Changes in Oxidative Status Biomarkers in Saliva and Serum in the Equine Gastric Ulcer Syndrome and Colic of Intestinal Aetiology: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diseased and Healthy Populations
2.2. Sampling
2.3. Salivary and Plasma Biomarkers
2.4. Analytical Validation of the Oxidative Stress Profile
2.5. Data Analysis
3. Results
3.1. Diseased and Healthy Populations
3.2. Analytical Validation of the Oxidative Stress Biomarkers
3.3. Adenosine Deaminase Results
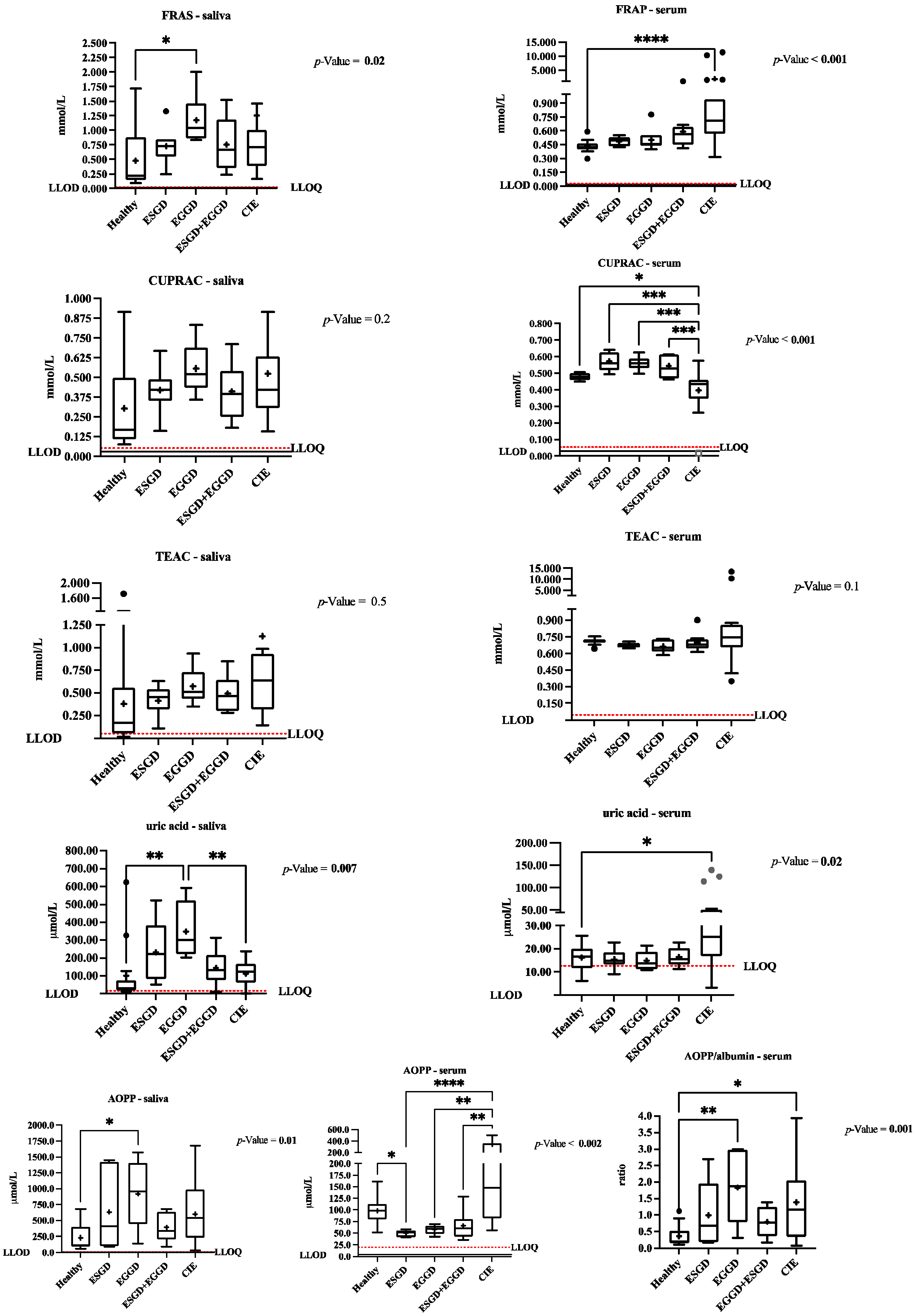
3.4. Changes in the Oxidative Stress Biomarkers in Saliva and Serum between the Healthy and Diseased Populations
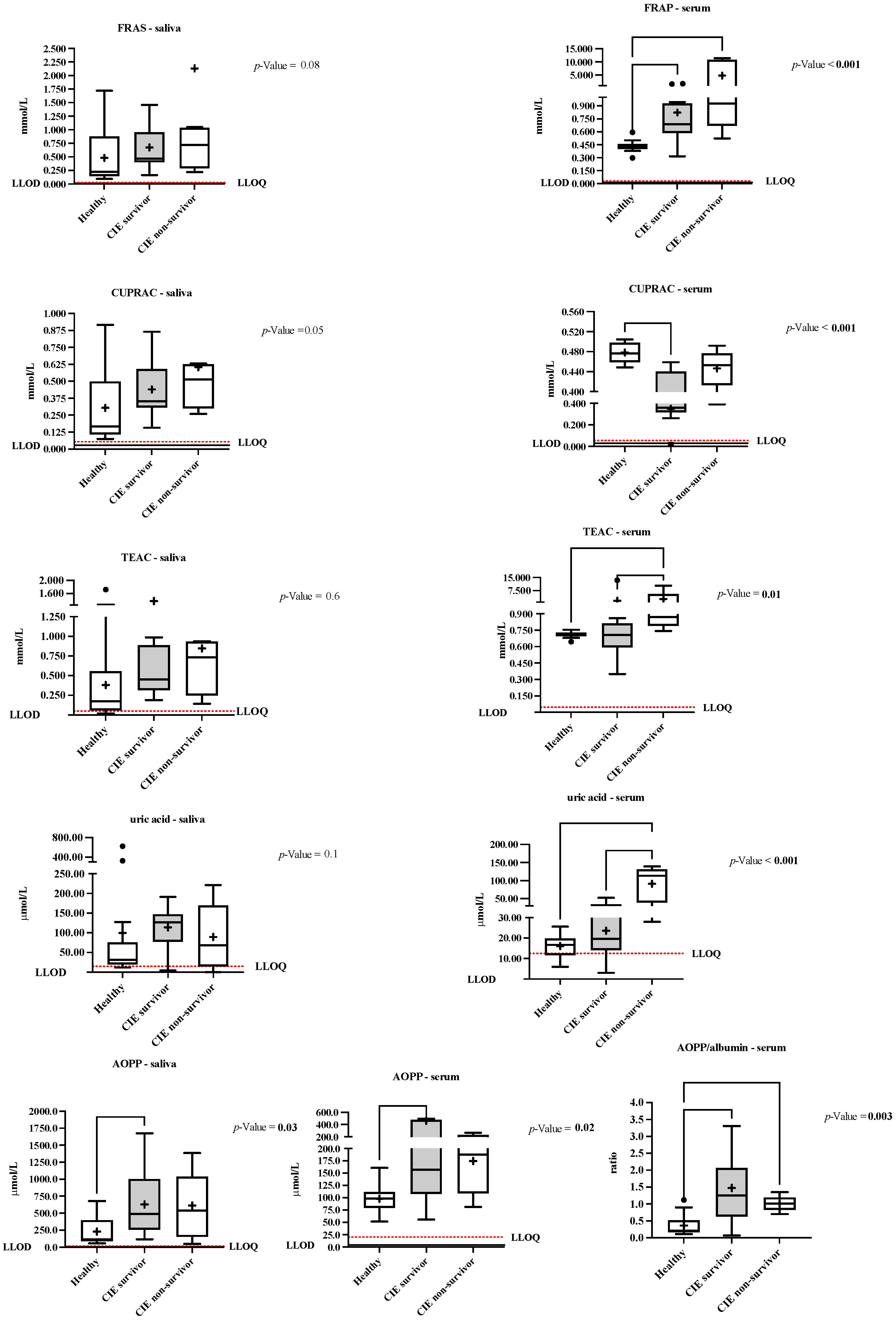
3.5. Differences in the Salivary and Serum Oxidative Stress Biomarkers depending on the Colic Outcome
3.6. Associations between the Salivary and Serum Redox Profiles with the Inflammatory and Immunity Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tvarijonaviciute, A.; Martínez-Subiela, S.; López-Jornet, P.; Lamy, E. (Eds.) Saliva in Health and Disease the Present and Future of a Unique Sample for Diagnosis, 1st ed.; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-37680-2. [Google Scholar]
- Lamy, E.; Mau, M. Saliva proteomics as an emerging, non-invasive tool to study livestock physiology, nutrition and diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.; Escribano, D.; Martínez-Subiela, S.; Martín-Cuervo, M.; Lamy, E.; Tecles, F.; Cerón, J.J. Changes in saliva analytes in equine acute abdominal disease: A sialochemistry approach. BMC Vet. Res. 2019, 15, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Aguilar, M.D.; Tvarijonaviciute, A.; Monkeviciene, I.; Martín-Cuervo, M.; González-Arostegui, L.G.; Franco-Martínez, L.; Cerón, J.J.; Tecles, F.; Escribano, D. Characterization of total adenosine deaminase activity (ADA) and its isoenzymes in saliva and serum in health and inflammatory conditions in four different species: An analytical and clinical validation pilot study. BMC Vet. Res. 2020, 16, 384. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.; Top Adler, D.M.; Bundgaard, L.; Sørensen, M.A.; Andersen, P.H.; Bendixen, E. The use of liquid chromatography tandem mass spectrometry to detect proteins in saliva from horses with and without systemic inflammation. Vet. J. 2014, 202, 483–488. [Google Scholar] [CrossRef]
- Rubio, C.P.; Contreras-Aguilar, M.D.; Quiles, A.; López-Arjona, M.; Cerón, J.J.; Martínez-Subiela, S.; Hevia, M.L.; Escribano, D.; Tecles, F. Biomarkers of oxidative stress in saliva in pigs: Analytical validation and changes in lactation. Res. Vet. Sci. 2019, 15, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, C.P.; Contreras-Aguilar, M.D.; Quiles, A.; López-Arjona, M.; Cerón, J.J.; Martínez-Subiela, S.; Hevia, M.L.; Escribano, D.; Tecles, F. Biomarkers of oxidative stress in saliva of sheep: Analytical performance and changes after an experimentally induced stress. Res. Vet. Sci. 2019, 123, 71–76. [Google Scholar] [CrossRef]
- Van Loon, J.P.A.M.; VanDierendonck, M.C. Monitoring acute equine visceral pain with the Equine Utrecht University Scale for Composite Pain Assessment (EQUUS-COMPASS) and the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS-FAP): A scale-construction study. Vet. J. 2015, 216, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Shawaf, T.; El-Deeb, W.M.; Elgioushy, M. The Contribution of Specific and Nonspecific Biomarkers in Diagnosis of Equine Gastric Ulcer Syndrome (EGUS) Under Field Condition. J. Equine Vet. Sci. 2020, 84, 102853. [Google Scholar] [CrossRef] [PubMed]
- Sykes, B.W.; Hewetson, M.; Hepburn, R.J.; Luthersson, N.; Tamzali, Y. European College of Equine Internal Medicine Consensus Statement-Equine Gastric Ulcer Syndrome in Adult Horses. J. Vet. Intern. Med. 2015, 29, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Banse, H.E.; Andrews, F.M. Equine glandular gastric disease: Prevalence, impact and management strategies. Vet. Med. Res. Rep. 2019, 10, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busechian, S.; Sgorbini, M.; Orvieto, S.; Pisello, L.; Zappulla, F.; Briganti, A.; Nocera, I.; Conte, G.; Rueca, F. Evaluation of a questionnaire to detect the risk of developing ESGD or EGGD in horses. Prev. Vet. Med. 2021, 188, 105285. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.A.; Ertzman-Ginsburg, R.; Steinman, A.; Milgram, J. Initial investigation of mortality rates and prognostic indicators in horses with colic in Israel: A retrospective study. Equine Vet. J. 2009, 41, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.; Blikslager, A. Advances in Diagnostics and Treatments in Horses with Acute Colic and Postoperative Ileus. Vet. Clin. N. Am. Equine Pract. 2018, 34, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Cook, V.L.; Hassel, D.M. Evaluation of the Colic in Horses. Decision for Referral. Vet. Clin. N. Am. Equine Pract. 2014, 30, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, N.; de Moffarts, B.; Lekeux, P. The oxidant/antioxidant equilibrium in horses. Vet. J. 2008, 177, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Dondi, F.; Lukacs, R.M.; Gentilini, F.; Rinnovati, R.; Spadari, A.; Romagnoli, N. Serum amyloid A, haptoglobin, and ferritin in horses with colic: Association with common clinicopathological variables and short-term outcome. Vet. J. 2015, 205, 50–55. [Google Scholar] [CrossRef]
- Roy, M.; Kwong, G.P.S.; Lambert, J.; Massie, S.; Lockhart, S. Prognostic value and development of a scoring system in horses with systemic inflammatory response syndrome. J. Vet. Intern. Med. 2017, 31, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Piras, M.A.; Gakis, C.; Budroni, M.; Andreoni, G. Adenosine deaminase activity in pleural effusions: An aid to differential diagnosis. Br. Med. J. 1978, 2, 1751–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes Baganha, M.; Pego, A.; Lima, M.A.; Gaspar, E.V.; Robalo Cordeiro, A. Serum and pleural adenosine deaminase. Correlation with lymphocytic populations. Chest 1990, 97, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Wise, J.C.; Wilkes, E.J.A.; Raidal, S.L.; Xie, G.; Crosby, D.E.; Hale, J.N.; Hughes, K.J. Interobserver and intraobserver reliability for 2 grading systems for gastric ulcer syndrome in horses. J. Vet. Intern. Med. 2021, 35, 571–579. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.; Martínez-Subiela, S.; Cerón, J.; Martín-Cuervo, M.; Tecles, F.; Escribano, D. Salivary alpha-amylase activity and concentration in horses with acute abdominal disease: Association with outcome. Equine Vet. J. 2019, 51, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Luisa, M.; Escribano, D.; Lamy, E.; Tecles, F.; Cerón, J.J. Effect of food contamination and collection material in the measurement of biomarkers in saliva of horses. Res. Vet. Sci. 2020, 129, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Miró, S.; López-Arjona, M.; Rubio, C.P.; Martínez-Subiela, S.; Cerón, J.J.; Tecles, F. Application of a score for evaluation of pain, distress and discomfort in pigs with lameness and prolapses: Correlation with saliva biomarkers and severity of the disease. Res. Vet. Sci. 2019, 126, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Vallejo-mateo, P.J.; Lamy, E.; Cer, J.J.; Tecles, F.; Rubio, C.P. Changes in Saliva Analytes in Dairy Cows during Peripartum: A Pilot Study. Animals 2021, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Merlo, M.; Da Dalt, L.; Stefani, A.; Barbato, O.; Gabai, G. Relationship between late embryonic mortality and the increase in plasma advanced oxidised protein products (AOPP) in dairy cows. Reprod. Fertil. Dev. 2011, 23, 527–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, S.; Kjelgaard-Hansen, M.; Hagbard Petersen, H.; Jensen, A.L. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. Vet. J. 2006, 172, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: London, UK, 2003; ISBN 0618124055. [Google Scholar]
- Food and Drug Administration Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health & Human Services: Washington, DC, USA, 2001; pp. 4–10.
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- MacDermott, R.P.; Tritsch, G.L.; Formeister, J.F. Adenosine deaminase and nucleoside phosphorylase activities in normal human blood mononuclear cell subpopulations. Clin. Exp. Immunol. 1980, 42, 303–307. [Google Scholar]
- Tecles, F.; Escribano, D.; Contreras-Aguilar, M.D.; Rubio, C.P.; Szczubiał, M.; Cerón, J.J.; Dąbrowski, R.; Tvarijonaviciute, A. Evaluation of adenosine deaminase in saliva and serum, and salivary α-amylase, in canine pyometra at diagnosis and after ovariohysterectomy. Vet. J. 2018, 236, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Gómez-García, F. Salivary Glands’ Anatomy and Physiology. In Saliva in Health and Disease: The Present and Future of a Unique Sample for Diagnosis; Tvarijonaviciute, A., Martínez-Subiela, S., López-Jornet, P., Lamy, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–21. ISBN 978-3-030-37681-9. [Google Scholar]
- Ibrahim, H.M.M. Oxidative stress associated with spasmodic, flatulent, and impaction colic in draft horses. J. Equine Vet. Sci. 2014, 34, 1205–1210. [Google Scholar] [CrossRef]
- Montero-Chacón, L.B.; Padilla-Cuadra, J.I.; Chiou, S.H.; Torrealba-Acosta, G. High-Density Lipoprotein, Mean Platelet Volume, and Uric Acid as Biomarkers for Outcomes in Patients With Sepsis: An Observational Study. J. Intensive Care Med. 2020, 35, 636–642. [Google Scholar] [CrossRef] [Green Version]
| Analytes | Pool Specimens | Saliva | Serum | ||||
|---|---|---|---|---|---|---|---|
| Mean Values | CV (%) | Mean CV (SD) | Mean Value | CV (%) | Mean CV (SD) | ||
| FRAS/FRAP (mmol/L) | High | 1.639 | 0.45 | 0.48 (0.14) | 0.884 | 0.58 | 0.51 (0.13) |
| Medium | 0.439 | 0.36 | 0.427 | 0.36 | |||
| Low | 0.243 | 0.63 | 0.360 | 0.59 | |||
| CUPRAC (mmol/L) | High | 0.867 | 0.54 | 0.81 (0.28) | 0.878 | 0.98 | 1.26 (0.49) |
| Medium | 0.446 | 0.80 | 0.402 | 1.82 | |||
| Low | 0.256 | 1.10 | 0.229 | 0.97 | |||
| TEAC (mmol/L) | High | 0.918 | 1.28 | 2.16 (1.67) | 0.881 | 0.46 | 0.82 (0.54) |
| Medium | 0.613 | 1.11 | 0.742 | 1.44 | |||
| Low | 0.105 | 4.08 | 0.670 | 0.56 | |||
| uric acid (μmol/L) | High | 515.69 | 0.35 | 1.33 (0.85) | 21.41 | 4.30 | 7.84 (3.62) |
| Medium | 93.38 | 1.67 | 13.68 | 11.53 | |||
| Low | 75.54 | 1.95 | 13.09 | 7.69 | |||
| AOPP (μmol/L) | High | 844.2 | 6.68 | 4.17 (2.72) | 141.7 | 4.69 | 3.66 (0.95) |
| Medium | 112.2 | 1.27 | 78.6 | 2.81 | |||
| Low | 90.2 | 4.55 | 59.2 | 3.48 | |||
| Analytes | Sample | Pool Specimens | Analyte Levels | Slope (95% CI) | Y-Intercept (95% CI) | p-Value | R2 |
|---|---|---|---|---|---|---|---|
| FRAS/FRAP (mmol/L) | Saliva | 1 | 1.644 | 0.92 (0.47–1.34) | 0.17 (−0.22–0.57) | 0.01 | 0.976 |
| 2 | 0.438 | 1.03 (0.30–1.76) | −0.05 (−0.25–1.59) | 0.03 | 0.949 | ||
| Serum | 1 | 0.877 | 1.04 (0.61–1.47) | −0.07 (−0.30–0.16) | 0.009 | 0.982 | |
| 2 | 0.426 | 1.05 (0.55–1.56) | −0.04 (−0.18–0.09) | 0.01 | 0.976 | ||
| CUPRAC (mmol/L) | Saliva | 1 | 0.878 | 1.11 (0.77–1.45) | −0.12 (−0.30–0.07) | 0.005 | 0.990 |
| 2 | 0.402 | 1.23 (1.00–1.45) | −0.10 (−0.16–0.04) | 0.002 | 0.996 | ||
| Serum | 1 | 0.426 | 1.28 (0.40–2.15) | −0.15 (−0.41–0.11) | 0.04 | 0.952 | |
| 2 | 0.446 | 1.31 (0.43–2.19) | −0.17 (−0.44–0.10) | 0.05 | 0.954 | ||
| TEAC (mmol/L) | Saliva | 1 | 0.918 | 0.96 (0.92–1.01) | 0.03 (0.01–0.06) | <0.001 | 1.000 |
| 2 | 0.613 | 0.95 (0.92–0.99) | 0.03 (0.02–0.04) | <0.001 | 1.000 | ||
| Serum | 1 | 0.879 | 0.99 (0.73–1.25) | −0.01 (−0.15–0.13) | 0.004 | 0.993 | |
| 2 | 0.742 | 0.96 (0.90–1.02) | 0.03 (0.00–0.05) | <0.001 | 1.000 | ||
| uric acid (μmol/L) | Saliva | 1 | 517.48 | 0.99 (0.95–1.04) | 0.09 (−0.14–0.31) | <0.001 | 1.000 |
| 2 | 91.00 | 1.02 (0.90–1.14) | −0.02 (−0.12–0.09) | <0.001 | 0.999 | ||
| Serum | 1 | 21.41 | 1.09 (0.76–1.42) | −0.04 (−0.11–0.04) | 0.005 | 0.990 | |
| 2 | 14.28 | 1.05 (0.75–1.34) | −0.04 (−0.05–0.03) | 0.004 | 0.992 | ||
| AOPP (μmol/L) | Saliva | 1 | 630.0 | 0.97 (0.66–1.29) | −1.63 (−120.3–117.0) | 0.006 | 0.989 |
| 2 | 112.2 | 1.00 (0.40–1.60) | −7.08 (−49.1–35.0) | 0.02 | 0.963 | ||
| Serum | 1 | 141.7 | 0.99 (0.99–1.00) | 0.17 (−0.17–0.52) | <0.001 | 1.000 | |
| 2 | 78.6 | 0.99 (0.93–1.05) | 0.50 (−2.43–3.43) | <0.001 | 1.000 |
| Parameters | Samples Types | FRAP | CUPRAC | AHH | Uric Acid | AOPP |
|---|---|---|---|---|---|---|
| mmol/L | mmol/L | mmol/L | µmol/L | µmol/L | ||
| LLOQ | SALIVA | <0.025 | <0.054 | 0.049 | <14.87 | <12.7 |
| SERUM | <0.029 | <0.056 | 0.046 | 12.49 | 20.2 | |
| LLOD | 0.015 | 0.029 | 0.000 | 0.00 | 3.8 |
| Horse Population | ADAt Serum (IU/L) | ADAt Saliva (IU/L) | ADA1 Saliva (IU/L) | ADA2 Saliva (IU/L) |
|---|---|---|---|---|
| Healthy horses | 0.30 [0.20–0.40] | 38.95 [15.58–98.75] | 38.27 [14.72–108.30] | 0.50 [0.30–1.45] |
| ESGD | 0.60 [0.40–0.60] | 397.60 (224.50–570.70) | 391.50 (165.50–616.60) | 5.74 (1.25–10.23) |
| EGGD | 0.45 [0.38–0.68] | 672.50 (206.80–1138.00) 1 | 662.20 (202.60–1122.00) 1 | 10.32 (3.80–16.84) 1 |
| ESGD + EGGD | 0.40 [0.30–0.60] | 623.90 (228.50–1019.00) 1 | 629.20 (188.20–1070.00) 1 | 11.09 (3.20–18.98) 1 |
| CIE | 0.30 [0.20–0.30] | 143.00 [42.60–513.30] | 138.00 [39.85–505.00] | 5.70 [2.48–8.25] 1 |
| survivors | 0.20 [0.10–0.30] | 85.10 [12.50–544.60] | 86.45 [14.68–298.70] | 5.35 [1.38–7.90] 2 |
| non-survivors | 0.30 [0.30–0.40] | 179.20 [108.30–550.10] | 184.00 [110.60–572.80] 2 | 4.85 [3.05–16.08] 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Aguilar, M.D.; Rubio, C.P.; González-Arostegui, L.G.; Martín-Cuervo, M.; Cerón, J.J.; Ayala, I.; Henriksen, I.-M.H.; Jacobsen, S.; Hansen, S. Changes in Oxidative Status Biomarkers in Saliva and Serum in the Equine Gastric Ulcer Syndrome and Colic of Intestinal Aetiology: A Pilot Study. Animals 2022, 12, 667. https://doi.org/10.3390/ani12050667
Contreras-Aguilar MD, Rubio CP, González-Arostegui LG, Martín-Cuervo M, Cerón JJ, Ayala I, Henriksen I-MH, Jacobsen S, Hansen S. Changes in Oxidative Status Biomarkers in Saliva and Serum in the Equine Gastric Ulcer Syndrome and Colic of Intestinal Aetiology: A Pilot Study. Animals. 2022; 12(5):667. https://doi.org/10.3390/ani12050667
Chicago/Turabian StyleContreras-Aguilar, María Dolores, Camila Peres Rubio, Luis Guillermo González-Arostegui, María Martín-Cuervo, Jose J. Cerón, Ignacio Ayala, Ida-Marie Holm Henriksen, Stine Jacobsen, and Sanni Hansen. 2022. "Changes in Oxidative Status Biomarkers in Saliva and Serum in the Equine Gastric Ulcer Syndrome and Colic of Intestinal Aetiology: A Pilot Study" Animals 12, no. 5: 667. https://doi.org/10.3390/ani12050667
APA StyleContreras-Aguilar, M. D., Rubio, C. P., González-Arostegui, L. G., Martín-Cuervo, M., Cerón, J. J., Ayala, I., Henriksen, I.-M. H., Jacobsen, S., & Hansen, S. (2022). Changes in Oxidative Status Biomarkers in Saliva and Serum in the Equine Gastric Ulcer Syndrome and Colic of Intestinal Aetiology: A Pilot Study. Animals, 12(5), 667. https://doi.org/10.3390/ani12050667







