A Multi-Point Identification Approach for the Recognition of Individual Leopards (Panthera pardus kotiya)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
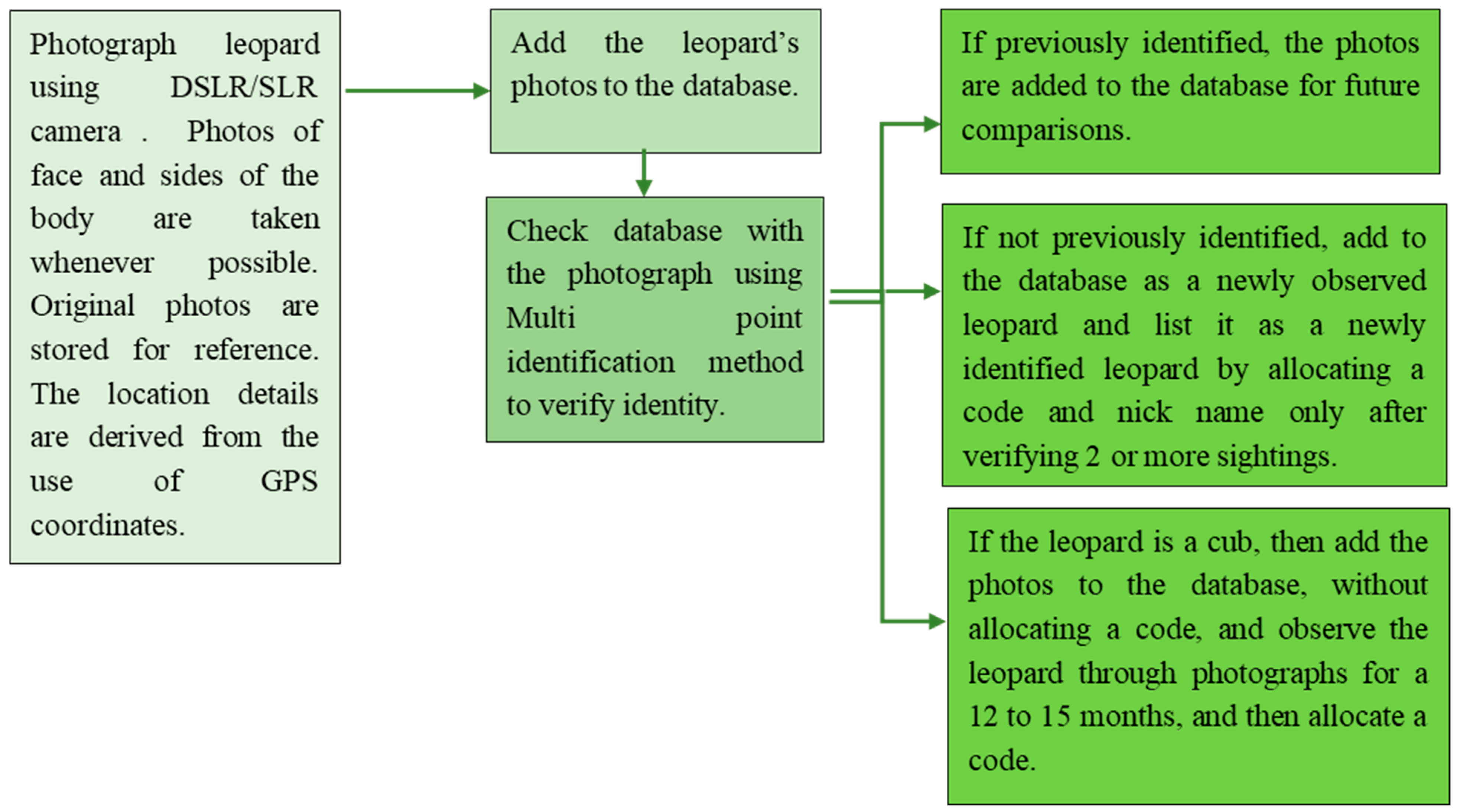
2.2. Methodology
2.3. The Visual Morphological Description of Sri Lankan Leopard
2.4. General Markings
2.5. Identification of a Leopard
2.6. Coding of Identified Leopards
2.7. Data Collection for Minimum Population Estimation of Leopards at YNP1
2.8. Data Analysis
3. Results
3.1. The Identified Leopards of Yala
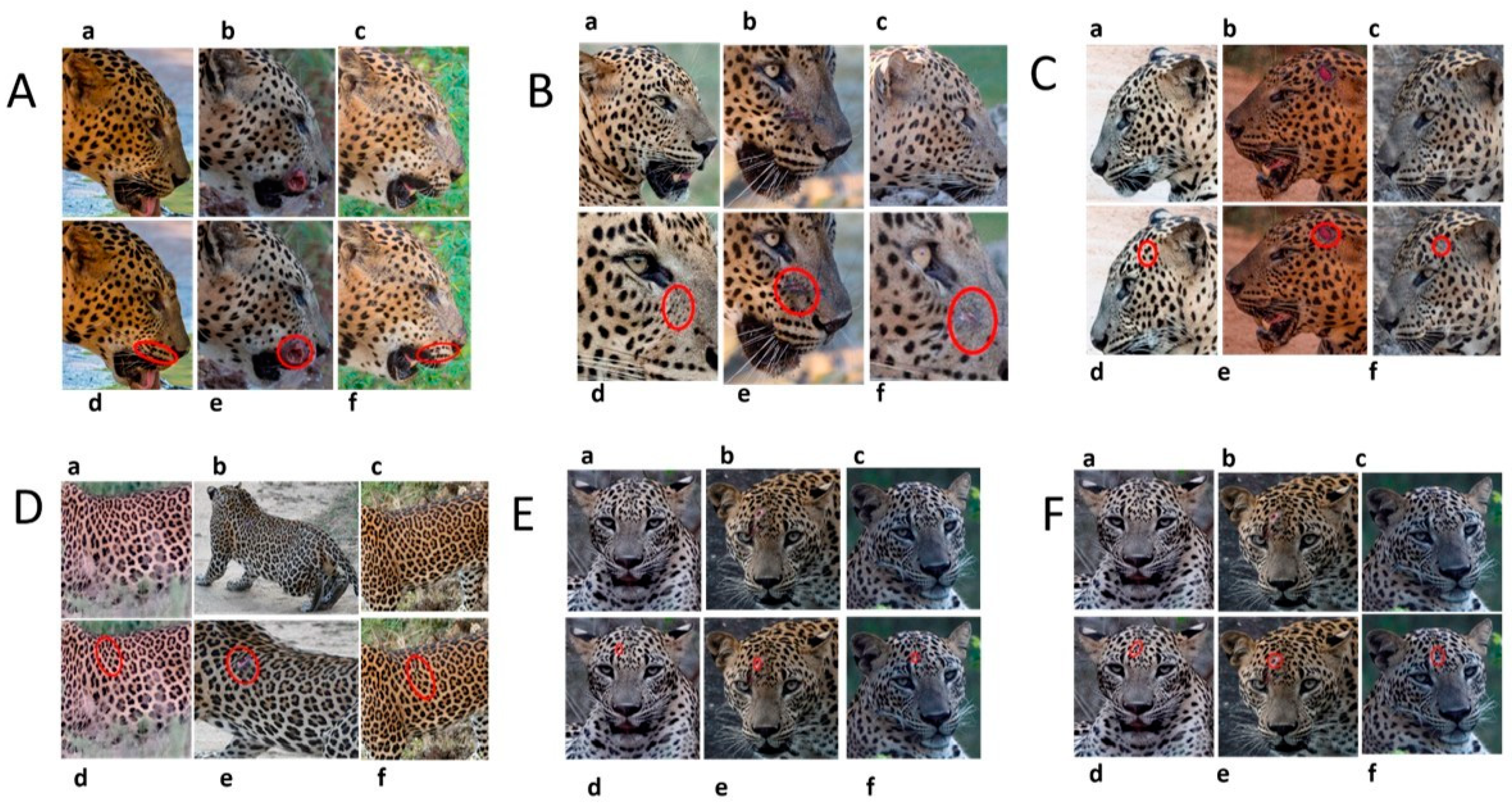
3.2. Changes in Spot Markings and Rosette Patterns of a Leopard
3.3. Changes to Spot Patterns with an Injury Being Documented
3.4. Changes to Spot Patterns without a Documented Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira-Santos, L.G.R.; Zucco, C.A.; Antunes, P.C.; Crawshaw, P.G. Is it possible to individually identify mammals with no natural markings using camera-traps? A controlled case-study with lowland tapirs. Mamm. Biol. 2010, 75, 375–378. [Google Scholar] [CrossRef]
- Rajani, C.V.; Patki, H.S.; Simanta, P.; Surjith, K.; Deepa, P.M.; Pradeep, M. Histomorphological differentiation of the skin of leopard (Panthera pardus), leopard cat (Prionailurus bengalensis), Bengal tiger (Panthera tigris), and golden jackal (Canis aureus). Vet. World 2020, 13, 827–832. [Google Scholar] [CrossRef]
- Liu, R.T.; Liaw, S.S.; Maini, P.K. Two-stage Turing model for generating pigment patterns on the leopard and the jaguar. Phys. Rev. E 2006, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, W.L.; Cuthill, I.C.; Scott-Samuel, N.E.; Baddeley, R. Why the leopard got its spots: Relating pattern development to ecology in felids. Proc. R. Soc. B Biol. Sci. 2011, 278, 1373–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittle, A.M.; Watson, A.C.; Fernando, T.S.P. The ecology and behaviour of a protected area Sri Lankan leopard (Panthera pardus kotiya) population. Trop. Ecol. 2017, 58, 71–86. [Google Scholar]
- Miththapala, S.; Seidensticker, J.; Phillips, L.G.; Fernando, S.B.U.; Smallwood, J.A. Identification of individual leopards (Panthera pardus kotiya) using spot pattern variation. J. Zool. 1989, 218, 527–536. [Google Scholar] [CrossRef]
- Stein, A.B.; Hayssen, V. Panthera pardus (Carnivora: Felidae). Mamm. Species 1856, 45, 30–48. [Google Scholar] [CrossRef] [Green Version]
- Manning, J.A.; Goldberg, C.S. Estimating population size using capture-recapture encounter histories created from point-coordinate locations of animals. Methods Ecol. Evol. 2010, 1, 389–397. [Google Scholar] [CrossRef]
- Donnelly, M.A.; Guyer, C. Estimating population size: Mark-recapture. Meas. Monit. Biol. Divers. Stand. Methods Amphib. 1994, 20, 183–200. [Google Scholar]
- Gray, T.N.E.; Vidya, T.N.C.; Maxwell, A.L.; Bharti, D.K.; Potdar, S.; Channa, P.; Sovanna, P. Using Fecal-DNA and Capture-Mark-Recapture to Establish a Baseline Asian Elephant Population for the Eastern Plains Landscape, Cambodia; WWF Greater Mekong Cambodia Country Program 1 Jawaharlal Nehru Centre for Advanced Scientific Research: Bangalore, India, 2011. [Google Scholar]
- O’brien, T.G.; Kinnaird, M.F. Density estimation of sympatric carnivores using spatially explicit capture-recapture methods and standard trapping grid. Ecol. Appl. 2011, 21, 2908–2916. [Google Scholar] [CrossRef]
- Krauss, S.L.; Roberts, D.G.; Phillips, R.D.; Edwards, C. Effectiveness of camera traps for quantifying daytime and nighttime visitation by vertebrate pollinators. Ecol. Evol. 2018, 8, 9304–9314. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Stuckas, H.; Bhaskar, R.; Khan, I.; Goyal, S.P.; Tiedemann, R. Genetically distinct population of Bengal tiger (Panthera tigris tigris) in Terai Arc Landscape (TAL) of India. Mamm. Biol. 2011, 76, 484–490. [Google Scholar] [CrossRef]
- Kawanishi, K.; Sunquist, M.E.; Eizirik, E.; Lynam, A.J.; Ngoprasert, D.; Wan Shahruddin, W.N.; Rayan, D.M.; Sharma, D.S.K.; Steinmetz, R. Near fixation of melanism in leopards of the Malay Peninsula. J. Zool. 2010, 282, 201–206. [Google Scholar] [CrossRef]
- Webb, E.L.; Choo, Y.R.; Kudavidanage, E.P.; Amarasinghe, T.R.; Sumith Indika Bandara, U.G.; Charitha Lakmali Wanninayaka, W.A.; Ravindrakumar, P.; Nimalrathna, T.S.; Liang, S.H.; Hwee Chua, M.A. Leopard activity patterns in a small montane protected area highlight the need for integrated, collaborative landscape conservation. Glob. Ecol. Conserv. 2020, 23, e01182. [Google Scholar] [CrossRef]
- Ghezzo, E.; Rook, L. The remarkable Panthera pardus (Felidae, Mammalia) record from Equi (Massa, Italy): Taphonomy, morphology, and paleoecology. Quat. Sci. Rev. 2015, 110, 131–151. [Google Scholar] [CrossRef]
- Harihar, A.; Pandav, B.; Goyal, S.P. Density of leopards (Panthera pardus) in the Chilla Range of Rajaji National Park, Uttarakhand, India. Mammalia 2009, 73, 68–71. [Google Scholar] [CrossRef]
- Park, H.; Lim, A.; Choi, T.Y.; Baek, S.Y.; Song, E.G.; Park, Y.C. Where to spot: Individual identification of leopard cats (Prionailurus bengalensis euptilurus) in South Korea. J. Ecol. Environ. 2019, 43, 1–5. [Google Scholar] [CrossRef]
- Da Silva, L.G.; Kawanishi, K.; Henschel, P.; Kittle, A.; Sanei, A.; Reebin, A.; Miquelle, D.; Stein, A.B.; Watson, A.; Kekule, L.B.; et al. Mapping black panthers: Macroecological modeling of melanism in leopards (Panthera pardus). PLoS ONE 2017, 12, e0170378. [Google Scholar] [CrossRef] [Green Version]
- Grey, J.N.C.; Kent, V.T.; Hill, R.A. Evidence of a high density Population of harvested leopards in a montane environment. PLoS ONE 2013, 8, e82832. [Google Scholar] [CrossRef] [Green Version]
- Green, A.M.; Chynoweth, M.W.; Şekercioğlu, Ç.H. Spatially Explicit Capture-Recapture Through Camera Trapping: A Review of Benchmark Analyses for Wildlife Density Estimation. Front. Ecol. Evol. 2020, 8. [Google Scholar] [CrossRef]
- Thompson, C.M.; Royle, J.A.; Garner, J.D. A framework for inference about carnivore density from unstructured spatial sampling of scat using detector dogs. J. Wildl. Manag. 2012, 76, 863–871. [Google Scholar] [CrossRef]
- Miththapala, S.; Seidensticker, J.; O’Brien, S.J. Phylogeographic subspecies recognition in leopards (Panthera pardus): Molecular genetic variation. Conserv. Biol. 1996, 10, 1115–1132. [Google Scholar] [CrossRef] [Green Version]
- Uphyrkina, O.; Johnson, W.E.; Quigley, H.; Miquelle, D.; Marker, L.; Bush, M.; O’Brien, S.J. Phylogenetics, genome diversity and origin of modern leopard, Panthera pardus. Mol. Ecol. 2001, 10, 2617–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittle, A.M.; Watson, A.C.; Kumara, P.H.S.C.; Sandanayake, S.D.K.C.; Sanjeewani, H.K.N.; Fernando, T.S.P. Notes on the diet and habitat selection of the Sri Lankan Leopard Panthera pardus kotiya (Mammalia: Felidae) in the central highlands of Sri Lanka. J. Threat. Taxa 2014, 6, 6214–6221. [Google Scholar] [CrossRef] [Green Version]
- Kittle, A.M.; Watson, A.C.; Cushman, S.A.; Macdonald, D.W. Forest cover and level of protection influence the island-wide distribution of an apex carnivore and umbrella species, the Sri Lankan leopard (Panthera pardus kotiya). Biodivers. Conserv. 2018, 27, 235–263. [Google Scholar] [CrossRef]
- Kittle, A.M.; Watson, A.C. Density of leopards (Panthera pardus kotiya) in Horton Plains National Park in the Central Highlands of Sri Lanka. Mammalia 2018, 82, 183–187. [Google Scholar] [CrossRef]
- Kittle, A.; Trust, W.C. Panthera pardus spp. Kotoya, The IUCN Red List of Threatened Species: Technical Report. e.T15959A50660847. 2020. Available online: https://www.iucnredlist.org/species/15959/50660847 (accessed on 1 January 2022). [CrossRef]
- Farhadinia, M.S.; Farahmand, H.; Gavashelishvili, A.; Kaboli, M.; Karami, M.; Khalili, B.; Montazamy, S. Molecular and craniological analysis of leopard, Panthera pardus (Carnivora: Felidae) in Iran: Support for a monophyletic clade in Western Asia. Biol. J. Linn. Soc. 2015, 114, 721–736. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.G. Ecology and Evolution of Melanism in Big Cats: Case Study with Black Leopards and Jaguars. Big Cats 2017, 6, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Wattegedera, M.; Siriwardena, R.; Silva, D. Leopard Diary; Yala National Park: Yala, Sri lanka, 2019; Volume 1, Available online: https://yalaleoparddiary.com/projects/leopard-identification (accessed on 1 January 2022).
- Jones, B.; Sall, J. JMP statistical discovery software. Using JMP Wiley Interdiscip. Rev. Comput. Stat. 2012, 3, 188–194. [Google Scholar] [CrossRef]
- Laake, J.L.; Buckland, S.T.; Anderson, D.R.; Burnham, K.P. Distance User’s Guide V2.2; Colorado Cooperative Fish & Wildlife Research Unit Colorado State University: Fort Collins, CO, USA, 1996; p. 82. [Google Scholar]
- Kelly, M.J.; Noss, A.J.; Di Bitetti, M.S.; Maffei, L.; Arispe, R.L.; Paviolo, A.; De Angelo, C.D.; Di Blanco, Y.E. Estimating puma densities from camera trapping across three study sites: Bolivia, Argentina, and Belize. J. Mammal. 2008, 89, 408–418. [Google Scholar] [CrossRef]
- Morrison, T.A.; Yoshizaki, J.; Nichols, J.D.; Bolger, D.T. Estimating survival in photographic capture-recapture studies: Overcoming misidentification error. Methods Ecol. Evol. 2011, 2, 454–463. [Google Scholar] [CrossRef]
- Seymour, B.K.L. Panthera onca. Am. Soc. Mammal. 1989, 340, 1–9. Available online: http://www.mammalogy.org/panthera-onca-2193 (accessed on 1 January 2022). [CrossRef]
- Henschel, P.; Ray, J. Leopards in African Rainforests: Survey and Monitoring Techniques. Tracks A J. Artist. Writ. 2003, 33, 54. [Google Scholar]
- Balme, G.A.; Batchelor, A.; De Woronin Britz, N.; Seymour, G.; Grover, M.; Hes, L.; Macdonald, D.W.; Hunter, L.T.B. Reproductive success of female leopards Panthera pardus: The importance of top-down processes. Mamm. Rev. 2013, 43, 221–237. [Google Scholar] [CrossRef]
- Friday, N.A.; Smith, T.D.; Stevick, P.T.; Allen, J.; Fernald, T. Balancing bias and precision in capture-recapture estimates of abundance. Mar. Mamm. Sci. 2008, 24, 253–275. [Google Scholar] [CrossRef]
- Van Valkenburgh, B.V.; Ruff, C.B. Canine tooth strength and large carnivores. J. Zool. 1987, 212, 379–397. [Google Scholar] [CrossRef]
- Wayne, R.K.; Modi, W.S.; O’Brien, S.J. Morphological Variability and Asymmetry in the Cheetah (Acinonyx jubatus), a Genetically Uniform Species. Evolution 1986, 40, 78–85. [Google Scholar] [CrossRef]
- Villumsen, A.L.; Zachau-Christiansen, B. Spontaneous alterations in position of the testes. Arch. Dis. Child. 1966, 41, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Powell, R.A. Animal Home Ranges and Territories and Home Range Estimators. Res. Tech. Anim. Ecol. Controv. Conseq. 2000, 442, 65–110. [Google Scholar]
- Mitchell, M.S.; Powell, R.A. Optimal use of resources structures home ranges and spatial distribution of black bears. Anim. Behav. 2007, 74, 219–230. [Google Scholar] [CrossRef]
- Boitani, L.; Fuller, T. Research Techniques in Animal Ecology; Columbia University Press: New York, NY, USA, 2000; p. 442. [Google Scholar]




| Skin Coat Segregation | Left Side Nasal Spot | Left Side Mystacial Row 1 | Left Side Mystacial Row 2 | Left Side of the Face | Neck—Left Side | Left Side Foreleg |
|---|---|---|---|---|---|---|
| MPC Point | 1 | 2 | 3 | 4 | 5 | 6 |
| Photograph of the segregation (Marked within the red area) |  |  |  |  |  |  |
| Skin Coat Segregation | Right Side Flank | Forehead | Left Side Flank | |||
| MPC Point | 9 | 8 | 7 | |||
| Photograph of the segregation (Marked within the red area) |  |  |  | |||
| Skin Coat Segregation | Right side Foreleg | Neck—Right Side | Right Side of the Face | Right side Nasal Spots | Right Side Mystacial Row 1 | Right Side Mystacial Row 2 |
| MPC Point | 10 | 11 | 12 | 13 | 14 | 15 |
| Photograph of the segregation (Marked within the red area) |  |  |  |  |  |  |
| Age Category | Abbreviation Used | Basis of Separation |
|---|---|---|
| Cub | Cub | A Leopard whose estimated age is 6 months. |
| Sub adult | SA | The period of 30 months after observation as a cub. |
| Early adult | EA | The period between 31 months and 45 months after observation as a cub or if the estimated age is less than 4 years. |
| Adult | A | An estimated age between 4 years and 8 years. |
| Aged adult | AA | An estimated age >8 years. |
| Spot Location | Picture—Guide | Picture—Described (PD) | Methodology Used * |
|---|---|---|---|
| Left-side Nasal spots (LNS)—Used for PCA |  |  | The LNS is the first row of spots in the left nasal area. The spot count in this area was carried out visually and recorded. The spot count was used for the analysis. These spots are commonly present in all leopards. |
| Left side Mystacial Row 1 (LM1)—Used for PCA |  |  | The cluster of spots immediately underneath the LNS. This cluster is within the mystacial area of the left side of the face. These spots are commonly present in all leopards. This cluster could be of varying patterns, and the patterns are not identical on both sides of the face. The spot count in this area was carried out visually and recorded. The spot count was used for analysis. |
| Left-side Mystacial 0Row 2 (LM2)—Used for PCA |  |  | The row of spots immediately underneath LM1. This consists of a row of spots in all leopards. This row is within the mystacial area of the left side of the face. These spots are commonly present in all leopards. The spot count in this area was carried out visually and recorded. The spot count was used for the analysis. |
| Left-side Extra Spots Above (LAM2)—Used for PCA |  |  | The spots that are positioned above LM2 but that are not a part of LM1. These spots are within the mystacial area of the left side of the face. These spots are not commonly present in all leopards. the spot count in this area was carried out visually and recorded. The spot count was used for the analysis. |
| Left-side Extra Spots Below (LBM2)—Used for PCA |  |  | The spots that are positioned just below LM2. These spots are above the mystacial row below LM2. LBM2 consists of less spots than LM2. These spots are not commonly present in all leopards. These spots are within the mystacial area of the left side of the face. The spot count in this area was carried out visually and recorded. The spot count was used for the analysis. |
| Right-side Nasal spots (RNS)—Used for PCA |  |  | The RNS is the first row of spots in the right nasal area. The spot count in this area was carried out visually and recorded. The spot count was used for the analysis. These spots are commonly present in all leopards. |
| Right-side Mystacial Row 1 (RM1)—Used for PCA |  |  | The cluster of spots immediately underneath the RNS. This cluster is within the mystacial area of the right side of the face. These spots are commonly present in all leopards. This cluster can be of varying patterns, and the patterns are not identical on both sides of the face. The spot count in this area was carried out visually and recorded. The spot count was used for the analysis. |
| Right-side Mystacial Row 2 (RM2)—Used for PCA |  |  | The row of spots immediately underneath the RM1. This consists of a row of spots in all leopards. This row is within the mystacial area of the right side of the face. These spots are commonly present in all leopards. The spot count in this area was carried out visually and recorded. The spot count was used for the analysis. |
| Right-side Extra Spots Above (RAM2)—Used for PCA |  |  | The spots that are positioned above RM2 but that are not a part of RM1. These spots are within the mystacial area of the right side of the face. These spots are not commonly present in all leopards. The spot count in this area was carried out visually and recorded. The spot count was used for the analysis. |
| Right-side Extra Spots Below (RBM2)—Used for PCA |  |  | The spots that are positioned just below RM2. These spots are above the mystacial row, which is below RM2. RBM2 consists of less spots than RM2. These spots are not present in all leopards. The spot count in this area was carried out visually and recorded. The RBM2 spots were counted for the analysis. |
| The Guide Arch of the Forehead |  |  | A prominent row of large spots starting from the top-inner corner of the area above each eye and that continues to the base of the ear lobe area. This row of spots forms a pattern similar with a less-angled arch and is present in all leopards. The formation of the pattern is not common to all leopards, as the spot sizes and shapes differ in each individual. We termed this pattern as the “guide arch”. |
| Elongated Spots in the guide arch of the Forehead |  |  | This is the first spot located in the guide arch area above the top-inner corner of each eye and has a comparatively longer shaped spot. We termed this spot as the “elongated spot”. Vibrissae is present in the periphery of the elongated spots on both sides of the forehead. |
| The genesis spots of the guide arch in the Forehead |  |  | The spot directly above the elongated spot in the guide arch was termed as the “genesis spot”. This is the 2nd spot of the guide arch and is located on each side of the forehead. |
| Forehead 1 (F1)—Used for PCA |  |  | The spot count of the row of spots positioned between the lower portion of the elongated spot of the guide arches on either side of the face. The lower area of the elongated spot is the lower corner of the spot facing towards the top inner corner of the eye. The spots in the F1 area spread upwards, towards the spots of F2 and downwards towards the area between the eyes. The spot markings in this area are present in all leopards. The clearly visible spots in the area below F2 near the muzzle have all been counted as F1 spots. |
| Forehead 2 (F2)—Used for PCA |  |  | The F2 spots comprise the prominent formation that starts from the inner side of the left genesis spots of the left guide arch and continue to the inner side of the right genesis spot of the right guide arch. The number of spots in the F2 have been counted for the analysis. Any spots above the F2 spot for pattern formation are taken as F3 spots. Any spots below the F2 spot for pattern formation are taken as F1 spots. |
| Forehead (F3)—Used for PCA |  |  | The F3 spots are the spots above and on the inner side of the F2 spot location for pattern formation. The F3 area continues until the 4th spot of the guide arch. The spots between the 4th spot of the left guide arch and the 4th spot of the right guide arch, both of which spread below F2 spot location for pattern formation were counted for the analysis. |
| Left side near eye (Leye)—Used for PCA |  |  | The number of spots in the semi-oval pattern starting above the inner area of the left eye and that continues to the outer end of the left eye. The number of spots was counted and used for the analysis. The pattern is present in all leopards. There are slight variations in the appearance of the pattern. |
| Side of the Face |  |  | The side of the face is the area between the guide arch in the forehead, the mystacial area, and the neck area. This area only consists of spots of varying sizes and shapes. No rosettes were observed on the sides of the face of any leopard. Both sides of the facial area were considered for leopard identification. |
| Mandibular Spot Formation of both Sides of the face |  |  | Distinctive pattern formation can be observed in the skin coat of the mandibular area on the side of the face of a leopard. A pattern of spots taking the shape of the circumference of an oblong or a circular pattern was present in all leopards observed by the team. The number of spots in the circumference of this pattern ranges between 9 and 13 spots. The inner side of the circumference of this pattern consists of a space with only between 1 to 3 spots and vibrissae in 1 or 2 points. This is the only area where vibrissae, apart from the mystacial area and the periphery of the elongated spots, is present in the side of the face. We termed this spot pattern as the “mandibular spot formation”. |
| The Upper Neck area (Both sides of the Leopard) |  |  | The upper neck area is the area between the side of the face and the foreleg. The upper neck is defined as the skin coat of the upper area between the rear upper corner of each earlobe and the front upper foreleg area. The upper neck area consists of rosettes and spots. Rosettes are more spaced out in the upper neck than in the other areas of the skin coat. This is present on both sides of the leopard. |
| Left-side Lower neck (LNeck)—Used for PCA |  |  | The number of broken lines consisting of spots starting from the left ear and ending in the lower neck area. The left foreleg area was visually observed and counted for the analysis. The pattern of broken lines consisting of spots is present in all leopards. The number of lines differ from each individual. |
| Right-side near eye (Reye)—Used for PCA |  |  | The number of spots in the semi-oval pattern starts above the inner area of the right eye and continues to the outer end of the right eye. The number of spots was counted and used for the analysis. The pattern is present in all leopards. There are slight variations in the appearance of the pattern. |
| Right-side Lower Neck (RNeck)—Used for PCA |  |  | The number of broken lines consisting of spots, starting from the right ear and ending in the right neck area and the right foreleg area, was visually observed and counted for the analysis. The pattern of broken lines consisting of spots is present in all leopards, but the number of lines differs. |
| Left-side Upper Foreleg-Rank (LForeleg-Rank)—Used for PCA |  |  | The spots formation along the left side of the upper foreleg was ranked considering the spacing of the spots. If the spacing between the spots is comparatively smaller, then the ranking is 2. If the spacing between the spots is higher, then the ranking is 3. This is a visual ranking based on the images collected. |
| Right-side Upper Foreleg-Rank (RForeleg-Rank)—Used for PCA |  |  | The spots formation of the right-side upper foreleg was ranked considering the spacing of the spots. If the spacing between the spots is comparatively smaller, the ranking is 2. If the spacing between the spots is higher, then the ranking is 3. This is a visual ranking based on the images collected. |
| Flank—Both sides of the Leopard |  |  | The flank of the leopard is described as the area between the tail and the lower foreleg area. The flank consists of spot and rosette formations in all leopards. The spot and rosette formations on both sides of the flank of the same leopard are different. The spot and rosette formations of each individual are different from each other. We did not come across any identical rosette formations in any of the leopards. We are continuing further studies on the spot and rosette formations on the flank. |
| Age Status | Female | Male | Minimum Population of Leopards (Combined Sexs) | Density of Leopards per Square Kilometer (km2) of the YNP1 (Area of YNP 1 Is 141 km2) |
|---|---|---|---|---|
| Listed as at the census date (31 March 2021) | ||||
| Sub Adult | 05 | 07 | 12 | |
| Early Adult | 04 | 06 | 10 | |
| Adult | 16 | 13 | 29 | |
| Aged Adult | 07 | 02 | 09 | |
| Total Listed Leopard at Census | 32 | 28 | 60 | |
| Unlisted but identified Leopards as at the Census date | ||||
| Cub | 01 | 02 | 03 | |
| Sub Adult | 03 | 05 | 08 | |
| Early Adult | Nil | Nil | Nil | |
| Adult | 03 | 02 | 05 | |
| Aged Adult | 01 | Nil | Nil | |
| Total Unlisted Leopards at Census | 08 | 09 | 17 | |
| Minimum Population of Leopards | 40 | 37 | 77 | 0.5 (~1) Leopard per km2 |
| Sightings | Density/km2 | % Co-efficient of variation | 95%Cl/km2 | Margin of error |
| 366 | 0.5461 | 53.04 | 19.25 ± 2.28 | 2.28 |
| Spot Area | Obliterate Changes | Rejig Changes | Probability of Occurrence |
|---|---|---|---|
| Forehead 1 | 8 | 6 | 0.2373 |
| Forehead 2 | 1 | 3 | 0.0677 |
| Forehead 3 | 0 | 3 | 0.0508 |
| Left Side Flank | 0 | 1 | 0.0169 |
| Left Side Mystacial Row 1 | 1 | 0 | 0.0169 |
| Left side of the face | 2 | 2 | 0.0677 |
| Right Side Mystacial Row 1 | 0 | 1 | 0.0169 |
| Right side Nasal Spots | 1 | 0 | 0.0169 |
| Right Side of the Face | 1 | 0 | 0.0169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattegedera, M.; Silva, D.; Sooriyabandara, C.; Wimaladasa, P.; Siriwardena, R.; Piyasena, M.; Marasinghe, R.M.S.L.R.P.; Hathurusinghe, B.M.; Nilanthi, R.M.R.; Gunawardena, S.; et al. A Multi-Point Identification Approach for the Recognition of Individual Leopards (Panthera pardus kotiya). Animals 2022, 12, 660. https://doi.org/10.3390/ani12050660
Wattegedera M, Silva D, Sooriyabandara C, Wimaladasa P, Siriwardena R, Piyasena M, Marasinghe RMSLRP, Hathurusinghe BM, Nilanthi RMR, Gunawardena S, et al. A Multi-Point Identification Approach for the Recognition of Individual Leopards (Panthera pardus kotiya). Animals. 2022; 12(5):660. https://doi.org/10.3390/ani12050660
Chicago/Turabian StyleWattegedera, Milinda, Dushyantha Silva, Chandana Sooriyabandara, Prasantha Wimaladasa, Raveendra Siriwardena, Mevan Piyasena, Ranjan M. S. L. R. P. Marasinghe, Bhagya M. Hathurusinghe, Rajapakse M. R. Nilanthi, Sadeepa Gunawardena, and et al. 2022. "A Multi-Point Identification Approach for the Recognition of Individual Leopards (Panthera pardus kotiya)" Animals 12, no. 5: 660. https://doi.org/10.3390/ani12050660
APA StyleWattegedera, M., Silva, D., Sooriyabandara, C., Wimaladasa, P., Siriwardena, R., Piyasena, M., Marasinghe, R. M. S. L. R. P., Hathurusinghe, B. M., Nilanthi, R. M. R., Gunawardena, S., Peiris, H., Seneviratne, P., Sendanayake, P. C., Dushmantha, C., Chandrasena, S., Gooneratne, S. S., Premaratne, P., Wickremaratne, S., & Mahela, M. (2022). A Multi-Point Identification Approach for the Recognition of Individual Leopards (Panthera pardus kotiya). Animals, 12(5), 660. https://doi.org/10.3390/ani12050660










