The Arrangement of the Peripheral Olfactory System of Pleuragramma antarcticum: A Well-Exploited Small Sensor, an Aided Water Flow, and a Prominent Effort in Primary Signal Elaboration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Tissues Preparation
2.2. Gross Morphology
2.3. Histology
2.4. Isotropic Fractionator
2.5. Building of the Dataset
| Species | Specimen | TL (mm) | Sex | RoL (mm) | RoW (mm) | LN | ES (mm2) | OB (mg) | OB Cells | OB neu. | Neu% | Cell/mg | Neu/mg | Cell/ mm2 | Neu/ mm2 | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. antarctica | 17.2 | 150 | - | - | - | - | 15 | 1 | 1.89 × 105 | 1.56 × 105 | 82.5 | 1.89 × 105 | 1.56 × 105 | 1.23 × 104 | 1.01 × 104 | present study |
| P. antarctica | 17.3 | 150 | - | 2.6 | 1.8 | 23 | 22 | 1 | 2.80 × 105 | 2.10 × 105 | 75.1 | 2.80 × 105 | 2.10 × 105 | 1.29 ×104 | 9.7 × 103 | present study |
| P. antarctica | 20.1 | 211 | F | 1 | 1.41 × 105 | 9.50 × 104 | 67.2 | 1.41 × 105 | 9.50 ×104 | present study | ||||||
| P. antarctica | 20.2 | 184 | F | 3.2 | 2 | 24 | 24 | present study | ||||||||
| P. antarctica | 20.3 | 181 | M | 3.2 | 2 | 27 | 26 | 1 | 1.71 × 105 | 1.53 × 105 | 89.1 | 1.71 × 105 | 1.53 × 105 | 6.59 × 103 | 5.87 × 103 | present study |
| P. antarctica | 20.4 | 214 | F | 3.3 | 2.3 | 24 | 32 | 1 | 7.70 × 104 | 4.87 × 104 | 63.2 | 7.70 × 104 | 4.87 × 104 | 2.43 ×103 | 1.53 × 103 | present study |
| D. mawsoni | Dm4 | 1290 | F | 11 | 12 | 42 | 1041 | [16] | ||||||||
| D. mawsoni | Dm5 | 1210 | M | 12 | 13 | 41 | 30 | 3.60 × 106 | 1.20 × 105 | [16] | ||||||
| G.melastomus | G1 | 360 | F | 3731 | 70 | 2.14 × 106 | 1.64 × 106 | 76.5 | 3.06 × 104 | 2.34 × 104 | 5.74 × 102 | 4.40 × 102 | [49] | |||
| G.melastomus | G2 | 300 | M | 3113 | 55 | 2.00 × 106 | 1.48 × 106 | 72.9 | 3.64 × 104 | 2.70 × 104 | 6.4 × 102 | 4.75 × 102 | [49] | |||
| G.melastomus | G3 | 270 | M | 2804 | 31 | 1.29 × 106 | 8.30 × 105 | 64.1 | 4.16 × 104 | 2.66 × 104 | 4.60 × 102 | 2.96 × 102 | [49] | |||
| G.melastomus | G4 | 180 | F | 1877 | 20 | 8.50 × 105 | 7.00 × 105 | 82.7 | 4.25 × 104 | 3.51 × 104 | 4.53 × 102 | 3.73 × 102 | [49] | |||
| G.melastomus | G5 | 170 | F | 1774 | 12 | 5.60 × 105 | 4.40 × 105 | 78.8 | 4.67 × 104 | 3.68 × 104 | 3.16 × 102 | 2.48 × 102 | [49] | |||
| G.melastomus | G6 | 150 | J | 1568 | 9 | 1.90 × 105 | 1.50 × 105 | 80 | 2.11 × 104 | 1.66 × 104 | 1.21 × 102 | 9.57 × 101 | [49] | |||
| G.melastomus | G7 | 135 | J | 1413 | 5 | 1.30 × 105 | 1.00 × 105 | 77.1 | 2.60 × 104 | 2.02 × 104 | 9.20 × 101 | 7.08 × 101 | [49] | |||
| G.melastomus | G8 | 135 | J | 1413 | 6 | 1.70 × 105 | 1.30 × 105 | 75 | 2.83 × 104 | 2.15 × 104 | 1.20 × 102 | 9.20 × 101 | [49] | |||
| G.melastomus | G9 | 150 | M | 1568 | 13 | 5.40 × 105 | 3.70 × 105 | 69.8 | 4.15 × 104 | 2.87 × 104 | 3.44 × 102 | 2.36 × 102 | [49] | |||
| G.melastomus | G10 | 130 | J | 1362 | 7 | 2.80 × 105 | 2.10 × 105 | 76.9 | 4.00 × 104 | 3.05 × 104 | 2.06 × 102 | 1.54 × 102 | [49] | |||
| G.melastomus | G11 | 110 | J | 1156 | 3 | 1.10 × 105 | 7.00 × 104 | 62.8 | 3.67 × 104 | 2.22 × 104 | 9.52 × 101 | 6.06 × 101 | [49] | |||
| S. canicula | S1 | 480 | M | 4081 | 99 | 2.45 × 106 | 1.85 × 106 | 75.7 | 2.47 × 104 | 1.87 × 104 | 6.00 × 102 | 4.53 × 102 | [49] | |||
| S. canicula | S2 | 330 | M | 2370 | 48 | 1.23 × 106 | 9.40 × 105 | 76.6 | 2.56 × 104 | 1.96 × 104 | 5.19 × 102 | 3.97 × 102 | [49] | |||
| S. canicula | S3 | 210 | M | 1001 | 19 | 4.50 × 105 | 3.30 × 105 | 75 | 1.74 × 104 | 1.76 × 104 | 4.50 × 102 | 3.30 × 102 | [49] | |||
| S. canicula | S4 | 200 | F | 888 | 21 | 3.50 × 105 | 2.70 × 105 | 76.5 | 1.76 × 104 | 1.27 × 104 | 3.94 × 102 | 3.04 × 102 | [49] | |||
| S. canicula | S5 | 285 | M | 1857 | 36 | 1.22 × 106 | 9.30 × 105 | 76.2 | 3.36 × 104 | 2.59 × 104 | 6.57 × 102 | 5.01 × 102 | [49] | |||
| S. canicula | S6 | 215 | F | 1958 | 23 | 3.30 × 105 | 2.50 × 105 | 74.6 | 3.96 × 104 | 1.07 × 104 | 1.69 × 102 | 1.28 × 102 | [49] | |||
| S. canicula | S7 | 210 | F | 1001 | 22 | 3.70 × 105 | 2.80 × 105 | 74.1 | 2.86 × 104 | 1.25 × 104 | 3.70 × 102 | 2.80 × 102 | [49] | |||
| S. canicula | S8 | 355 | M | 2655 | 49 | 1.21 × 106 | 9.30 × 105 | 76.5 | 2.31 × 104 | 1.89 × 104 | 4.56 × 102 | 3.50 × 102 | [49] | |||
| S. canicula | S9 | 305 | M | 2085 | 42 | 9.10 × 105 | 6.80 × 105 | 74.1 | 3.64 × 104 | 1.61 × 104 | 4.36 × 102 | 3.26 × 102 | [49] | |||
| S. canicula | S10 | 275 | F | 1742 | 23 | 6.30 × 105 | 4.80 × 105 | 76 | 5.35 × 104 | 2.09 × 104 | 3.62 × 102 | 2.76 × 102 | [49] | |||
| S. canicula | S11 | 275 | F | 1742 | 28 | 1.13 × 106 | 7.50 × 105 | 66 | 3.43 × 104 | 2.67 × 104 | 6.49 × 102 | 4.31 × 102 | [49] | |||
| S. canicula | S12 | 290 | F | 1914 | 26 | 1.53 × 106 | 1.05 × 106 | 68.3 | 2.42 × 104 | 4.02 × 104 | 7.99 × 102 | 5.49 × 102 | [49] | |||
| S. canicula | S13 | 280 | M | 1799 | 33 | 1.23 × 106 | 9.20 × 105 | 74.7 | 3.73 × 104 | 2.78 × 104 | 6.84 × 102 | 5.11 × 102 | [49] | |||
| S. canicula | S14 | 270 | M | 1685 | 24 | 9.60 × 105 | 7.40 × 105 | 76.7 | 4.00 × 104 | 3.08 × 104 | 5.70 × 102 | 4.39 × 102 | [49] | |||
| S. canicula | S15 | 245 | M | 1400 | 22 | 6.30 × 105 | 4.40 × 105 | 69 | 2.86 × 104 | 1.98 × 104 | 4.50 × 102 | 3.14 × 102 | [49] | |||
| S. canicula | S16 | 250 | F | 1457 | 17 | 7.00 × 105 | 5.30 × 105 | 76.2 | 4.12 × 104 | 3.12 × 104 | 4.80 × 102 | 3.64 × 102 | [49] | |||
| S. canicula | S17 | 225 | M | 1172 | 20 | 7.30 × 105 | 5.30 × 105 | 72.4 | 3.65 × 104 | 2.63 × 104 | 6.23 × 102 | 4.52 × 102 | [49] |
3. Results
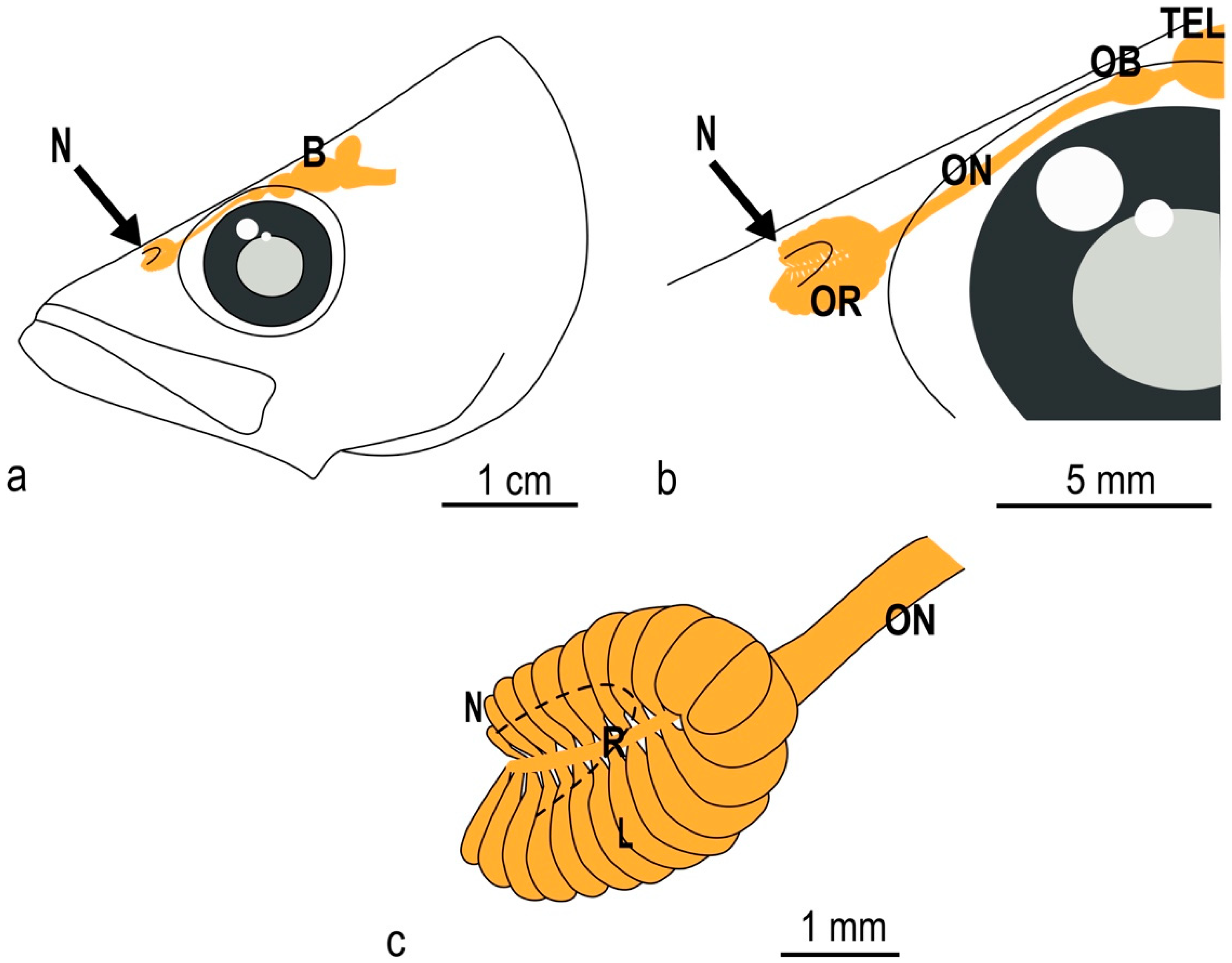
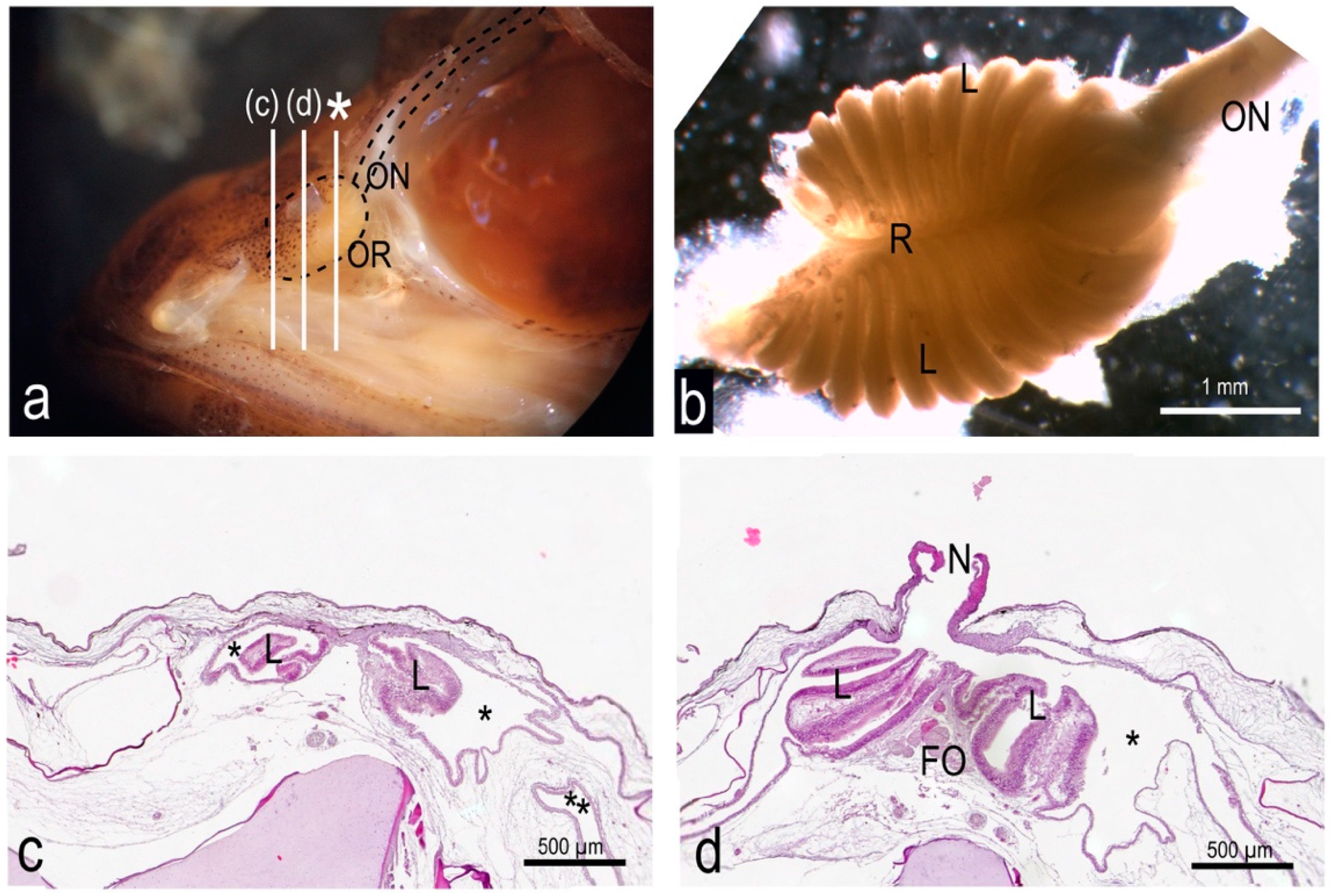
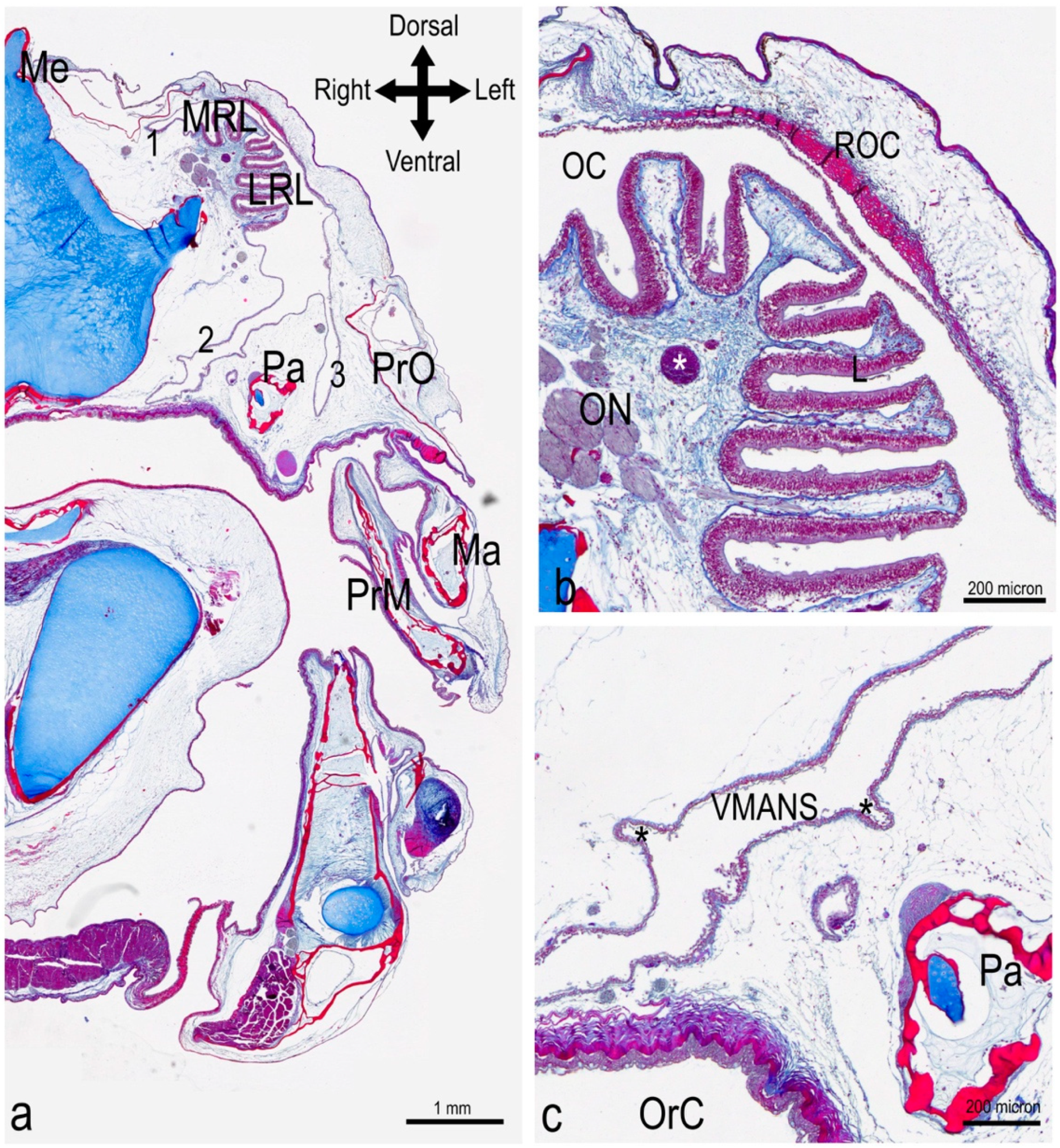
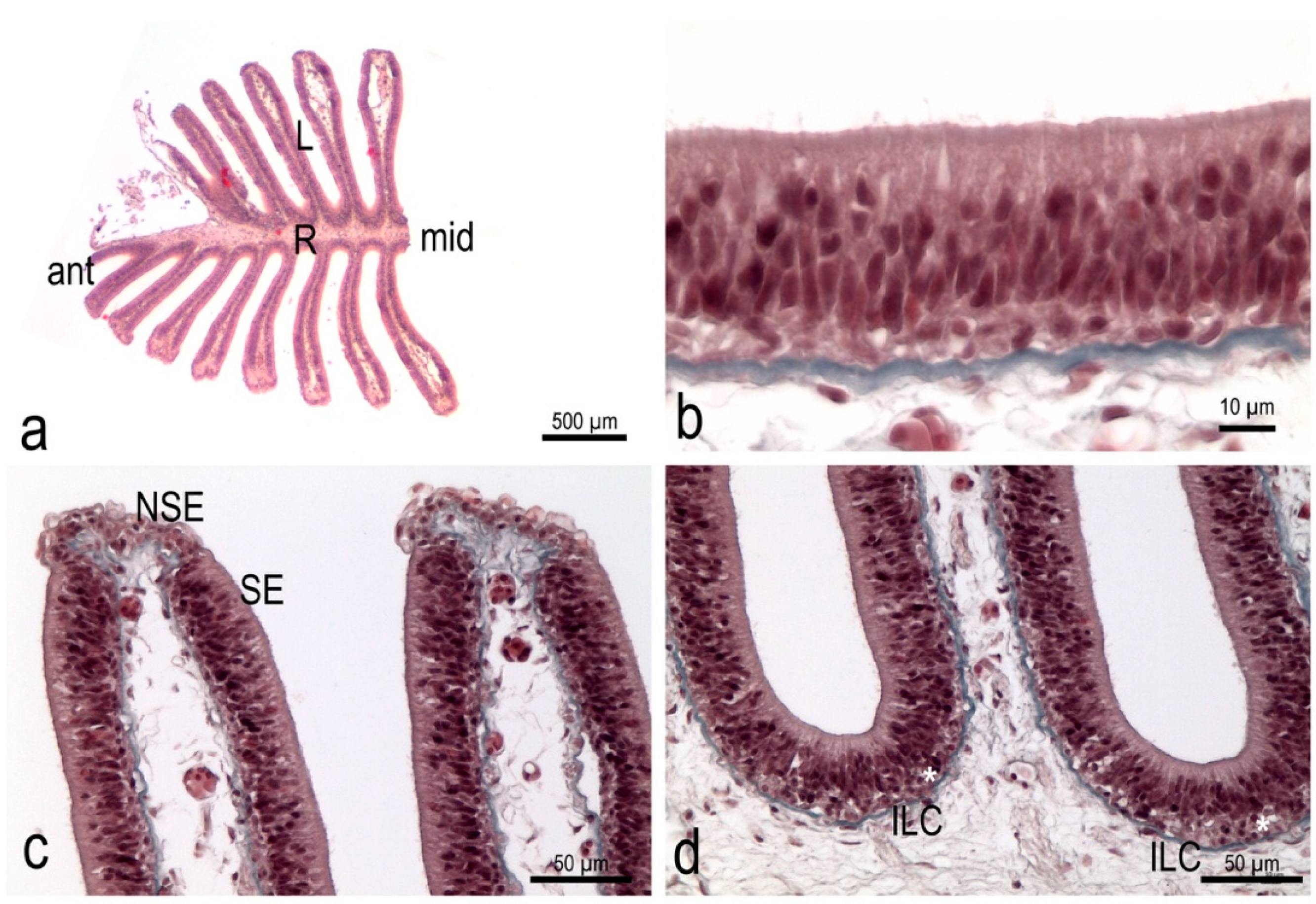
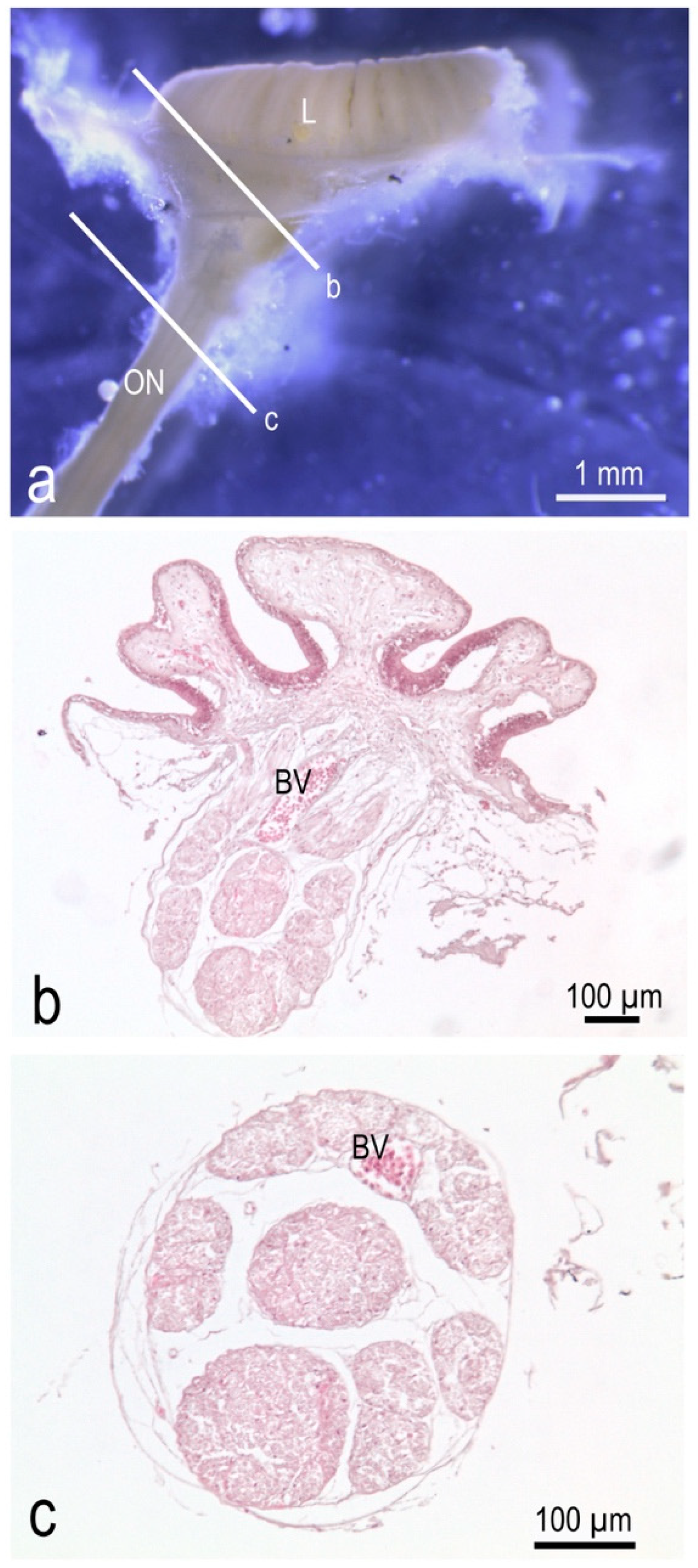
3.1. The Olfactory Organ of Adult P. antarcticum Is an Asymmetrical Rosette
3.2. The Nasal Chamber Has a Reinforced Roof and Is Connected to Accessory Nasal Sacs
3.3. The Olfactory Organ Surface Area of an Adult P. antarcticum Is Almost 50 mm2
3.4. In P. antarcticum the Olfactory Nerve Is Long, while the Olfactory Tract Is Short
3.5. In the Olfactory Bulb of P. antarcticum, Many Cells Elaborate the Signal from a Small Olfactory Organ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burne, R.H. The Anatomy of the Olfactory Organ of Teleostean Fishes. Proc. Zool. Soc. Lond. 1909, 2, 610–663. [Google Scholar]
- Døving, K.B. Functional Properties of the Fish Olfactory System. Prog. Sens. Physiol. 1986, 6, 39–104. [Google Scholar]
- Helfman, G.S.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution, and Ecology, 2nd ed.; Wiley-Blackwell, A John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kasumyan, A.O. The Olfactory System in Fish: Structure, Function, and Role in Behavior. J. Ichthyol. 2004, 44, S180–S223. [Google Scholar]
- Yamamoto, M. Comparative Morphology of the Peripheral Olfactory Organ in Teleosts. In Chemoreception in Fishes; Hara, T.J., Ed.; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands, 1982; pp. 39–59. [Google Scholar]
- Cox, J.P.L. Hydrodynamic Aspects of Fish Olfaction. J. R. Soc. Interface 2008, 5, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Schild, D.; Restrepo, D. Transduction Mechanisms in Vertebrate Olfactory Receptor Cells. Physiol. Rev. 1998, 78, 429–466. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, B.; Polese, G.; Luongo, L.; Scandurra, A.; Magliozzi, L.; Aria, M.; Pinelli, C. Neuroanatomical Relationships between FMRFamide-Immunoreactive Components of the Nervus Terminalis and the Topology of Olfactory Bulbs in Teleost Fish. Cell Tissue Res. 2016, 364, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Baier, H.; Korsching, S. Olfactory Glomeruli in the Zebrafish Form an Invariant Pattern and Are Identifiable across Animals. J. Neurosci. 1994, 14, 219–230. [Google Scholar] [CrossRef]
- Mombaerts, P. Axonal Wiring in the Mouse Olfactory System. Annu. Rev. Cell Dev. Biol. 2006, 22, 713–737. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Arratia, G. Olfactory Organ of Acipenseriformes and Comparison with Other Actinopterygians: Patterns of Diversity. J. Morphol. 1994, 222, 241–267. [Google Scholar] [CrossRef]
- Diaz, J.P.; Prié-Granié, M.; Blasco, C.; Noëll, T.; Connes, R. Ultrastructural Study of the Olfactory Organ in Adult and Developing European Sea Bass, Dicentrarchus Labrax. Can. J. Zool. 2002, 80, 1610–1622. [Google Scholar] [CrossRef]
- Pashchenko, N.I.; Kasumyan, A.O. Development of the Olfactory Organ in the Ontogeny of Carps (Cyprinidae). J. Ichthyol. 2017, 57, 136–151. [Google Scholar] [CrossRef]
- Pashchenko, N.I.; Kasumyan, A.O. Scanning Electron Microscopy of Development of the Olfactory Organ in Ontogeny of Grass Carp Ctenopharyngodon idella. J. Ichthyol. 2015, 55, 880–899. [Google Scholar] [CrossRef]
- Atta, K.I. Morphological, Anatomical and Histological Studies on the Olfactory Organs and Eyes of Teleost Fish: Anguilla anguilla in Relation to Its Feeding Habits. J. Basic Appl. Zool. 2013, 66, 101–108. [Google Scholar] [CrossRef]
- Ferrando, S.; Amaroli, A.; Gallus, L.; Di Blasi, D.; Carlig, E.; Rottigni, M.; Vacchi, M.; Parker, S.J.; Ghigliotti, L. Olfaction in the Antarctic Toothfish Dissostichus mawsoni: Clues from the Morphology and Histology of the Olfactory Rosette and Bulb. Polar Biol. 2019, 42, 1081–1091. [Google Scholar] [CrossRef]
- Teichmann, H. Vergleichende Untersuchungen an der Nase der Fische. Z. Für Morphol. Okologie Tiere 1954, 43, 171–212. [Google Scholar] [CrossRef]
- Døving, K.B.; Gemne, G. Electrophysiological and histological properties of the olfactory tract of the burbot (Lota Lota L.). J. Neurophysiol. 1965, 28, 139–153. [Google Scholar] [CrossRef]
- Lisney, T.J.; Wagner, H.-J.; Collin, S.P. Ontogenetic Shifts in the Number of Axons in the Olfactory Tract and Optic Nerve in Two Species of Deep-Sea Grenadier Fish (Gadiformes: Macrouridae: Coryphaenoides). Front. Ecol. Evol. 2018, 6, 168. [Google Scholar] [CrossRef]
- Westerman, R.A.; Wilson, J.A.F. The Fine Structure of the Olfactory Tract in the Teleost Carassius carassius L. Z. Für Zellforsch. Mikrosk. Anat. 1968, 91, 186–199. [Google Scholar] [CrossRef]
- Easton, D.M. Garfish Olfactory Nerve: Easily Accessible Source of Numerous Long, Homogeneous, Nonmyelinated Axons. Science 1971, 172, 952–955. [Google Scholar] [CrossRef]
- Gemne, G.; Døving, K.B. Ultrastructural Properties of Primary Olfactory Neurons in Fish ( Lota Lota L.): Primary olfactory neurons in fish. Am. J. Anat. 1969, 126, 457–475. [Google Scholar] [CrossRef]
- Kreutzberg, G.W.; Gross, G.W. General Morphology and Axonal Ultrastructure of the Olfactory Nerve of the Pike, Esox lucius. Cell Tissue Res. 1977, 181, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Shinto, M.; Sakurai, Y.; Kaeriyama, M. Morphometry of Olfactory Lamellae and Olfactory Receptor Neurons During the Life History of Chum Salmon (Oncorhynchus keta). Chem. Senses 2009, 34, 617–624. [Google Scholar] [CrossRef]
- Eastman, J.T. Antarctic Fish Biology. Evolution in a Unique Environment; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Eastman, J.T.; La Mesa, M. Neuromorphological Disparity in Deep-Living Sister Species of the Antarctic Fish Genus Trematomus. Polar Biol. 2021, 44, 315–334. [Google Scholar] [CrossRef]
- Eastman, J.T.; Lannoo, M.J. Brain and Sense Organ Anatomy and Histology of the Falkland Islands Mullet, Eleginops maclovinus (Eleginopidae), the Sister Group of the Antarctic Notothenioid Fishes (Perciformes: Notothenioidei). J. Morphol. 2008, 269, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.T.; Lannoo, M.J. Brain and Sense Organ Anatomy and Histology of Two Species of Phyletically Basal Non-Antarctic Thornfishes of the Antarctic Suborder Notothenioidei (Perciformes: Bovichtidae). J. Morphol. 2007, 268, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.T.; Lannoo, M.J. Brain and Sense Organ Anatomy and Histology in Hemoglobinless Antarctic Icefishes (Perciformes: Notothenioidei: Channichthyidae). J. Morphol. 2004, 260, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.T.; Lannoo, M.J. Anatomy and Histology of the Brain and Sense Organs of the Antarctic Plunderfish Dolloidraco longedorsalis (Perciformes: Notothenioidei: Artedidraconidae), with Comments on the Brain Morphology of Other Artedidraconids and Closely Related Harpagiferids. J. Morphol. 2003, 255, 358–377. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.T.; Lannoo, M.J. Diversification of Brain and Sense Organ Morphology in Antarctic Dragonfishes (Perciformes: Notothenioidei: Bathydraconidae). J. Morphol. 2003, 258, 130–150. [Google Scholar] [CrossRef]
- Iwami, T. A Note on the Nasal Structures of Fishes of the Suborder Notothenioidei (Pisces, Perciformes). Mem. Natl. Inst. Polar Res. 1986, 44, 151–152. [Google Scholar]
- Jakubowski, M. Anatomical Structure of Olfactory Organs Provided with Internal Nares in the Antarctic Fish Gymnodraco Acuticeps Boul. (Bathydraconidae). Bull. Acad. Pol. Sci. 1975, 23, 115–120. [Google Scholar]
- Eastman, J.T. Phyletic Devergence and Specialization for Pelagic Life in the Antarctic Nototheniid Fish Pleuragramma antarcticum. Comp. Biochem. Physiol. A Physiol. 1997, 118, 1095–1101. [Google Scholar] [CrossRef]
- Hagen, W.; Kattner, G. The Role of Lipids in the Life History of the Antarctic Silverfish Pleuragramma antarctica. In The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem; Vacchi, M., Pisano, E., Ghigliotti, L., Eds.; Advances in Polar Ecology; Springer International Publishing: Cham, Switzerland, 2017; Volume 3, pp. 131–148. ISBN 978-3-319-55891-2. [Google Scholar]
- Voskoboinikova, O.; Detrich, H.W.; Albertson, R.C.; Postlethwait, J.H.; Ghigliotti, L.; Pisano, E. Evolution Reshaped Life for the Water Column: The Skeleton of the Antarctic Silverfish Pleuragramma antarctica Boulenger, 1902. In The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem; Vacchi, M., Pisano, E., Ghigliotti, L., Eds.; Advances in Polar Ecology; Springer International Publishing: Cham, Switzerland, 2017; Volume 3, pp. 3–26. ISBN 978-3-319-55891-2. [Google Scholar]
- Koubbi, P.; O’Brien, C.; Loots, C.; Giraldo, C.; Smith, M.; Tavernier, E.; Vacchi, M.; Vallet, C.; Chevallier, J.; Moteki, M. Spatial Distribution and Inter-Annual Variations in the Size Frequency Distribution and Abundances of Pleuragramma antarcticum Larvae in the Dumont d’Urville Sea from 2004 to 2010. Polar Sci. 2011, 5, 225–238. [Google Scholar] [CrossRef]
- Sheiko, B.A. Comments on the Nomenclature of Genus- and Family-Series Taxa of Notothenioid Fishes (Perciformes, Notothenioidei). Bionomina 2019, 16, 46–82. [Google Scholar] [CrossRef]
- Eastman, J.T.; Lannoo, M.J. Divergence of Brain and Retinal Anatomy and Histology in Pelagic Antarctic Notothenioid Fishes of the Sister Taxa Dissostichus and Pleuragramma. J. Morphol. 2011, 272, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Clark, M. Voyage Report TAN04-02 Western Ross Sea, Hydrographic and Biodiversity Survey RV Tangaroa 27 January to 13 March 2004; NIWA: Auckland, New Zealand, 2004; pp. 1–52. [Google Scholar]
- O’Driscoll, R.; Bowden; Gutierrez Rodriguez, A.; Safi, K.; McMillan, P.; Escobar-Flores, P. Voyage Report TAN1901, Ross Sea Environment and Ecosystem Voyage 2019, 4 January to 13 February 2019; NIWA: Auckland, New Zealand, 2019; pp. 1–57. [Google Scholar]
- Hubold, G.; Tomo, A.P. Age and Growth of Antarctic Silverfish Pleuragramma antarcticum Boulenger, 1902, from the Southern Weddell Sea and Antarctic Peninsula. Polar Biol. 1989, 9, 205–212. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Herculano-Houzel, S.; Lent, R. Isotropic Fractionator: A Simple, Rapid Method for the Quantification of Total Cell and Neuron Numbers in the Brain. J. Neurosci. 2005, 25, 2518–2521. [Google Scholar] [CrossRef]
- Marhounová, L.; Kotrschal, A.; Kverková, K.; Kolm, N.; Němec, P. Artificial Selection on Brain Size Leads to Matching Changes in Overall Number of Neurons. Evolution 2019, 73, 2003–2012. [Google Scholar] [CrossRef]
- Ngwenya, A.; Patzke, N.; Manger, P.R.; Herculano-Houzel, S. Continued Growth of the Central Nervous System without Mandatory Addition of Neurons in the Nile Crocodile (Crocodylus niloticus). Brain Behav. Evol. 2016, 87, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Olkowicz, S.; Kocourek, M.; Lučan, R.K.; Porteš, M.; Fitch, W.T.; Herculano-Houzel, S.; Němec, P. Birds Have Primate-like Numbers of Neurons in the Forebrain. Proc. Natl. Acad. Sci. USA 2016, 113, 7255–7260. [Google Scholar] [CrossRef]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a Neuronal Specific Nuclear Protein in Vertebrates. Development 1992, 116, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.F.M.; Manger, P.R.; Catania, K.C.; Kaas, J.H.; Herculano-Houzel, S. Greater Addition of Neurons to the Olfactory Bulb than to the Cerebral Cortex of Eulipotyphlans but Not Rodents, Afrotherians or Primates. Front. Neuroanat. 2014, 8, 23. [Google Scholar] [CrossRef][Green Version]
- Froese, R.; Pauly, D. Editors. FishBase. World Wide Web Electronic Publication. 2022. Available online: www.fishbase.org (accessed on 3 March 2022).
- Aicardi, S.; Amaroli, A.; Gallus, L.; Di Blasi, D.; Ghigliotti, L.; Betti, F.; Vacchi, M.; Ferrando, S. Quantification of Neurons in the Olfactory Bulb of the Catsharks Scyliorhinus canicula (Linnaeus, 1758) and Galeus melastomus (Rafinesque, 1810). Zoology 2020, 141, 125796. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Ferrando, S.; Amaroli, A.; Gallus, L.; Aicardi, S.; Di Blasi, D.; Christiansen, J.S.; Vacchi, M.; Ghigliotti, L. Secondary Folds Contribute Significantly to the Total Surface Area in the Olfactory Organ of Chondrichthyes. Front. Physiol. 2019, 10, 245. [Google Scholar] [CrossRef]
- Adams, D.R. Olfactory and Non-Olfactory Epithelia in the Nasal Cavity of the Mouse, Peromyscus. Am. J. Anat. 1972, 133, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Green, P.A.; Van Valkenburgh, B.; Pang, B.; Bird, D.; Rowe, T.; Curtis, A. Respiratory and Olfactory Turbinal Size in Canid and Arctoid Carnivorans. J. Anat. 2012, 221, 609–621. [Google Scholar] [CrossRef]
- Harkema, J.R. Comparative Aspects of Nasal Airway Anatomy: Relevance to Inhalation Toxicology. Toxicol. Pathol. 1991, 19, 321–336. [Google Scholar] [CrossRef]
- Martinez, Q.; Clavel, J.; Esselstyn, J.A.; Achmadi, A.S.; Grohé, C.; Pirot, N.; Fabre, P.-H. Convergent Evolution of Olfactory and Thermoregulatory Capacities in Small Amphibious Mammals. Proc. Natl. Acad. Sci. USA 2020, 117, 8958–8965. [Google Scholar] [CrossRef]
- Smith, T.D.; Bhatnagar, K.P.; Tuladhar, P.; Burrows, A.M. Distribution of Olfactory Epithelium in the Primate Nasal Cavity: Are Microsmia and Macrosmia Valid Morphological Concepts? Anat. Rec. 2004, 281A, 1173–1181. [Google Scholar] [CrossRef]
- van Valkenburgh, B.; Pang, B.; Bird, D.; Curtis, A.; Yee, K.; Wysocki, C.; Craven, B.A. Respiratory and Olfactory Turbinals in Feliform and Caniform Carnivorans: The Influence of Snout Length: Turbinals in Feliform and Caniform Carnivora. Anat. Rec. 2014, 297, 2065–2079. [Google Scholar] [CrossRef]
- Freudenthal, M.; Martín-Suárez, E. Estimating Body Mass of Fossil Rodents. Scr. Geol. 2013, 145, 1–513. [Google Scholar]
- Sharifi, M. Postnatal Growth in Myotis Blythii (Chiroptera, Vespertilionidae). Mammalia 2004, 68, 283–289. [Google Scholar] [CrossRef]
- McGann, J.P. Poor Human Olfaction is a 19th-Century Myth. Science 2017, 356, eaam7263. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.C.; Wheeler, P. The Expensive-Tissue Hypothesis: The Brain and the Digestive System in Human and Primate Evolution. Curr. Anthropol. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Kotrschal, A.; Rogell, B.; Bundsen, A.; Svensson, B.; Zajitschek, S.; Brännström, I.; Immler, S.; Maklakov, A.A.; Kolm, N. Artificial Selection on Relative Brain Size in the Guppy Reveals Costs and Benefits of Evolving a Larger Brain. Curr. Biol. 2013, 23, 168–171. [Google Scholar] [CrossRef]
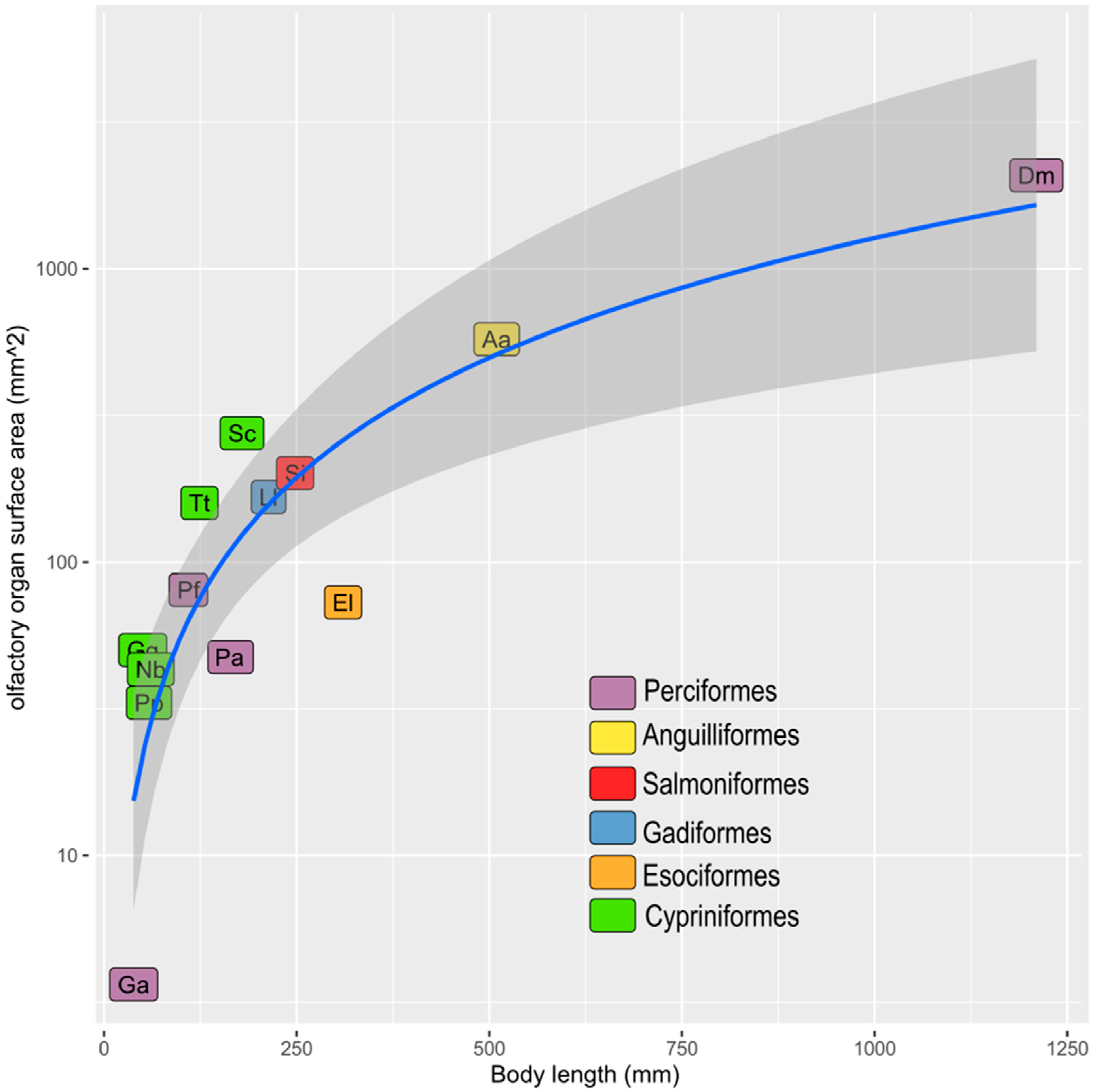

| Order | Species | SL or TL (mm) | Whole ES (mm2) | ES (mm2) | Source |
|---|---|---|---|---|---|
| Anguilliformes | Anguilla anguilla | 509.6 | 575.16 | 287.58 | Teichmann, 1954 [17] |
| Cypriniformes | Phoxinus phoxinus | 58.58 | 33.15 | 16.575 | Teichmann, 1954 [17] |
| Cypriniformes | Gobio gobio | 50.41 | 50.16 | 25.08 | Teichmann, 1954 [17] |
| Cypriniformes | Squalius cephalus | 179.2 | 275.09 | 137.545 | Teichmann, 1954 [17] |
| Cypriniformes | Tinca tinca | 123.93 | 159.09 | 79.545 | Teichmann, 1954 [17] |
| Cypriniformes | Nemachilus barbatulus | 60.64 | 43.09 | 21.545 | Teichmann, 1954 [17] |
| Esociformes | Esox lucius | 310.2 | 72.82 | 36.41 | Teichmann, 1954 [17] |
| Gadiformes | Lota lota | 213.5 | 166.532 | 83.266 | Teichmann, 1954 [17] |
| Perciformes | Perca fluviatilis | 109.62 | 80.35 | 40.175 | Teichmann, 1954 [17] |
| Perciformes | Gasterosteus aculeatus | 38.48 | 3.64 | 1.82 | Teichmann, 1954 [17] |
| Perciformes | Pleuragramma antarcticum | 158.4 | 47.42 | 23.71 | present paper |
| Perciformes | Dissostichus mawsoni | 121 | 2082 | 1041 | Ferrando et al. 2019 [16] |
| Salmoniformes | Salmo irideus | 248.15 | 201.01 | 100.505 | Teichmann, 1954 [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aicardi, S.; Bozzo, M.; Amaroli, A.; Gallus, L.; Risso, B.; Carlig, E.; Di Blasi, D.; Vacchi, M.; Ghigliotti, L.; Ferrando, S. The Arrangement of the Peripheral Olfactory System of Pleuragramma antarcticum: A Well-Exploited Small Sensor, an Aided Water Flow, and a Prominent Effort in Primary Signal Elaboration. Animals 2022, 12, 663. https://doi.org/10.3390/ani12050663
Aicardi S, Bozzo M, Amaroli A, Gallus L, Risso B, Carlig E, Di Blasi D, Vacchi M, Ghigliotti L, Ferrando S. The Arrangement of the Peripheral Olfactory System of Pleuragramma antarcticum: A Well-Exploited Small Sensor, an Aided Water Flow, and a Prominent Effort in Primary Signal Elaboration. Animals. 2022; 12(5):663. https://doi.org/10.3390/ani12050663
Chicago/Turabian StyleAicardi, Stefano, Matteo Bozzo, Andrea Amaroli, Lorenzo Gallus, Beatrice Risso, Erica Carlig, Davide Di Blasi, Marino Vacchi, Laura Ghigliotti, and Sara Ferrando. 2022. "The Arrangement of the Peripheral Olfactory System of Pleuragramma antarcticum: A Well-Exploited Small Sensor, an Aided Water Flow, and a Prominent Effort in Primary Signal Elaboration" Animals 12, no. 5: 663. https://doi.org/10.3390/ani12050663
APA StyleAicardi, S., Bozzo, M., Amaroli, A., Gallus, L., Risso, B., Carlig, E., Di Blasi, D., Vacchi, M., Ghigliotti, L., & Ferrando, S. (2022). The Arrangement of the Peripheral Olfactory System of Pleuragramma antarcticum: A Well-Exploited Small Sensor, an Aided Water Flow, and a Prominent Effort in Primary Signal Elaboration. Animals, 12(5), 663. https://doi.org/10.3390/ani12050663











