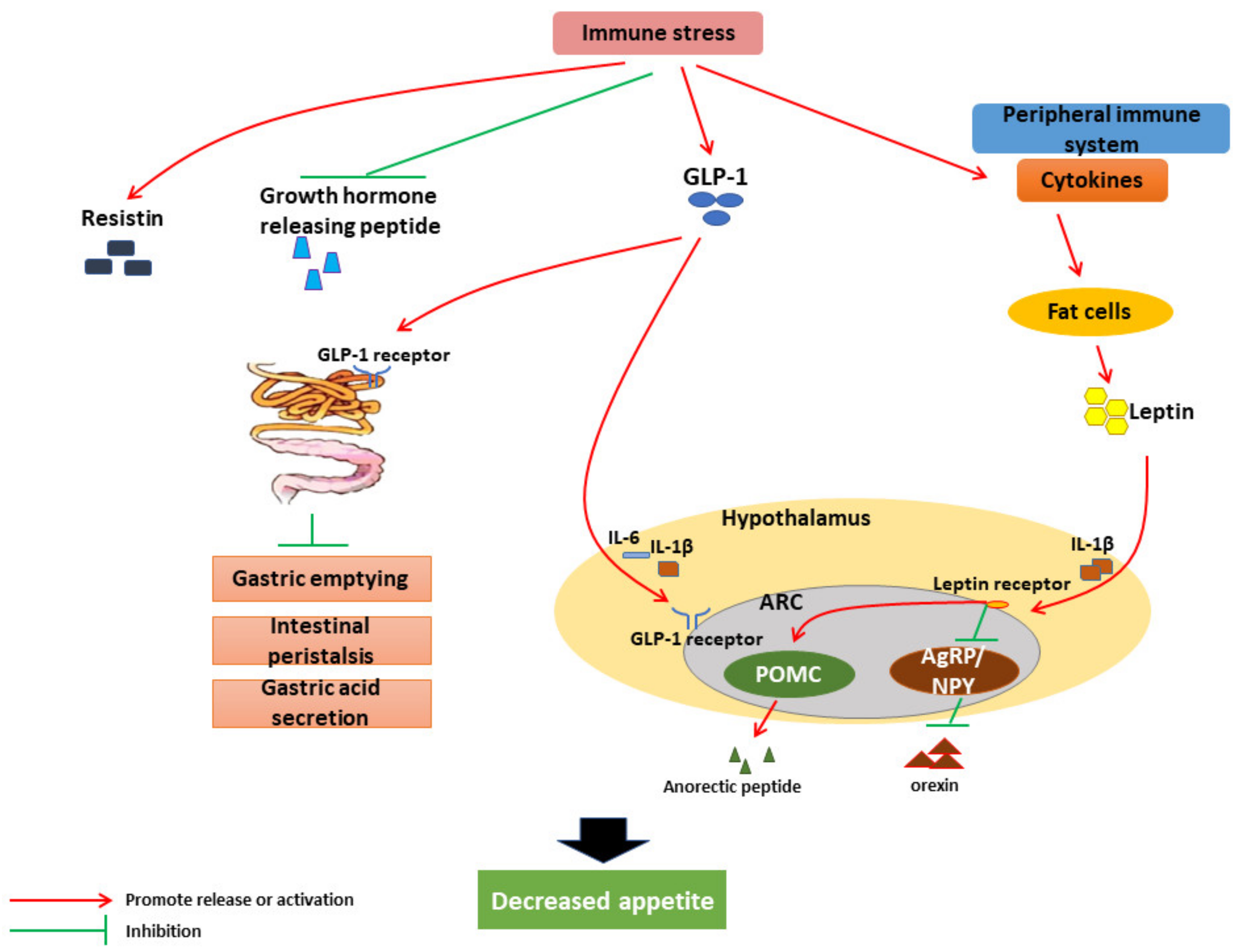
3.1. Effects and Mechanism of IS on Livestock Feed Intake
3.2. Effect of IS on the Digestion and Absorption of Livestock and Poultry
3.3. Effect of IS on Nutrient Metabolism in Livestock
3.4. Other Effects of IS on Livestock
4. Prevention and Control Technologies for IS
4.1. Vaccination Program
4.2. Improved Feeding Regime
4.3. Nutritional Regulation
4.3.1. Amino Acid Additives
4.3.2. Fatty Acid Additives
4.3.3. Vitamin Additives
4.3.4. Trace Element Additives
4.3.5. Probiotics Additives
4.3.6. Plant Extract Additives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C. Study on Regulation and Mechanism of Resveratrol on Immunological Stress in Chickens. Ph.D. Thesis, Henan Agricultural University, Henan, China, May 2014. [Google Scholar]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Nordgreen, J.; Munsterhjelm, C.; Aae, F.; Popova, A.; Boysen, P.; Ranheim, B.; Heinonen, M.; Raszplewicz, J.; Piepponen, P.; Lervik, A.; et al. The effect of lipopolysaccharide (LPS) on inflammatory markers in blood and brain and on behavior in individually-housed pigs. Physiol. Behav. 2018, 195, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.Y.; Yang, Z.H.; Li, X.R.; Wang, H.; Li, L. Protective effects of melatonin against the damages of neuroendocrine-immune induced by lipopolysaccharide in diabetic rats. Exp. Clin. Endocrinol. Diabetes 2009, 117, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Gao, W.; Tao, H.; Yang, J.; Huang, T. The regulation effects of danofloxacin on pig immune stress induced by LPS. Res. Vet. Sci. 2017, 110, 65–71. [Google Scholar] [CrossRef]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Liu, S.; Guo, Y.; Applegate, T.J.; Eicher, S.D. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014, 111, 1394–1404. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.J.; Zhou, Y.M.; Wu, Y.N.; Zhang, L.L.; Wang, T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet. Immunol. Immunopathol. 2013, 153, 70–76. [Google Scholar] [CrossRef]
- Zhang, F.X.; Kirschning, C.J.; Mancinelli, R.; Xu, X.P.; Jin, Y.; Faure, E.; Mantovani, A.; Rothe, M.; Muzio, M.; Arditi, M. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J. Biol. Chem. 1999, 274, 7611–7614. [Google Scholar] [CrossRef] [Green Version]
- Hayley, S.; Mangano, E.; Strickland, M.; Anisman, H. Lipopolysaccharide and a social stressor influence behaviour, corticosterone and cytokine levels: Divergent actions in cyclooxygenase-2 deficient mice and wild type controls. J. Neuroimmunol. 2008, 197, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.; Owens, M.D. Thrombocytes respond to lipopolysaccharide through Toll-like receptor-4, and MAP kinase and NF-kappaB pathways leading to expression of interleukin-6 and cyclooxygenase-2 with production of prostaglandin E2. Mol. Immunol. 2008, 45, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Ostareck, D.H.; Ostareck-Lederer, A. RNA-Binding Proteins in the Control of LPS-Induced Macrophage Response. Front. Genet. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhang, L.; Chen, L.; Zhu, Q.; Wang, W.; Qiao, J. Lactobacillus acidophilus alleviates the inflammatory response to enterotoxigenic Escherichia coli K88 via inhibition of the NF-κB and p38 mitogen-activated protein kinase signaling pathways in piglets. BMC Microbiol. 2016, 16, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhang, H.; Han, Q.; Lan, J.; Chen, G.; Cao, G.; Yang, C. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1096–1105. [Google Scholar] [CrossRef]
- Alhadidi, Q.; Shah, Z.A. Cofilin Mediates LPS-Induced Microglial Cell Activation and Associated Neurotoxicity Through Activation of NF-κB and JAK-STAT Pathway. Mol. Neurobiol. 2018, 55, 1676–1691. [Google Scholar] [CrossRef]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. Interactions of cytokines with the blood-brain barrier: Implications for feeding. Curr. Pharm. Des. 2003, 9, 827–831. [Google Scholar] [CrossRef]
- Guyon, A.; Massa, F.; Rovère, C.; Nahon, J.L. How cytokines can influence the brain: A role for chemokines? J. Neuroimmunol. 2008, 198, 46–55. [Google Scholar] [CrossRef]
- Watkins, L.R.; Goehler, L.E.; Relton, J.K.; Tartaglia, N.; Silbert, L.; Martin, D.; Maier, S.F. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: Evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 1995, 183, 27–31. [Google Scholar] [CrossRef]
- Ericsson, A.; Kovács, K.J.; Sawchenko, P.E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994, 14, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, K.; Kobayashi, S. Signaling the brain in inflammation: The role of endothelial cells. Front. Biosci. 2004, 9, 2819–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaftel, S.S.; Carlson, T.J.; Olschowka, J.A.; Kyrkanides, S.; Matousek, S.B.; O’Banion, M.K. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 2007, 27, 9301–9309. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef] [Green Version]
- Dornelles, G.L.; de Oliveira, J.S.; de Almeida, E.J.R.; Mello, C.B.E.; BR, E.R.; da Silva, C.B.; Petry, L.D.S.; Pillat, M.M.; Palma, T.V.; de Andrade, C.M. Ellagic Acid Inhibits Neuroinflammation and Cognitive Impairment Induced by Lipopolysaccharides. Neurochem. Res. 2020, 45, 2456–2473. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Müller, N.; Myint, A.M.; Schwarz, M.J. Inflammatory biomarkers and depression. Neurotox. Res. 2011, 19, 308–318. [Google Scholar] [CrossRef]
- Chen, S.; Yong, Y.; Ju, X. Effect of heat stress on growth and production performance of livestock and poultry: Mechanism to prevention. J. Therm. Biol. 2021, 99, 103019. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [Green Version]
- Herman, J.P.; Nawreen, N.; Smail, M.A.; Cotella, E.M. Brain mechanisms of HPA axis regulation: Neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress 2020, 23, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Fan, H.; Lan, T.; Yang, X.L.; Shi, W.F.; Zhang, W.; Zhu, Y.; Zhang, Y.W.; Xie, Q.M.; Mani, S.; et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 2018, 556, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Oppong, E.; Cato, A.C. Effects of Glucocorticoids in the Immune System. Adv. Exp. Med. Biol. 2015, 872, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Bertoni, G. Some physiological and biochemical methods for acute and chronic stress evaluationin dairy cows. Italian J. Anim. Sci. 2009, 8, 265–286. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Mao, H.; Yang, C.; Du, H.; Wang, H.; Tu, J. Effects of chitosan nanoparticle supplementation on growth performance, humoral immunity, gut microbiota and immune responses after lipopolysaccharide challenge in weaned pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 597–605. [Google Scholar] [CrossRef]
- Zhang, P.F.; Shi, B.L.; Su, J.L.; Yue, Y.X.; Cao, Z.X.; Chu, W.B.; Li, K.; Yan, S.M. Relieving effect of Artemisia argyi aqueous extract on immune stress in broilers. J. Anim. Physiol. Anim. Nutr. 2017, 101, 251–258. [Google Scholar] [CrossRef]
- Stasi, A.; Franzin, R.; Divella, C.; Sallustio, F.; Curci, C.; Picerno, A.; Pontrelli, P.; Staffieri, F.; Lacitignola, L.; Crovace, A.; et al. PMMA-Based Continuous Hemofiltration Modulated Complement Activation and Renal Dysfunction in LPS-Induced Acute Kidney Injury. Front. Immunol. 2021, 12, 605212. [Google Scholar] [CrossRef]
- Ma, C.; Li, G.; Chen, W.; Jia, Z.; Yang, X.; Pan, X.; Ma, D. Eimeria tenella: IMP1 protein delivered by Lactococcus lactis induces immune responses against homologous challenge in chickens. Vet. Parasitol. 2021, 289, 109320. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, H.K.; Klompen, A.L.; De Vries Reilingh, G.; Lammers, A. Effect of concurrent intratracheal lipopolysaccharide and human serum albumin challenge on primary and secondary antibody responses in poultry. Vaccine 2008, 26, 5510–5520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, G.; Huang, X.; Li, C.; Huang, X.; Zhang, X.; Lin, Q.; Liu, S.; Dai, Q. Evaluation of serum antioxidative status, immune status and intestinal condition of Linwu duck challenged by lipopolysaccharide with various dosages and replications. Poult. Sci. 2021, 100, 101199. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.; Jiang, Y.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Effects of Artemisia argyi flavonoids on growth performance and immune function in broilers challenged with lipopolysaccharide. Anim. Biosci. 2021, 34, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Wick, M.; Lilburn, M.S. Effect of a post-hatch lipopolysaccharide challenge in Turkey poults and ducklings after a primary embryonic heat stress. Dev. Comp. Immunol. 2019, 101, 103436. [Google Scholar] [CrossRef]
- Liu, Y.L.; Li, D.F.; Gong, L.M.; Yi, G.F.; Gaines, A.M.; Carroll, J.A. Effects of fish oil supplementation on the performance and the immunological, adrenal, and somatotropic responses of weaned pigs after an Escherichia coli lipopolysaccharide challenge. J. Anim. Sci. 2003, 81, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Van Heugten, E.; Coffey, M.T.; Spears, J.W. Effects of immune challenge, dietary energy density, and source of energy on performance and immunity in weanling pigs. J. Anim. Sci. 1996, 74, 2431–2440. [Google Scholar] [CrossRef]
- Mao, X.F.; Piao, X.S.; Lai, C.H.; Li, D.F.; Xing, J.J.; Shi, B.L. Effects of beta-glucan obtained from the Chinese herb Astragalus membranaceus and lipopolysaccharide challenge on performance, immunological, adrenal, and somatotropic responses of weanling pigs. J. Anim. Sci. 2005, 83, 2775–2782. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; He, X.; Yuan, J.; Yang, Y.; Wang, Z. Growth performance and immune responses in chickens after challenge with lipopolysaccharide and modulation by dietary different oils. Animal 2008, 2, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Xiang-hong, J.; Yan-hong, Y.; Han-jin, X.; Li-long, A.; Yingmei, X. Impacts of heat stress on baseline immune measures and a subset of T cells in Bama miniature pigs. Livest. Sci. 2011, 135, 289–292. [Google Scholar] [CrossRef]
- Cangiano, L.R.; Zenobi, M.G.; Nelson, C.D.; Ipharraguerre, I.R.; Dilorenzo, N. A bioactive extract from Olea europaea protects newly weaned beef heifers against experimentally induced chronic inflammation1. J. Anim. Sci. 2019, 97, 4349–4361. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Liu, S.P.; Yuan, Z.H.; Yi, J.E.; Tian, Y.N.; Wu, J.; Wen, L.X. Effects of induced stress from the live LaSota Newcastle disease vaccination on the growth performance and immune function in broiler chickens. Poult. Sci. 2020, 99, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of L-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate. Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Chen, Y.P.; Yang, M.X.; Zhang, L.L.; Lu, Z.X.; Zhou, Y.M.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015, 94, 1504–1511. [Google Scholar] [CrossRef]
- Yang, X.J.; Li, W.L.; Feng, Y.; Yao, J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011, 90, 2740–2746. [Google Scholar] [CrossRef]
- Wu, J.; He, C.; Bu, J.; Luo, Y.; Yang, S.; Ye, C.; Yu, S.; He, B.; Yin, Y.; Yang, X. Betaine attenuates LPS-induced downregulation of Occludin and Claudin-1 and restores intestinal barrier function. BMC Vet. Res. 2020, 16, 75. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Yu, Y.H. Bacillus subtilis-fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult. Sci. 2021, 100, 875–886. [Google Scholar] [CrossRef]
- Xie, M.Y.; Hou, L.J.; Sun, J.J.; Zeng, B.; Xi, Q.Y.; Luo, J.Y.; Chen, T.; Zhang, Y.L. Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-κB and p53 Pathways in Intestinal Epithelial Cells. J. Agric. Food Chem. 2019, 67, 9477–9491. [Google Scholar] [CrossRef]
- Oh, J.; Harper, M.; Giallongo, F.; Bravo, D.M.; Wall, E.H.; Hristov, A.N. Effects of rumen-protected Capsicum oleoresin on immune responses in dairy cows intravenously challenged with lipopolysaccharide. J. Dairy Sci. 2017, 100, 1902–1913. [Google Scholar] [CrossRef]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef]
- Bliss, E.S.; Whiteside, E. The Gut-Brain Axis, the Human Gut Microbiota and Their Integration in the Development of Obesity. Front. Physiol. 2018, 9, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachot, C.; Poole, S.; Luheshi, G.N. Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. J. Physiol. 2004, 561, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Kelley, K.W.; Dantzer, R.; Johnson, R.W. In vivo and in vitro evidence for the involvement of tumor necrosis factor-alpha in the induction of leptin by lipopolysaccharide. Endocrinology 1998, 139, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Luheshi, G.N.; Gardner, J.D.; Rushforth, D.A.; Loudon, A.S.; Rothwell, N.J. Leptin actions on food intake and body temperature are mediated by IL-1. Proc. Natl. Acad. Sci. USA 1999, 96, 7047–7052. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, M.; Jonaidi, H.; Sadeghi, B. Influence of peripheral lipopolysaccharide (LPS) on feed intake, body temperature and hypothalamic expression of neuropeptides involved in appetite regulation in broilers and layer chicks. Br. Poult. Sci. 2021, 62, 110–117. [Google Scholar] [CrossRef]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef]
- Shirazi, R.; Palsdottir, V.; Collander, J.; Anesten, F.; Vogel, H.; Langlet, F.; Jaschke, A.; Schürmann, A.; Prévot, V.; Shao, R.; et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc. Natl. Acad. Sci. USA 2013, 110, 16199–16204. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.C.; Shieh, W.Y.; Chen, C.Y.; Hsu, S.C.; Chen, H.L. Lipopolysaccharide increases resistin gene expression in vivo and in vitro. FEBS Lett. 2002, 530, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Basa, N.R.; Wang, L.; Arteaga, J.R.; Heber, D.; Livingston, E.H.; Taché, Y. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci. Lett. 2003, 343, 25–28. [Google Scholar] [CrossRef]
- Howick, K.; Griffin, B.T.; Cryan, J.F.; Schellekens, H. From Belly to Brain: Targeting the Ghrelin Receptor in Appetite and Food Intake Regulation. Int. J. Mol. Sci. 2017, 18, 273. [Google Scholar] [CrossRef] [Green Version]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Li, J.; Wang, M.; Wang, Q.; Li, J.; Ding, N.; Yang, H.; Yin, Y. Dietary high protein-induced diarrhea and intestinal inflammation by activation of NF-κB signaling in piglets. Anim. Nutr. 2021, 7, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.E.; Hume, I.D. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol. Rev. 1998, 78, 393–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhao, L.; Cao, F.; Ahmad, H.; Wang, G.; Wang, T. Effects of feeding fermented Ginkgo biloba leaves on small intestinal morphology, absorption, and immunomodulation of early lipopolysaccharide-challenged chicks. Poult. Sci. 2013, 92, 119–130. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, X.; Wang, X.; Wu, H.; Zhu, H.; Hou, Y.; Dai, B.; Liu, X.; Liu, Y. Glutamate alleviates intestinal injury, maintains mTOR and suppresses TLR4 and NOD signaling pathways in weanling pigs challenged with lipopolysaccharide. Sci. Rep. 2018, 8, 15124. [Google Scholar] [CrossRef] [Green Version]
- Yi, D.; Hou, Y.; Xiao, H.; Wang, L.; Zhang, Y.; Chen, H.; Wu, T.; Ding, B.; Hu, C.A.; Wu, G. N-Acetylcysteine improves intestinal function in lipopolysaccharides-challenged piglets through multiple signaling pathways. Amino Acids 2017, 49, 1915–1929. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol Improves Barrier Function and Attenuates Inflammatory Responses in Porcine Intestinal Epithelial Cells during Lipopolysaccharide (LPS)-Induced Inflammation. J. Agric. Food. Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef]
- Li, Y.; Song, Z.; Kerr, K.A.; Moeser, A.J. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PLoS ONE 2017, 12, e0171617. [Google Scholar] [CrossRef]
- Xie, Y.; Wen, M.; Zhao, H.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; Jia, G. Effect of zinc supplementation on growth performance, intestinal development, and intestinal barrier function in Pekin ducks with lipopolysaccharide challenge. Poult. Sci. 2021, 100, 101462. [Google Scholar] [CrossRef]
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.X.; He, J.; Wu, S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92. [Google Scholar] [CrossRef]
- Xu, X.; Hua, H.; Wang, L.; He, P.; Zhang, L.; Qin, Q.; Yu, C.; Wang, X.; Zhang, G.; Liu, Y. Holly polyphenols alleviate intestinal inflammation and alter microbiota composition in lipopolysaccharide-challenged pigs. Br. J. Nutr. 2020, 123, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Wang, L.; Yu, C.; Zhang, G.; Zhu, H.; Wang, C.; Zhao, S.; Hu, C.A.; Liu, Y. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Cheng, Y.; Fu, J.; Lu, Z.; Wang, F.; Jin, M.; Zong, X.; Wang, Y. Gut Immunity and Microbiota Dysbiosis Are Associated with Altered Bile Acid Metabolism in LPS-Challenged Piglets. Oxid Med. Cell Longev. 2021, 2021, 6634821. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qi, L.; Wei, Q.; Shi, F. Maternal stevioside supplementation ameliorates intestinal mucosal damage and modulates gut microbiota in chicken offspring challenged with lipopolysaccharide. Food Funct. 2021, 12, 6014–6028. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, R.; Liu, Y.; Zhu, W.; Mao, S. Intravenous lipopolysaccharide challenge alters ruminal bacterial microbiota and disrupts ruminal metabolism in dairy cattle. Br. J. Nutr. 2014, 112, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Zhang, Q.; Wang, C.; Wu, H.; Jiao, L.; Hong, Q.; Hu, C. LPS challenge increased intestinal permeability, disrupted mitochondrial function and triggered mitophagy of piglets. Innate. Immun. 2018, 24, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.Y.; Sun, L.H.; Lin, Y.C.; Ma, X.Y.; Zheng, C.T.; Zhou, G.L.; Chen, F.; Zou, S.T. Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J. Anim. Sci. 2009, 87, 4050–4056. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, L.; Li, F. Effects of glucocorticoids on the gene expression of nutrient transporters in different rabbit intestinal segments. Animal 2020, 14, 1693–1700. [Google Scholar] [CrossRef]
- Spurlock, M.E. Regulation of metabolism and growth during immune challenge: An overview of cytokine function. J. Anim. Sci. 1997, 75, 1773–1783. [Google Scholar] [CrossRef] [Green Version]
- Mani, V.; Weber, T.E.; Baumgard, L.H.; Gabler, N.K. Growth and Development Symposium: Endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 2012, 90, 1452–1465. [Google Scholar] [CrossRef]
- Johnson, R.W. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 1997, 75, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.Q.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Wang, J.P.; Peng, H.W.; Zeng, Q.F. High dietary energy content increases inflammatory markers after lipopolysaccharide challenge in meat ducks. Poult. Sci. 2019, 98, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X.; Ma, X.; Gao, K.; Hu, Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015, 28, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.L.T.; Alghirani, M.M.; Kamalludin, M.H.; Nayan, N.; Jesse, F.F.A.; Wei, O.T.A.; Stephen, M.; Reduan, M.F.H.; Loh, T.C. Do different vaccination regimes affect the growth performance, immune status, carcase characteristics and meat quality of broilers? Br. Poult. Sci. 2021, 62, 32–37. [Google Scholar] [CrossRef]
- Klasing, K.C. Nutritional aspects of leukocytic cytokines. J. Nutr. 1988, 118, 1436–1446. [Google Scholar] [CrossRef]
- De Boever, S.; Beyaert, R.; Vandemaele, F.; Baert, K.; Duchateau, L.; Goddeeris, B.; De Backer, P.; Croubels, S. The influence of age and repeated lipopolysaccharide administration on body temperature and the concentration of interleukin-6 and IgM antibodies against lipopolysaccharide in broiler chickens. Avian Pathol. 2008, 37, 39–44. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Zheng, A.; Zhang, A.; Chen, Z.; Pirzado, S.A.; Chang, W.; Cai, H.; Bryden, W.L.; Liu, G. Molecular mechanisms of growth depression in broiler chickens (Gallus Gallus domesticus) mediated by immune stress: A hepatic proteome study. J. Anim. Sci. Biotechnol. 2021, 12, 90. [Google Scholar] [CrossRef]
- Klasing, K.C.; Korver, D.R. Leukocytic Cytokines Regulate Growth Rate and Composition Following Activation of the Immune System. J. Anim. Sci. 1997, 75, 58–67. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Wang, J.; Yi, J.; Yuan, Z.; Wu, J.; Wen, L.; Li, R. Effects of ND vaccination combined LPS on growth performance, antioxidant performance and lipid metabolism of broiler. Res. Vet. Sci 2021, 135, 317–323. [Google Scholar] [CrossRef]
- Song, M.; Lin, X.; Zhao, J.; Wang, X.; Jiao, H.; Li, H.; Sun, S.; Lin, H. High frequency vaccination-induced immune stress reduces bone strength with the involvement of activated osteoclastogenesis in layer pullets. Poult. Sci. 2020, 99, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food. Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.L.; Qiao, M.; Smith, D.P. The relationship of raw broiler breast meat color and pH to cooked meat color and pH. Poult. Sci. 2000, 79, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef] [Green Version]
- Sauber, T.E.; Stahly, T.S.; Nonnecke, B.J. Effect of level of chronic immune system activation on the lactational performance of sows. J. Anim. Sci. 1999, 77, 1985–1993. [Google Scholar] [CrossRef]
- Nie, W.; Wang, B.; Gao, J.; Guo, Y.; Wang, Z. Effects of dietary phosphorous supplementation on laying performance, egg quality, bone health and immune responses of laying hens challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. Biotechnol. 2018, 9, 53. [Google Scholar] [CrossRef]
- Sharma, J.M. Introduction to poultry vaccines and immunity. Adv. Vet. Med. 1999, 41, 481–494. [Google Scholar] [CrossRef]
- Marangon, S.; Busani, L. The use of vaccination in poultry production. Rev. Sci. Tech. 2007, 26, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Cader, M.S.; Palomino-Tapia, V.; Amarasinghe, A.; Ahmed-Hassan, H.; De Silva Senapathi, U.; Abdul-Careem, M.F. Hatchery Vaccination Against Poultry Viral Diseases: Potential Mechanisms and Limitations. Viral Immunol. 2018, 31, 23–33. [Google Scholar] [CrossRef]
- Yang, X.M. A review of combined immunization: Current research situation and its promising future. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 120–126. [Google Scholar] [CrossRef]
- Hilke, J.; Strobel, H.; Woelke, S.; Stoeter, M.; Voigt, K.; Grimm, L.; Meilwes, J.; Punsmann, T.; Blaha, I.; Salditt, A.; et al. A comparison of different vaccination schemes used in sheep combining inactivated bluetongue vaccines against serotypes 4 and 8. Vaccine 2019, 37, 5844–5853. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.; Park, K.H.; Yang, S.; Jeong, J.; Kang, I.; Park, C.; Chae, C. Evaluation of the efficacy of a trivalent vaccine mixture against a triple challenge with Mycoplasma hyopneumoniae, PCV2, and PRRSV and the efficacy comparison of the respective monovalent vaccines against a single challenge. BMC Vet. Res. 2019, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Bourry, O.; Fablet, C.; Simon, G.; Marois-Créhan, C. Efficacy of combined vaccination against Mycoplasma hyopneumoniae and porcine reproductive and respiratory syndrome virus in dually infected pigs. Vet. Microbiol. 2015, 180, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, D.; Riblet, S.M.; Newman, L.; Koopman, R.; Barbosa, T.; García, M. Evaluation of vaccination against infectious laryngotracheitis (ILT) with recombinant herpesvirus of turkey (rHVT-LT) and chicken embryo origin (CEO) vaccines applied alone or in combination. Avian Pathol. 2019, 48, 573–581. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmucker, S.S.; Bessei, W.; Grashorn, M.; Stefanski, V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals 2020, 10, 1138. [Google Scholar] [CrossRef]
- Yin, L.Y.; Wang, Z.Y.; Yang, H.M.; Xu, L.; Zhang, J.; Xing, H. Effects of stocking density on growth performance, feather growth, intestinal development, and serum parameters of geese. Poult. Sci. 2017, 96, 3163–3168. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, J.; Hou, Y.; Zhu, H.; Zhao, S.; Ding, B.; Yin, Y.; Yi, G.; Shi, J.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yi, D.; Hou, Y.; Ding, B.; Li, K.; Li, B.; Zhu, H.; Liu, Y.; Wu, G. Dietary Supplementation with α-Ketoglutarate Activates mTOR Signaling and Enhances Energy Status in Skeletal Muscle of Lipopolysaccharide-Challenged Piglets. J. Nutr. 2016, 146, 1514–1520. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Yi, D.; Li, Y.; Ding, B.; Zhu, H.; Liu, J.; Xiao, H.; Wu, G. Dietary supplementation with glutamate precursor α-ketoglutarate attenuates lipopolysaccharide-induced liver injury in young pigs. Amino Acids 2015, 47, 1309–1318. [Google Scholar] [CrossRef]
- Li, R.; Song, Z.; Zhao, J.; Huo, D.; Fan, Z.; Hou, D.X.; He, X. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers. Anim. Nutr. 2018, 4, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ohta, N.; Akiba, Y. Influences of dietary methionine and cysteine on metabolic responses to immunological stress by Escherichia coli lipopolysaccharide injection, and mitogenic response in broiler chickens. Br. J. Nutr. 1997, 78, 815–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Wang, L.; Zhang, W.; Yang, Z.; Ding, B.; Zhu, H.; Liu, Y.; Qiu, Y.; Yin, Y.; Wu, G. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Hou, Y.; Wang, L.; Ding, B.; Yang, Z.; Li, J.; Long, M.; Liu, Y.; Wu, G. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Br. J. Nutr. 2014, 111, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Aoki, A.; Takimoto, T.; Akiba, Y. Dietary supplementation of glycine modulates inflammatory response indicators in broiler chickens. Br. J. Nutr. 2008, 100, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.B.; Lee, J.W.; Huang, H.J.; Wang, C.H.; Lee, T.T.; Yen, H.T.; Yu, B. Effects of supplemental glutamine on growth performance, plasma parameters and LPS-induced immune response of weaned barrows after castration. Asian-Australas. J. Anim. Sci. 2012, 25, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Li, S.; Pi, D.; Zhu, H.; Hou, Y.; Shi, H.; Leng, W. Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br. J. Nutr. 2015, 114, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.; Yin, J.; Li, D.; Zhao, L.; Chen, X. Effects of dietary conjugated linoleic acid supplementation on performance and immune function of weaned pigs. Arch. Anim. Nutr. 2005, 59, 41–51. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, J.C.; Mullan, B.P.; Pluske, J.R.; Kim, I.H. Vitamin E and omega-3 fatty acids independently attenuate plasma concentrations of proinflammatory cytokines and prostaglandin E3 in Escherichia coli lipopolysaccharide-challenged growing-finishing pigs. J. Anim. Sci. 2015, 93, 2926–2934. [Google Scholar] [CrossRef] [Green Version]
- Yakah, W.; Ramiro-Cortijo, D.; Singh, P.; Brown, J.; Stoll, B.; Kulkarni, M.; Oosterloo, B.C.; Burrin, D.; Maddipati, K.R.; Fichorova, R.N.; et al. Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs. Nutrients 2021, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Korver, D.R.; Klasing, K.C. Dietary fish oil alters specific and inflammatory immune responses in chicks. J. Nutr. 1997, 127, 2039–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, L.F.; Neves Neto, J.T.; Pedrico, A.; Ferrazza, R.A.; Lima, F.S.; Bisinotto, R.S.; Martinez, N.; Garcia, M.; Ribeiro, E.S.; Gomes, G.C.; et al. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on performance and inflammatory responses to a lipopolysaccharide challenge in lactating Holstein cows. J. Dairy Sci. 2015, 98, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Xu, Z.P.; Wang, W.; Cao, J.B.; Fu, Q.; Zhao, W.X.; Li, Y.; Huo, X.L.; Zhang, L.M.; Li, Y.F.; et al. Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018, 65, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Eicher, S.D.; McKee, C.A.; Carroll, J.A.; Pajor, E.A. Supplemental vitamin C and yeast cell wall beta-glucan as growth enhancers in newborn pigs and as immunomodulators after an endotoxin challenge after weaning. J. Anim. Sci. 2006, 84, 2352–2360. [Google Scholar] [CrossRef]
- Zhao, G.P.; Han, M.J.; Zheng, M.Q.; Zhao, J.P.; Chen, J.L.; Wen, J. Effects of dietary vitamin E on immunological stress of layers and their offspring. J. Anim. Physio.l Anim. Nutr. 2011, 95, 343–350. [Google Scholar] [CrossRef]
- Niu, Z.Y.; Liu, F.Z.; Yan, Q.L.; Li, W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009, 88, 2101–2107. [Google Scholar] [CrossRef]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Yu, J.; Ma, C.; Zhuang, P.; Zeng, J.; Zhang, J.; Deng, G.; Wang, Y. Magnesium sulfate attenuates lipopolysaccharides-induced acute lung injury in mice. Chin. J Physiol. 2019, 62, 203–209. [Google Scholar] [CrossRef]
- Gao, F.; Ding, B.; Zhou, L.; Gao, X.; Guo, H.; Xu, H. Magnesium sulfate provides neuroprotection in lipopolysaccharide-activated primary microglia by inhibiting NF-κB pathway. J. Surg. Res. 2013, 184, 944–950. [Google Scholar] [CrossRef]
- Song, C.; Jiang, J.; Han, X.; Yu, G.; Pang, Y. Effect of immunological stress to neuroendocrine and gene expression in different swine breeds. Mol. Biol. Rep. 2014, 41, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhao, T.; Liu, L.; Jiao, H.; Lin, H. Effect of copper on antioxidant ability and nutrient metabolism in broiler chickens stimulated by lipopolysaccharides. Arch. Anim. Nutr. 2011, 65, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Abouelezz, K.F.M.; Li, L.; Gou, Z.; Wang, Y.; Lin, X.; Ye, J.; Jiang, S. Influence of Mushroom Polysaccharide, Nano-Copper, Copper Loaded Chitosan, and Lysozyme on Intestinal Barrier and Immunity of LPS-mediated Yellow-Feathered Chickens. Animals 2020, 10, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Practical aspects of the use of probiotics in pig production: A review. Livest. Sci. 2019, 223, 84–96. [Google Scholar] [CrossRef]
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18(+) in pigs. Anim. Nutr. 2021, 7, 326–333. [Google Scholar] [CrossRef]
- Gadde, U.D.; Oh, S.; Lee, Y.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017, 114, 236–243. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, T.; Yi, D.; Wang, L.; Li, P.; Zhang, J.; Hou, Y.; Wu, G. Dietary Supplementation with Lactobacillus casei Alleviates Lipopolysaccharide-Induced Liver Injury in a Porcine Model. Int. J. Mol. Sci. 2017, 18, 2535. [Google Scholar] [CrossRef] [Green Version]
- Burdick Sanchez, N.C.; Carroll, J.A.; Broadway, P.R.; Bass, B.E.; Frank, J.W. Supplementation of a Lactobacillus acidophilus fermentation product can attenuate the acute phase response following a lipopolysaccharide challenge in weaned pigs. Animal 2019, 13, 144–152. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Chen, Q.; Yang, L.; Yin, J.; Li, Y.; Huang, X. Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor-Bruton’s Tyrosine Kinase-Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide. Antioxidants 2021, 10, 468. [Google Scholar] [CrossRef]
- Wang, K.; Chen, G.; Cao, G.; Xu, Y.; Wang, Y.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, intestinal structure, and inflammation in lipopolysaccharide-challenged weaned piglets. J. Anim. Sci. 2019, 97, 4140–4151. [Google Scholar] [CrossRef]
- Fan, C.; Han, J.; Liu, X.; Zhang, F.; Long, Y.; Xie, Q. Modulation of hypoxia-inducible factor-1α/cyclo-oxygenase-2 pathway associated with attenuation of intestinal mucosa inflammatory damage by Acanthopanax senticosus polysaccharides in lipopolysaccharide-challenged piglets. Br. J. Nutr. 2019, 122, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Bian, L.; Liu, X.; Zhang, F.; Zhang, Y.; Yu, N. Effects of Acanthopanax senticosus Polysaccharide Supplementation on Growth Performance, Immunity, Blood Parameters and Expression of Pro-inflammatory Cytokines Genes in Challenged Weaned Piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamboh, A.A.; Zhu, W.Y. Individual and combined effects of genistein and hesperidin on immunity and intestinal morphometry in lipopolysacharide-challenged broiler chickens. Poult. Sci. 2014, 93, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Muhammad, T.; Ikram, M.; Kim, M.O. Dietary Supplementation of the Antioxidant Curcumin Halts Systemic LPS-Induced Neuroinflammation-Associated Neurodegeneration and Memory/Synaptic Impairment via the JNK/NF-κB/Akt Signaling Pathway in Adult Rats. Oxid. Med. Cell Longev. 2019, 2019, 7860650. [Google Scholar] [CrossRef] [Green Version]
- Lu, N.; Li, X.; Yu, J.; Li, Y.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Curcumin Attenuates Lipopolysaccharide-Induced Hepatic Lipid Metabolism Disorder by Modification of m(6) A RNA Methylation in Piglets. Lipids 2018, 53, 53–63. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tsai, M.H.; Sheu, W.H.; Hsieh, S.C.; Chiang, A.N. The therapeutic potential and mechanisms of action of quercetin in relation to lipopolysaccharide-induced sepsis in vitro and in vivo. PLoS ONE 2013, 8, e80744. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, G.; Liang, X.; Wang, M.; Zhu, X.; Luo, Y.; Shang, Y.; Yang, J.Q.; Zhou, P.; Gu, X.L. Effects of berberine on the growth performance, antioxidative capacity and immune response to lipopolysaccharide challenge in broilers. Anim. Sci. J. 2019, 90, 1229–1238. [Google Scholar] [CrossRef]
- Heim, G.; O’Doherty, J.V.; O’Shea, C.J.; Doyle, D.N.; Egan, A.M.; Thornton, K.; Sweeney, T. Maternal supplementation of seaweed-derived polysaccharides improves intestinal health and immune status of suckling piglets. J. Nutr. Sci. 2015, 4, e27. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica Polysaccharide Alleviated Lipopolysaccharide-induced Oxidative Stress of Broilers via Nrf2/Keap1 and TLR4/NF-κB Pathway. Ecotoxicol. Environ. Saf. 2021, 223, 112566. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.X.; Shao, Q.; Chen, W.B.; Ma, L.; Xu, W.H.; Li, Y.X.; Huang, S.C.; Ma, Y.B. Effects of Glycyrrhiza polysaccharide in diet on growth performance, serum antioxidant capacity, and biochemistry of broilers. Poult. Sci. 2021, 100, 100927. [Google Scholar] [CrossRef]
- Jiang, J.; Qi, L.; Lv, Z.; Jin, S.; Wei, X.; Shi, F. Dietary Stevioside Supplementation Alleviates Lipopolysaccharide-Induced Intestinal Mucosal Damage through Anti-Inflammatory and Antioxidant Effects in Broiler Chickens. Antioxidants 2019, 8, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Wang, Y.Q.; Qi, Y.X. Influence of procyanidin supplementation on the immune responses of broilers challenged with lipopolysaccharide. Anim. Sci. J. 2017, 88, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Yashaswini, P.S.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. In vivo modulation of LPS induced leukotrienes generation and oxidative stress by sesame lignans. J. Nutr. Biochem. 2017, 41, 151–157. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Qiao, Q.; Sun, Y.; Liu, Q.; Ren, B.; Liu, X. Sesamol supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of nuclear factor kappaB. Mol. Nutr. Food. Res. 2017, 61, 1600734. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Wang, T.; Cai, M.; Qian, F.; Sun, Y.; Wang, Y. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019, 10, 3898–3908. [Google Scholar] [CrossRef]
- Liu, S.D.; Song, M.H.; Yun, W.W.; Lee, J.H.; Kim, H.B.; Cho, J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019, 98, 2026–2033. [Google Scholar] [CrossRef]
| Type | Name | Structural Formula | Mechanism of Mitigating IS | Related Literature |
|---|---|---|---|---|
| Polysaccharides | Acanthopanax senticosus polysaccharide | mixture | Downregulates the HIF-1α/COX-2 pathway and NF-κB to inhibit the release of inflammatory cytokines, increases the activity of diamine oxidase and lactase, improves intestinal morphology, increases GH and IGF-I. | [152,153] |
| Astragalus polysaccharides | mixture | Inhibits the TLR4/NF-κB signaling pathway, downregulates inflammatory cytokines, and upregulates intestinal tight junction proteins. | [16] | |
| Ginseng polysaccharides | mixture | Inhibits the TLR4/NF-κB signaling pathway, downregulates inflammatory cytokines, upregulates intestinal tight junction proteins. | [16] | |
| Seaweed polysaccharides | mixture | Downregulates IL-1β and IL-6, increases breast milk IgG content, improves intestinal morphology. | [159] | |
| Artemisia ordosica polysaccharide | mixture | Reduces LPS-induced oxidative stress by inhibiting Nrf2/Keap1 and TLR4/NF-κB pathways. | [160] | |
| Glycyrrhiza polysaccharide | mixture | Downregulates the expression of IL-1β and IFN-γ, increases SOD activity, reduces MDA content. | [161] | |
| Glycosides | Stevioside | 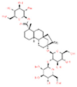 | Reduces MDA content and increases antioxidant enzyme activity. | [162] |
| Salidroside |  | Reduces the levels of NE and 5-HT in the prefrontal cortex, upregulates the BDNF/TrkB signaling pathway, inhibits inflammatory cytokines. | [163] | |
| Hesperidin | 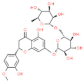 | Enhances the activity of monocytes and macrophages, enhances the ratio of intestinal V/C. | [154] | |
| Phenols | Procyanidin | mixture | Inhibits the activities of inflammatory factors (IFN-γ, IL-1β, IL-2, IL-4, IL-6, and IL-10) and nitrogen oxides (NOx). | [164] |
| Thymol | 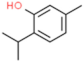 | Enhances the barrier function of epithelial cells, reduces the production of ROS and expression of proinflammatory cytokine genes. | [78] | |
| Sesamol | 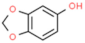 | Reduces MCP-1, inhibits NF-κB and inflammatory cytokines TNF-α and IL-1β, prevents lipid peroxidation in serum and liver, increases catalase and glutathione reductase activities. | [165,166] | |
| Curcumin |  | Regulates the JNK/NF- κ B/Akt signaling pathway, plays an anti-inflammatory and antioxidant effect, may alleviate liver damage and liver lipid metabolism disorders by increasing m6A RNA methylation. | [155,156] | |
| Green tea polyphenols | mixture | Inhibits NF-κB signaling and inhibit NLRP3 inflammasome activation. | [167] | |
| Carvacrol | 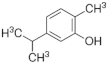 | Downregulates the expression of inflammatory cytokines by inhibiting the TLRs/NF-κB pathway. | [168] | |
| Flavonoids | Artemisia argyi flavonoids | mixture | Decreases the expression of NF-κB, IL-1β, and IL-6. | [45] |
| Quercetin | 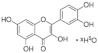 | Inhibits the activity of IκBα and NF-κB into the nucleus, reduces the expression of TNF-α and IL-1β. | [157] | |
| Genistein | 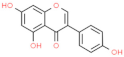 | Enhances the activity of monocytes and macrophages, increases the ratio of intestinal V/C. | [154] | |
| Esters | Ellagic Acid | 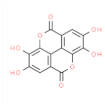 | Antioxidant activity, inhibits AChE activity. | [27] |
| Sesame lignans | 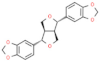 | Reduces MCP-1, inflammatory cytokines TNF-α and IL-1β, prevents lipid peroxidation. | [165] | |
| Alkaloids | Berberine | 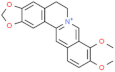 | Inhibits NF-κB signal transduction and the expression of inflammatory mediators, enhances the activity of antioxidant enzymes. | [158] |
| Crude extract | Artemisia argyi aqueous extract | mixture | Inhibits the release of CORT and IL-2. | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Ding, Y.; Chen, S.; Gooneratne, R.; Ju, X. Effect of Immune Stress on Growth Performance and Immune Functions of Livestock: Mechanisms and Prevention. Animals 2022, 12, 909. https://doi.org/10.3390/ani12070909
Niu X, Ding Y, Chen S, Gooneratne R, Ju X. Effect of Immune Stress on Growth Performance and Immune Functions of Livestock: Mechanisms and Prevention. Animals. 2022; 12(7):909. https://doi.org/10.3390/ani12070909
Chicago/Turabian StyleNiu, Xueting, Yuexia Ding, Shengwei Chen, Ravi Gooneratne, and Xianghong Ju. 2022. "Effect of Immune Stress on Growth Performance and Immune Functions of Livestock: Mechanisms and Prevention" Animals 12, no. 7: 909. https://doi.org/10.3390/ani12070909
APA StyleNiu, X., Ding, Y., Chen, S., Gooneratne, R., & Ju, X. (2022). Effect of Immune Stress on Growth Performance and Immune Functions of Livestock: Mechanisms and Prevention. Animals, 12(7), 909. https://doi.org/10.3390/ani12070909





