Molecular Characterization of Embryos with Different Buoyancy Levels in the Yellowtail Kingfish (Seriola lalandi)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Design
2.3. Batches and Sample Characterization
2.4. RNA Isolation and Real-Time Polymerase Chain Reaction (qPCR)
2.5. Enzymatic Activity Assays
2.6. Free Amino Acid (FAA) Determination
2.7. Statistical Analysis
3. Results
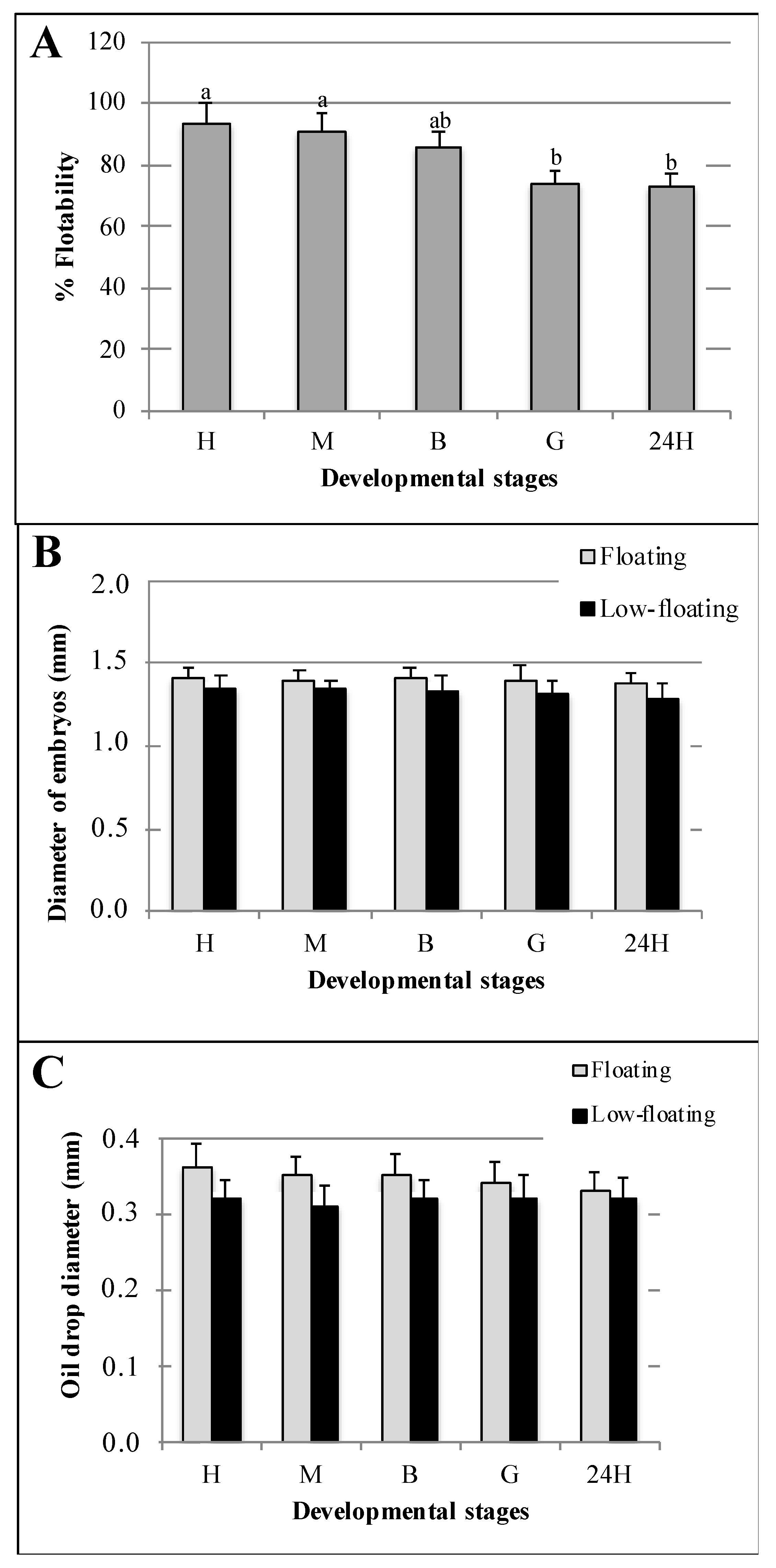
3.1. Characterization of Batches and Samples
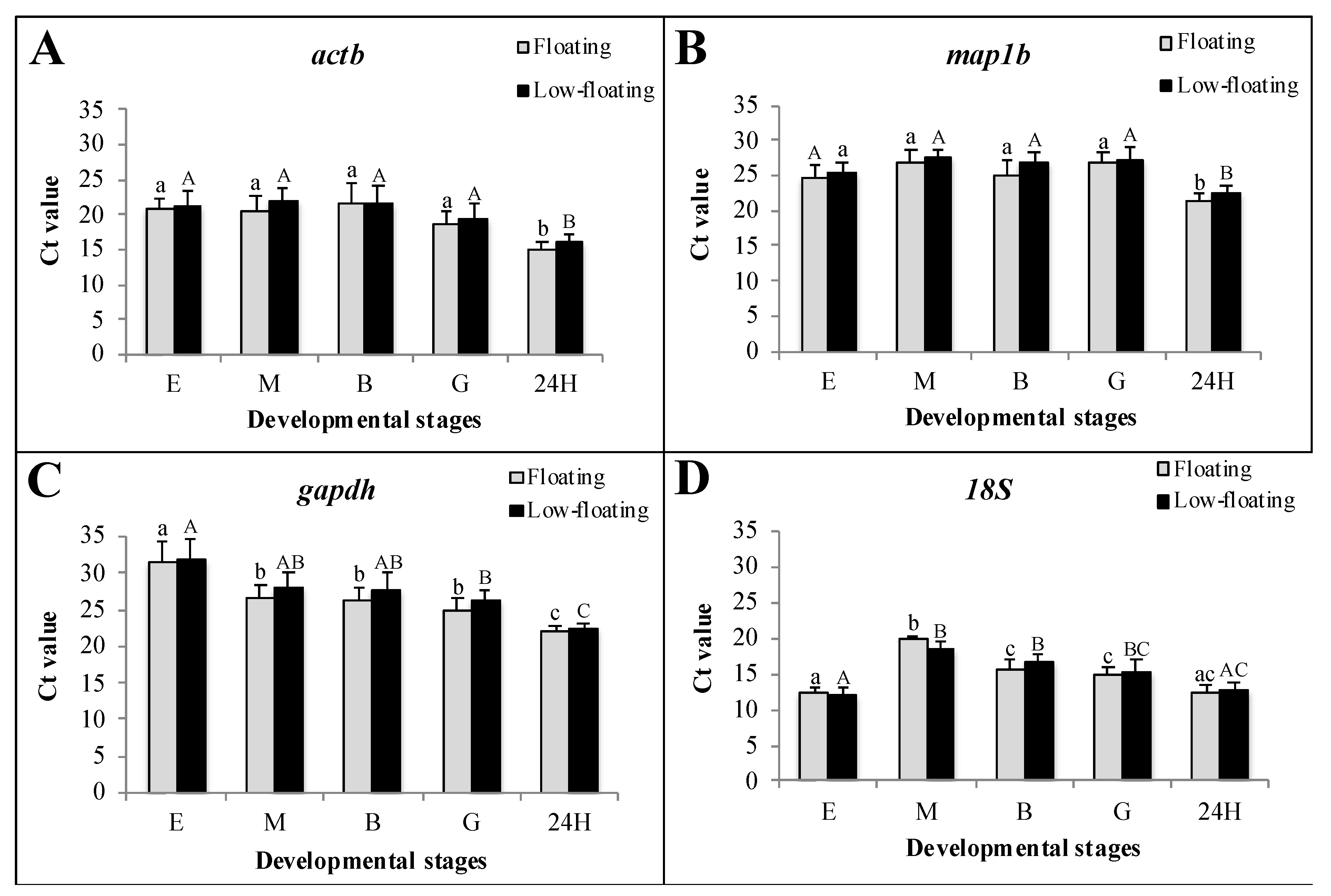
3.2. Transcriptional Status of the Samples
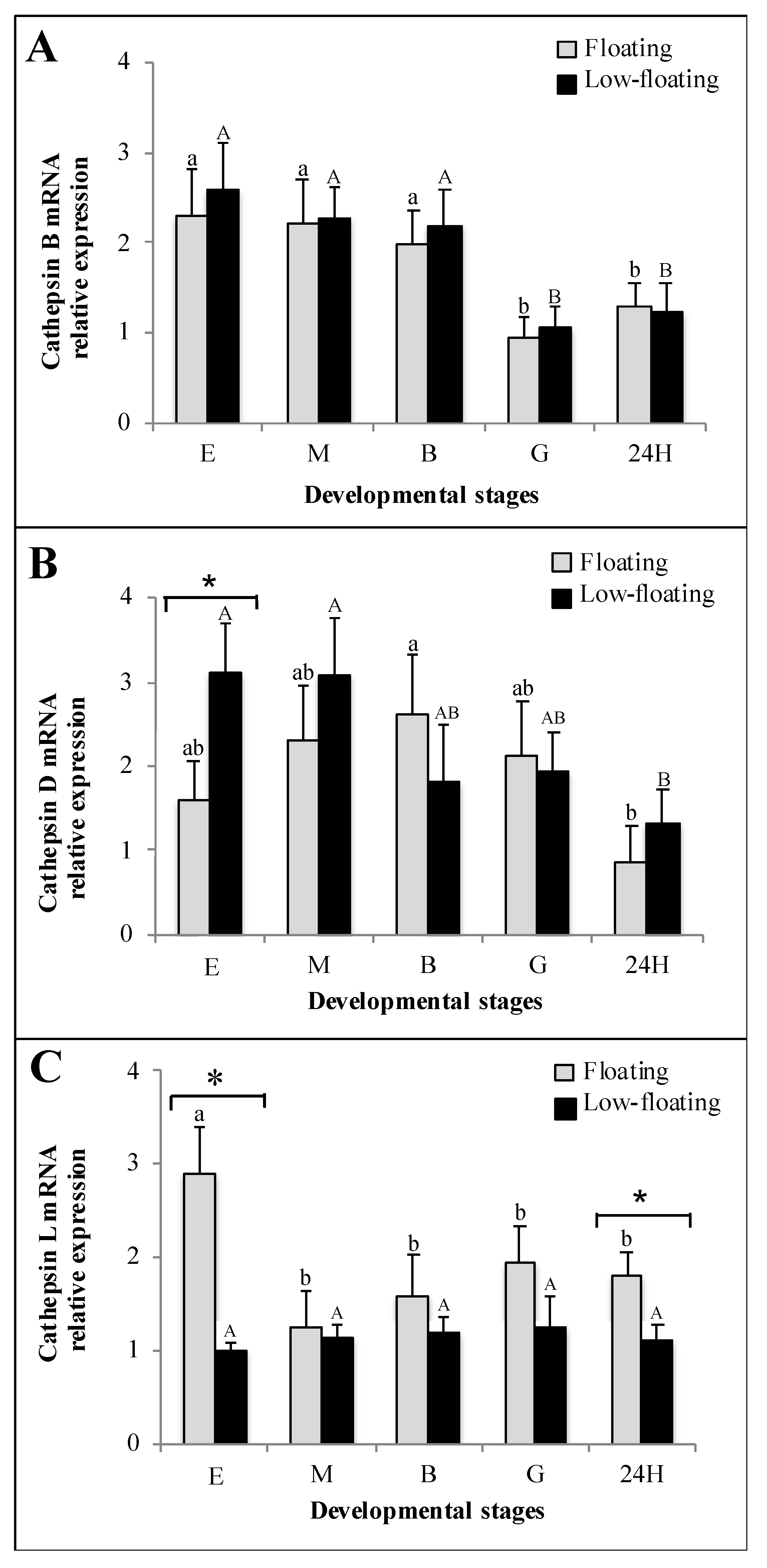
3.3. Cathepsin mRNA Expression Analysis
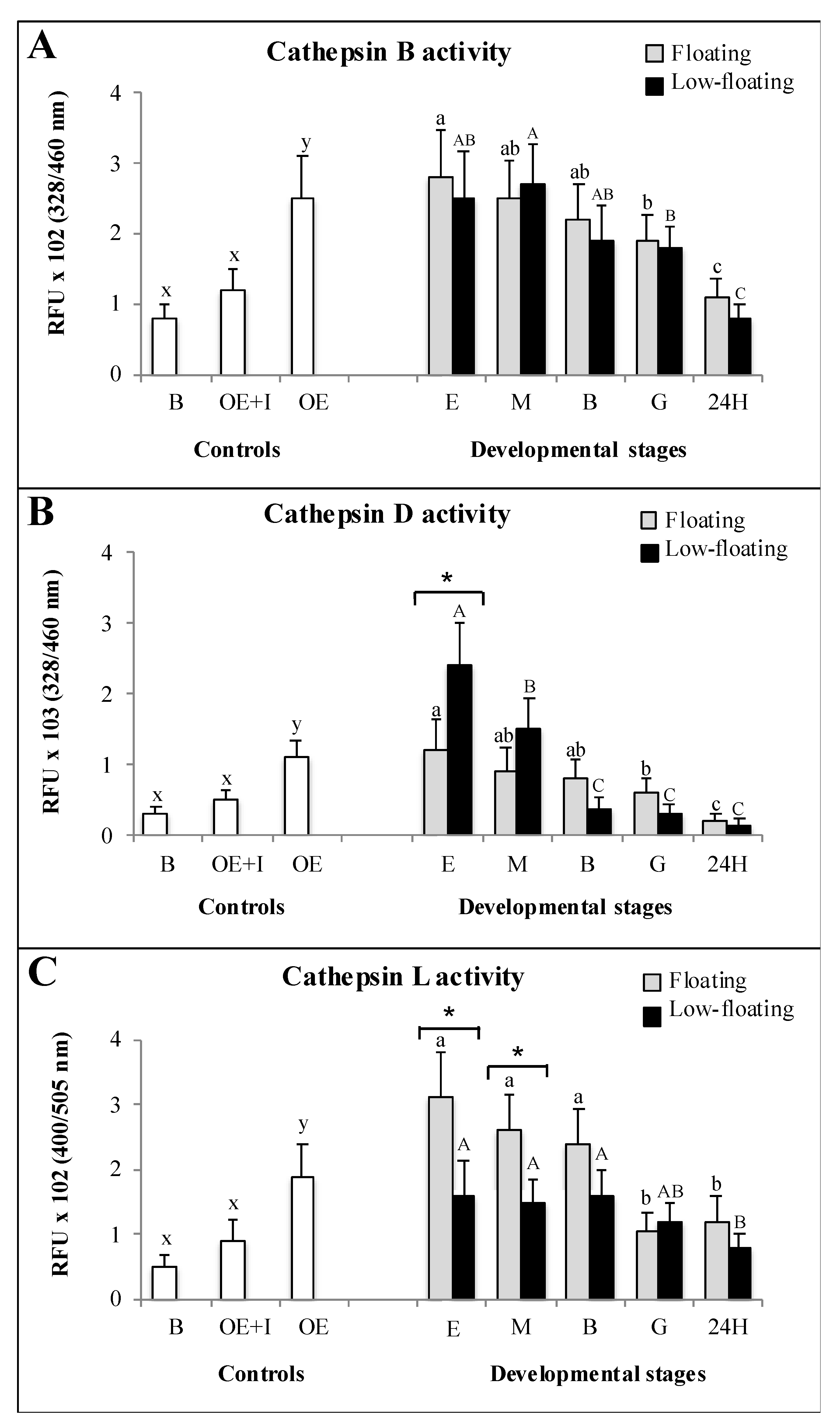
3.4. Enzymatic Activity
3.5. FAA Composition
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50 (Suppl. S1), S195–S219. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, S.; Kagawa, H.; Ohkubo, N.; Hiramatsu, N.; Sullivan, C.V.; Matsubara, T. Molecular characterization of three forms of vitellogenin and their yolk protein products during oocyte growth and maturation in red seabream (Pagrus major), a marine teleost spawning pelagic eggs. Mol. Reprod. Dev. 2006, 73, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdá, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Polzonetti-Magni, A.M.; Mosconi, G.; Soverchia, L.; Kikuyama, S.; Carnevali, O. Multihormonal control of vitellogenesis in lower vertebrates. Int. Rev. Cytol. 2004, 239, 1–46. [Google Scholar] [CrossRef]
- Wang, H.; Tan, J.T.; Emelyanov, A.; Korzh, V.; Gong, Z. Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio. Gene 2005, 356, 91–100. [Google Scholar] [CrossRef]
- Carnevali, O.; Mosconi, G.; Cambi, A.; Ridolfi, S.; Zanuy, S.; Polzonetti-Magni, A.M. Changes of lysosomal enzyme activities in sea bass (Dicentrarchus labrax) eggs and developing embryos. Aquaculture 2001, 202, 249–256. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Prat, F.; Randall, C.; Tyler, C. Molecular characterization of putative yolk processing enzymes and their expression during oogenesis and embryogenesis in rainbow trout (Oncorhynchus mykiss). Biol. Reprod. 2001, 65, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.; Herrera, G.; Dettleff, P.; Patel, A.; Torres-Fuentes, J.L.; Martínez, V. Assessment of cathepsin mRNA expression and enzymatic activity during early embryonic development in the yellowtail kingfish Seriola lalandi. Anim. Reprod. Sci. 2017, 80, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, I.; Fyhn, H.J. Metabolic aspects of free amino acids in developing marine fish eggs and larvae. Rev. Fish. Sci. 1993, 1, 239–259. [Google Scholar] [CrossRef]
- Carnevali, O.; Carletta, R.; Cambi, A.; Vita, A.; Bromage, N. Yolk formation and degradation during oocyte maturation in seabream Sparus aurata: Involvement of two lysosomal proteinases. Biol. Reprod. 1999, 60, 140–146. [Google Scholar] [CrossRef]
- Fabra, M.; Raldúa, D.A.; Power, P.M.T.; Deen, J.; Cerdà, J. Marine fish egg hydration is aquaporin mediated. Science 2005, 307, 545. [Google Scholar] [CrossRef]
- Carnevali, O.; Cionna, C.; Tosti, L.; Lubzens, E.; Maradonna, F. Role of cathepsins in ovarian follicle growth and maturation. Gen. Comp. Endocrinol. 2006, 146, 195–203. [Google Scholar] [CrossRef]
- Sundby, S.; Kristiansen, T. The principles of buoyancy in marine fish eggs and their vertical distributions across the world oceans. PLoS ONE 2015, 10, e0138821. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Wamboldt, M.; Fyhn, H.J. Differential processing of yolk proteins during oocyte hydration in marine fishes (Labridae) that spawn benthic and pelagic eggs. Mar. Ecol. Prog. Ser. 2002, 237, 217–226. [Google Scholar] [CrossRef]
- Carnevali, O.; Centonze, F.; Brooks, S.; Marota, I.; Sumpter, Y.J.P. Molecular cloning and expression of ovarian cathepsin D in sea bream Sparus aurata. Biol. Reprod. 1999, 61, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, O.; Mosconi, G.; Cardinali, M.; Meiri, I.; Polzoneti-Magni, A. Molecular components related to egg viability in the gilthead sea bream, Sparus aurata. Mol. Reprod. Dev. 2001, 58, 330–335. [Google Scholar] [CrossRef]
- Seoka, M.; Yamada, S.; Iwata, Y.; Yanagisawa, T.; Nakagawa, T.; Kumai, H. Differences in the biochemical content of buoyant and non-buoyant eggs of the Japanese eel, A. japonica. Aquaculture 2003, 216, 355–362. [Google Scholar] [CrossRef]
- Jung, K.M.; Folkvord, A.; Kjesbu, O.S.; Sundby, S. Experimental parameterization of principal physics in buoyancy variations of marine teleost eggs. PLoS ONE 2014, 9, e104089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samaee, S.M.; Mente, E.; Estevez, A.; Gimenez, G.; Lahnsteiner, F. Embryo and larva development in common dentex (Dentex dentex), a pelagophil teleost: The quantitative composition of egg-free amino acids and their interrelations. Theriogenology 2010, 73, 909–919. [Google Scholar] [CrossRef]
- Moran, D.; Smith, C.; Gara, B.; Poortenaar, C. Reproductive behavior and early development in yellowtail kingfish (Seriola lalandi Valenciennes 1833). Aquaculture 2007, 262, 95–104. [Google Scholar] [CrossRef]
- Orellana, J.; Waller, U.; Wecker, B. Culture of yellowtail kingfish (Seriola lalandi) in a marine recirculating aquaculture system (RAS) with artificial seawater. Aquac. Eng. 2014, 58, 20–28. [Google Scholar] [CrossRef]
- Sanchís-Benlloch, P.J.; Nocillado, J.; Ladisa, C. In-vitro and in vivo biological activity of recombinant yellowtail kingfish (Seriola lalandi) follicle stimulating hormone. Gen. Comp. Endocrinol. 2017, 241, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Poortenaar, C.W.; Hooker, S.H.; Sharp, N. Assessment of yellowtail kingfish Seriola lalandi reproductive physiology, as a basis for aquaculture development. Aquaculture 2001, 201, 271–286. [Google Scholar] [CrossRef]
- Palomino, J.; Gómez, C.; Otarola, M.; Dettleff, P.; Patiño-García, D.; Orellana, R.; Moreno, R.D. Embryo buoyancy and cell death markers in yellowtail kingfish (Seriola lalandi Valenciennes 1833) during early embryogenesis. Front. Cell. Dev. Biol. 2021, 9, 630947. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.G.; Hur, S.W.; Ji, S.C. Morphological development of embryo, larvae and juvenile in yellowtail kingfish, Seriola lalandi. Dev. Reprod. 2016, 20, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S. Quantification of mRNA using real-time reverse transcription (RT-qPCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Sokal, R.R. Biometry: The Principles and Practice of Statistic in Biological Research; W. H. Freeman: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Jung, K.M.; Folkvord, A.; Kjesbu, O.S.; Agnalt, A.L.; Thorsen, A.; Sundby, S. Egg buoyancy variability in local populations of Atlantic cod (Gadus morhua). Mar. Biol. 2012, 159, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Álvarez, A.; Palomera, I.; Parada, C. Changes in egg buoyancy during development and its effects on the vertical distribution of anchovy eggs. Fish. Res. 2012, 117–118, 86–95. [Google Scholar] [CrossRef]
- Adlandsvik, B.; Coombs, S.; Sundby, S.; Temple, G. Buoyancy and vertical distribution of eggs and larvae of blue whiting (Micromesistius poutassou): Observations and modelling. Fish. Res. 2001, 50, 59–72. [Google Scholar] [CrossRef]
- Pérez-Robles, J.; Diaz, F.; Ibarra-Castro, L.; Giffard-Mena, I.; Re, A.D.; Ibarra, L.E.R.; Soto, J.A.I. Effects of salinity on osmoregulation during the embryonic development of the bullseye puffer (Sphoeroides annulatus Jenyns 1842). Aquac. Res. 2014, 47, 838–846. [Google Scholar] [CrossRef]
- Riis-Vestergaard, J. Physiology of teleost embryos related to environmental challenges. Sarsia 1987, 72, 351–358. [Google Scholar] [CrossRef]
- Finn, R.N.; Rønnestad, I. The effect of acute changes in temperature on the aerobic metabolism of embryos and yolk-sac larvae of turbot (Scophthalmus maximus). Can. J. Fish. Aquat. Sci. 2003, 60, 1324–1331. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Carnevali, O.; Cerdà, J. Cathepsin B differential expression and enzyme processing and activity during Fundulus heteroclitus embryogenesis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.H.; Kim, H.K.; Baek, H.J.; Lee, Y.-W.; Kwon, J.Y. Cathepsin B & D and the survival of early embryos in Red Spotted Grouper, Ephinephelus akaara. Dev. Reprod. 2017, 21, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.; Thomé, R.; Santos, H.; Bazzoli, N.; Rizzo, E. Relationship between bcl-2, bax, beclin-1, and cathepsin-D proteins during postovulatory follicular regression in fish ovary. Theriogenology 2016, 85, 1118–1131. [Google Scholar] [CrossRef]
- Carnevali, O.; Polzonetti, V.; Cardinali, M.; Pugnaloni, A.; Natalini, P.; Zmora, N.; Mosconi, G.; Polzonetti-Magni, A. Apoptosis in sea bream Sparus aurata eggs. Mol. Reprod. Dev. 2003, 66, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, N.; Matsubara, T. Sequential utilization of free amino acids, yolk proteins and lipids in developing eggs and yolk-sac larvae of barfin flounder Verasper moseri. Mar. Biol. 2002, 140, 187–196. [Google Scholar] [CrossRef]
- Moran, D.; Gara, B.; Wells, R. Energetics and metabolism of yellowtail kingfish (Seriola lalandi Valenciennes 1833) during embryogenesis. Aquaculture 2007, 265, 359–369. [Google Scholar] [CrossRef]
- Finn, R.N.; Fyhn, H.J. Requirement for amino acids in ontogeny of fish. Aquac. Res. 2010, 41, 684–716. [Google Scholar] [CrossRef]
- Rønnestad, I.; Fyhn, H.J.; Gravningen, K. The importance of free amino acids to the energy metabolism of eggs and larvae of turbot (Scophthalmus maximus). Mar. Biol. 1992, 114, 517–525. [Google Scholar] [CrossRef]
- Rayner, T.A.; Hwang, J.-S.; Hansen, B.W. Anticipating the free amino acid concentrations in newly hatched pelagic fish larvae based on recently fertilized eggs and temperature. J. Plankton Res. 2017, 39, 1012–1019. [Google Scholar] [CrossRef]
- Rønnestad, I.; Robertson, R.; Fyhn, H.J. Free amino acids and protein content in pelagic and demersal eggs of tropical marine fishes. In The Fish Egg; MacKinlay, D.D., Eldridge, M., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 81–84. [Google Scholar]
- Rønnestad, I.; Finn, R.N.; Groot, E.P.; Fyhn, H.J. Utilization of free amino acids related to energy metabolism of developing eggs and larvae of lemon sole Microstomus kitt reared in the laboratory. Mar. Ecol. Prog. Ser. 1992, 8, 195–205. [Google Scholar] [CrossRef]

| Gene | Primer Sequences (5′-3′) | TM (°C) | Efficiency | Amplicon (bp) |
|---|---|---|---|---|
| catb | F: GTAATGGTGGCTACCCTTCA | 54.3 | 1.97 | 279 |
| R: CATACTGAATCTGCTCCTCG | 52.5 | |||
| catd | F: GCCAAGTCCAGCACATACG | 56.3 | 2.19 | 215 |
| R: ACAGAGATGCGTGGGTAGG | 56.6 | |||
| catl | F: ACTACAACTCTGCCAACGAC | 54.8 | 1.97 | 165 |
| R: AACTGGAAAGACTCGTGACC | 54.6 | |||
| actb | F: AGGGAAATCGTGCGTGACAT | 57 | 2.04 | 563 |
| R: GCTGAAGTTGTTGGGCGTTT | 56.7 | |||
| map1b | F: TCATCAAGATTATCAGGAGGCG | 54.3 | 1.98 | 158 |
| R: GGAAGCATACACCATGTAGAGG | 55 | |||
| gapdh | F: CCCTTCATCGACCTGGAGTA | 55.8 | 1.99 | 459 |
| R: GAGCAGAGGCCTTCTCAATG | 55.6 | |||
| 18S | F: GCTCGTAGTTGGATCTCGGG | 57.3 | 2.01 | 597 |
| R: GGTGAGGTTTCCCGTGTTGA | 57.5 |
| Floating Developing Embryos | Low-Floating Developing Embryos | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | M | B | G | 24H | H | M | B | G | 24H | |
| Nonessential FAAs | ||||||||||
| Aspartic acid | 0.71 ± 0.06 | 0.55 ± 0.04 | 0.53 ± 0.02 | 0.49 ± 0.03 | 0.41 ± 0.02 | 0.43 ± 0.02 | 0.26 ± 0.03 | 0.50 ± 0.03 | 0.44 ± 0.03 | 0.39 ± 0.02 |
| Glutamic acid | 1.91 ± 0.11 | 1.77 ± 0.09 | 1.56 ± 0.15 | 1.45 ± 0.13 | 1.12 ± 0.08 | 0.96 ± 0.09 | 1.06 ± 0.02 | 1.06 ± 0.08 | 0.95 ± 0.10 | 0.81 ± 0.09 |
| Serine | 7.37 ± 1.02 a | 7.38 ± 0.96 a | 6.64 ± 0.82 ab | 6.42 ± 0.87 ab | 5.21 ± 0.49 bc | 4.91 ± 0.40 c | 4.12 ± 0.34 d | 4.61 ± 0.55 cd | 4.75 ± 0.58 cd | 4.80 ± 0.64 cd |
| Gycine | 2.43 ± 0.42 | 2.38 ± 0.39 | 2.31 ± 0.45 | 2.16 ± 0.34 | 1.54 ± 0.21 | 1.62 ± 0.15 | 1.29 ± 0.07 | 1.52 ± 0.11 | 1.62 ± 0.16 | 1.40 ± 0.09 |
| Alanine | 9.57 ± 2.35 a | 8.44 ± 2.54 a | 7.68 ± 1.87 a | 7.73 ± 2.21 a | 7.03 ± 1.95 ab | 5.68 ± 0.75 b | 4.72 ± 0.63 b | 5.21 ± 0.75 b | 5.95 ± 0.85 b | 5.35 ± 0.85 b |
| Proline | 1.82 ± 0.14 | 1.77 ± 0.12 | 1.67 ± 0.14 | 1.63 ± 0.08 | 1.41 ± 0.09 | 1.26 ± 0.10 | 1.09 ± 0.06 | 1.14 ± 0.09 | 1.22 ± 0.08 | 1.06 ± 0.07 |
| Cysteine | 0.60 ± 0.03 | 0.41 ± 0.03 | 0.28 ± 0.03 | 0.16 ± 0.02 | 0.14 ± 0.02 | 0.07 ± 0.02 | 0.29 ± 0.09 | 0.15 ± 0.03 | 0.12 ± 0.02 | 0.11 ± 0.02 |
| ∑ NEFAAs | 24.41 ± 4.13 a | 22.00 ± 4.20 ab | 20.67 ± 3.21 ab | 20.04 ± 3.68 ab | 16.86 ± 2.86 bc | 14.92 ± 1.53 c | 12.80 ± 1.24 c | 14.20 ± 1.64 c | 15.04 ± 1.82 c | 13.93 ± 1.78 c |
| Essential FAAs | ||||||||||
| Histidine | 1.43 ± 0.07 | 1.38 ± 0.09 | 1.33 ± 0.07 | 1.26 ± 0.05 | 0.86 ± 0.04 | 0.89 ± 0.02 | 0.77 ± 0.02 | 0.89 ± 0.03 | 0.94 ± 0.05 | 0.81 ± 0.02 |
| Arginine | 3.36 ± 0.38 | 3.35 ± 0.41 | 3.10 ± 0.35 | 2.98 ± 0.28 | 2.22 ± 0.20 | 2.23 ± 0.22 | 1.81 ± 0.12 | 2.06 ± 0.18 | 2.21 ± 0.20 | 2.14 ± 0.21 |
| Threonine | 3.11 ± 0.25 | 3.17 ± 0.30 | 2.79 ± 0.25 | 2.72 ± 0.28 | 2.24 ± 0.22 | 2.10 ± 0.17 | 1.92 ± 0.10 | 2.02 ± 0.20 | 2.13 ± 0.17 | 1.94 ± 0.09 |
| Tyrosine | 1.82 ± 0.18 | 1.50 ± 0.17 | 1.43 ± 0.15 | 1.39 ± 0.08 | 1.14 ± 0.09 | 1.11 ± 0.06 | 0.99 ± 0.06 | 0.97 ± 0.08 | 1.07 ± 0.05 | 0.95 ± 0.06 |
| Valine | 5.15 ± 0.65 | 5.09 ± 0.60 | 4.62 ± 0.54 | 4.44 ± 0.41 | 4.23 ± 0.33 | 3.37 ± 0.35 | 3.22 ± 0.30 | 3.51 ± 0.39 | 3.41 ± 0.39 | 3.26 ± 0.38 |
| Methionine | 1.70 ± 0.12 | 1.70 ± 0.15 | 1.45 ± 0.12 | 1.34 ± 0.09 | 0.99 ± 0.05 | 1.00 ± 0.05 | 1.04 ± 0.04 | 0.98 ± 0.07 | 0.94 ± 0.05 | 0.72 ± 0.04 |
| Isoleucine | 5.18 ± 0.77 | 4.97 ± 0.85 | 4.44 ± 0.83 | 4.30 ± 0.63 | 4.17 ± 0.45 | 3.46 ± 0.41 | 3.21 ± 0.35 | 4.14 ± 0.50 | 3.55 ± 0.45 | 3.13 ± 0.38 |
| Leucine | 7.82 ± 1.55 a | 7.77 ± 1.98 a | 7.44 ± 2.04 a | 6.69 ± 1.60 ab | 6.07 ± 1.34 ab | 5.08 ± 1.24 b | 4.88 ± 0.58 b | 5.07 ± 1.54 b | 5.24 ± 1.47 b | 4.78 ± 0.55 b |
| Phenylalanine | 2.57 ± 0.16 | 2.13± 0.15 | 2.09 ± 0.16 | 2.03 ± 0.12 | 1.76 ± 0.12 | 1.55 ± 0.06 | 1.31 ± 0.07 | 1.96 ± 0.14 | 1.50 ± 0.06 | 1.43 ± 0.06 |
| Lysine | 7.02 ± 1.56 a | 6.35 ± 1.36 a | 6.16 ± 1.56 a | 5.74 ± 1.46 ab | 5.27 ± 1.20 ab | 4.46 ± 0.94 bc | 3.68 ± 0.45 c | 4.60 ± 1.35 bc | 4.16 ± 0.47 bc | 4.14 ± 0.48 bc |
| Total EFAAs | 39.16 ± 5.69 a | 37.40 ± 6.06 ab | 34.85 ± 6.07 ab | 32.89 ± 5.01 ab | 28.95 ± 4.14 bc | 25.25 ± 3.48 cd | 22.82 ± 2.09 cd | 26.21 ± 5.02 cd | 25.15 ± 3.36 cd | 23.29 ± 2.27 d |
| Total FAAs | 63.57 ± 9.82 a | 59.40 ± 10.26 ab | 55.52 ± 9.28 ab | 52.93 ± 8.69 ab | 45.85 ± 7.00 bc | 40.17 ± 5.01 c | 35.62 ± 3.33 c | 40.41 ± 6.66 c | 40.19 ± 5.18 c | 37.22 ± 4.05 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettleff, P.; Rodríguez, J.; Patiño-García, D.; Orellana, R.; Castro, R.; Escobar-Aguirre, S.; Moreno, R.D.; Palomino, J. Molecular Characterization of Embryos with Different Buoyancy Levels in the Yellowtail Kingfish (Seriola lalandi). Animals 2022, 12, 720. https://doi.org/10.3390/ani12060720
Dettleff P, Rodríguez J, Patiño-García D, Orellana R, Castro R, Escobar-Aguirre S, Moreno RD, Palomino J. Molecular Characterization of Embryos with Different Buoyancy Levels in the Yellowtail Kingfish (Seriola lalandi). Animals. 2022; 12(6):720. https://doi.org/10.3390/ani12060720
Chicago/Turabian StyleDettleff, Phillip, Javiera Rodríguez, Daniel Patiño-García, Renan Orellana, Rodrigo Castro, Sebastián Escobar-Aguirre, Ricardo Daniel Moreno, and Jaime Palomino. 2022. "Molecular Characterization of Embryos with Different Buoyancy Levels in the Yellowtail Kingfish (Seriola lalandi)" Animals 12, no. 6: 720. https://doi.org/10.3390/ani12060720
APA StyleDettleff, P., Rodríguez, J., Patiño-García, D., Orellana, R., Castro, R., Escobar-Aguirre, S., Moreno, R. D., & Palomino, J. (2022). Molecular Characterization of Embryos with Different Buoyancy Levels in the Yellowtail Kingfish (Seriola lalandi). Animals, 12(6), 720. https://doi.org/10.3390/ani12060720






