Ultrasound-Guided Saphenous Nerve Block in Rabbits (Oryctolagus cuniculus): A Cadaveric Study Comparing Two Injectate Volumes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
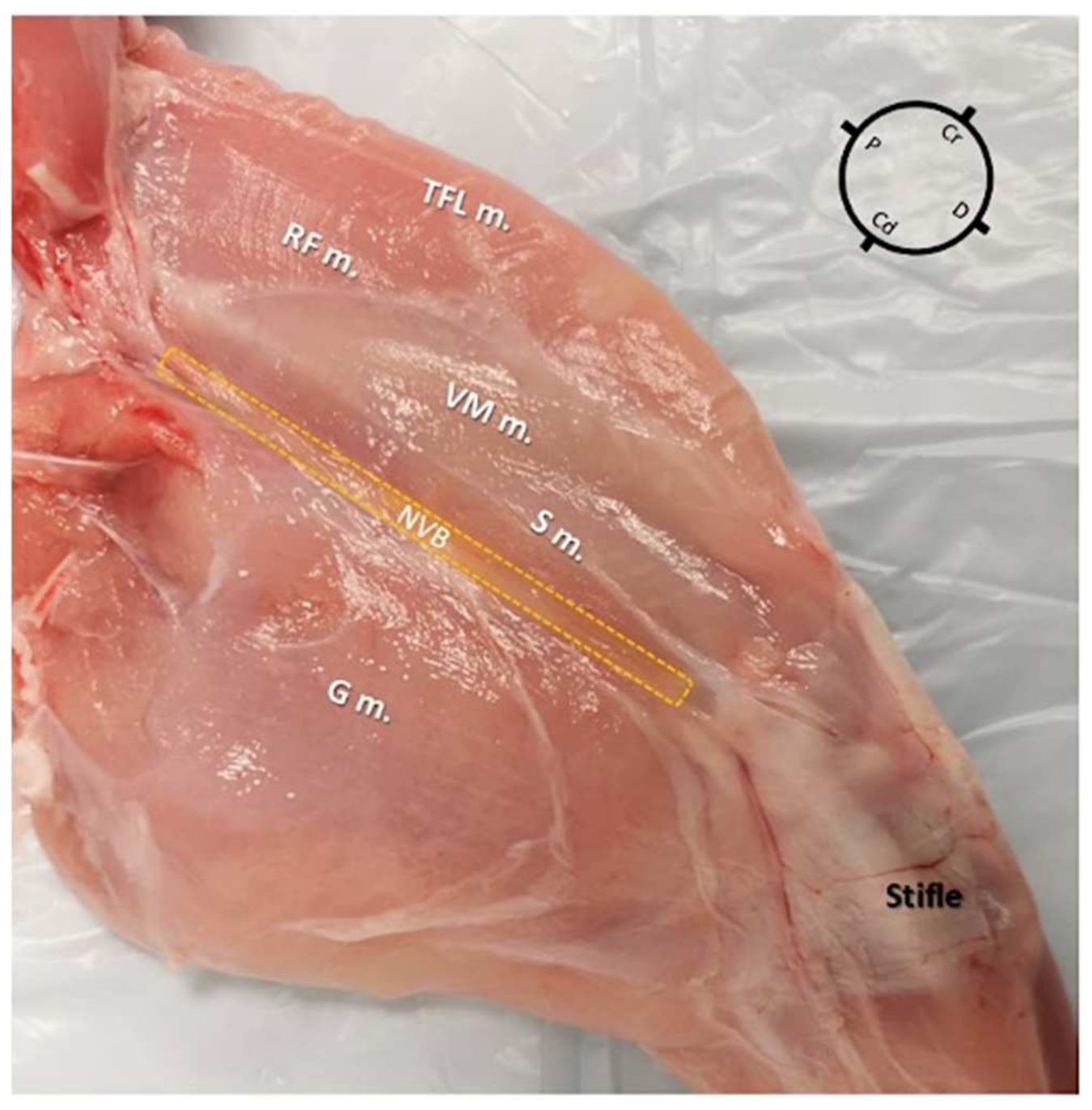
2.1. Gross Anatomical Investigation
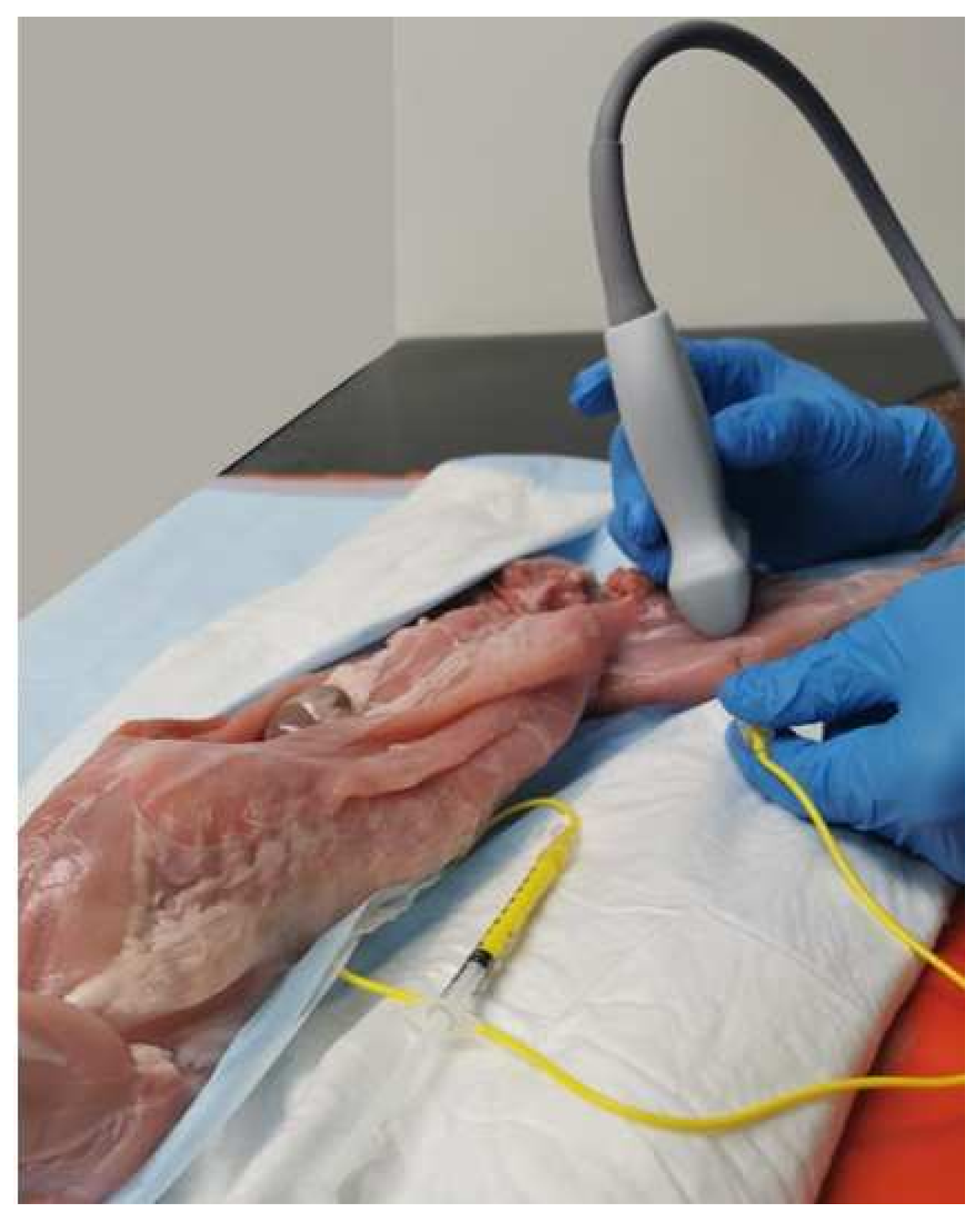
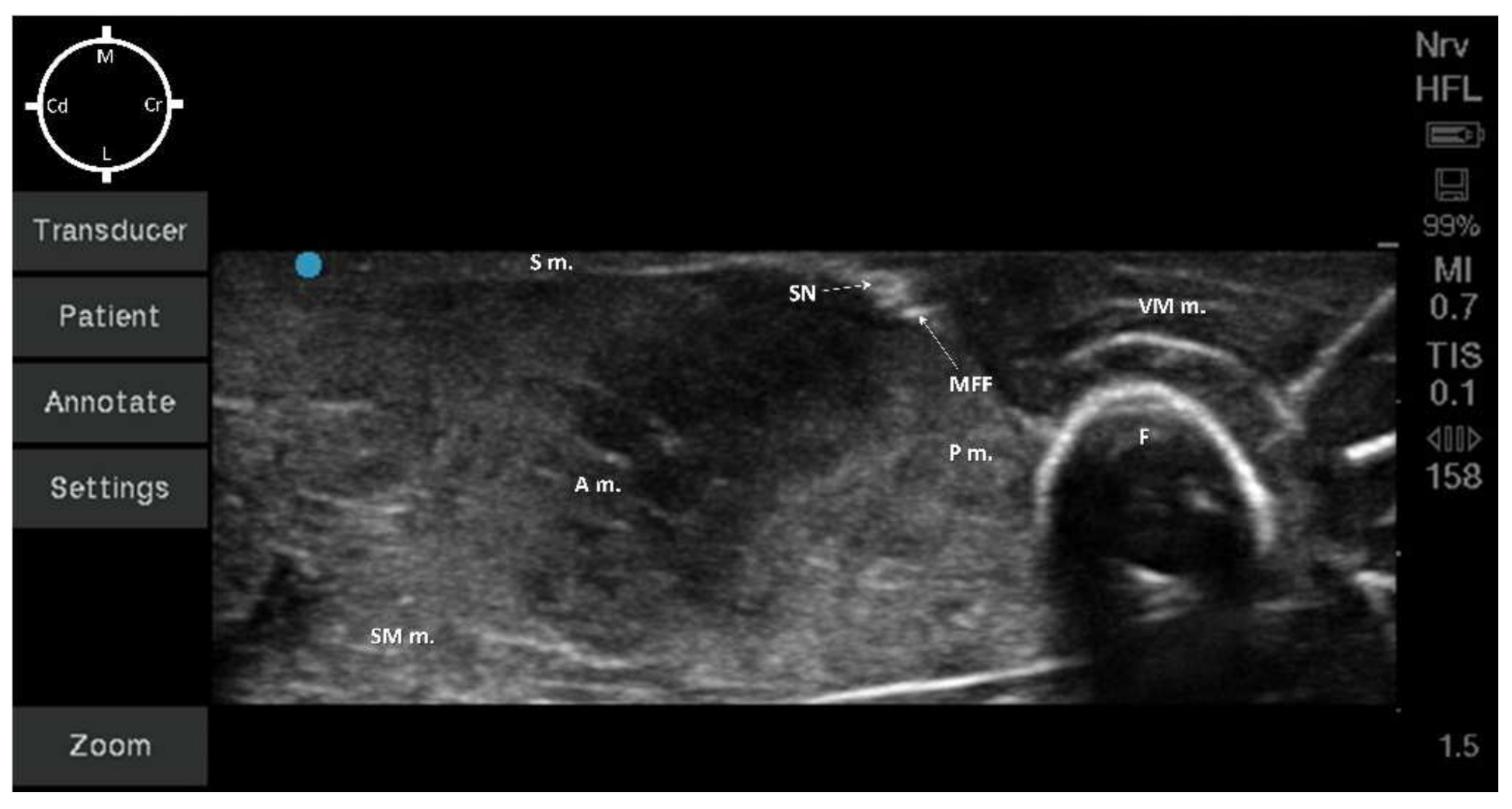
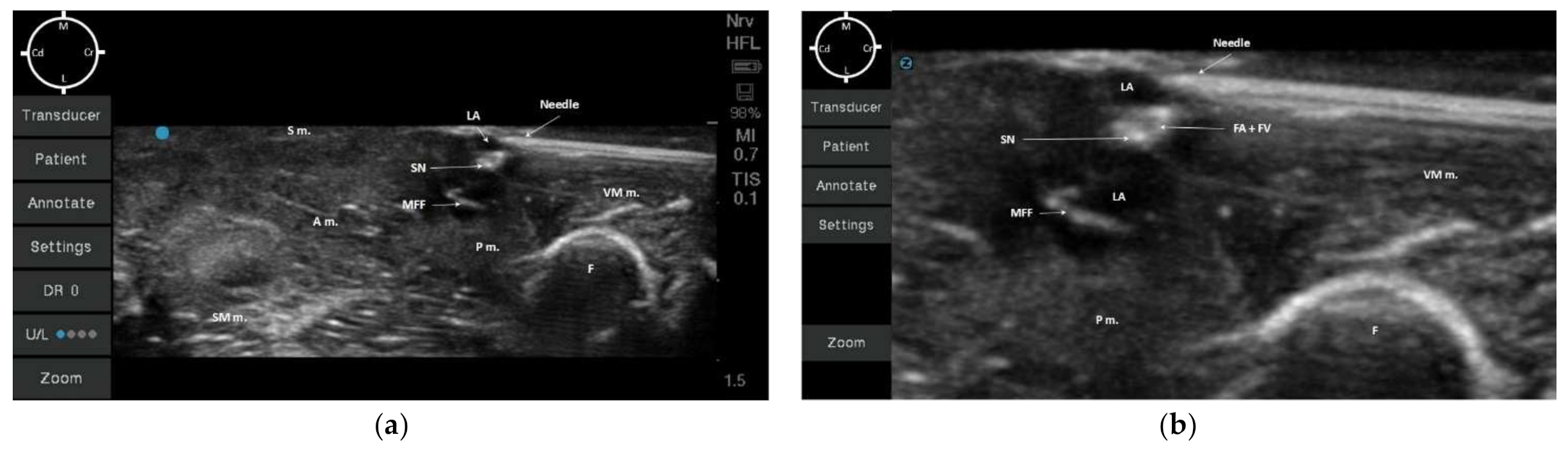
2.2. Sono-Anatomy Study and US-Guided Saphenous Nerve Block Design
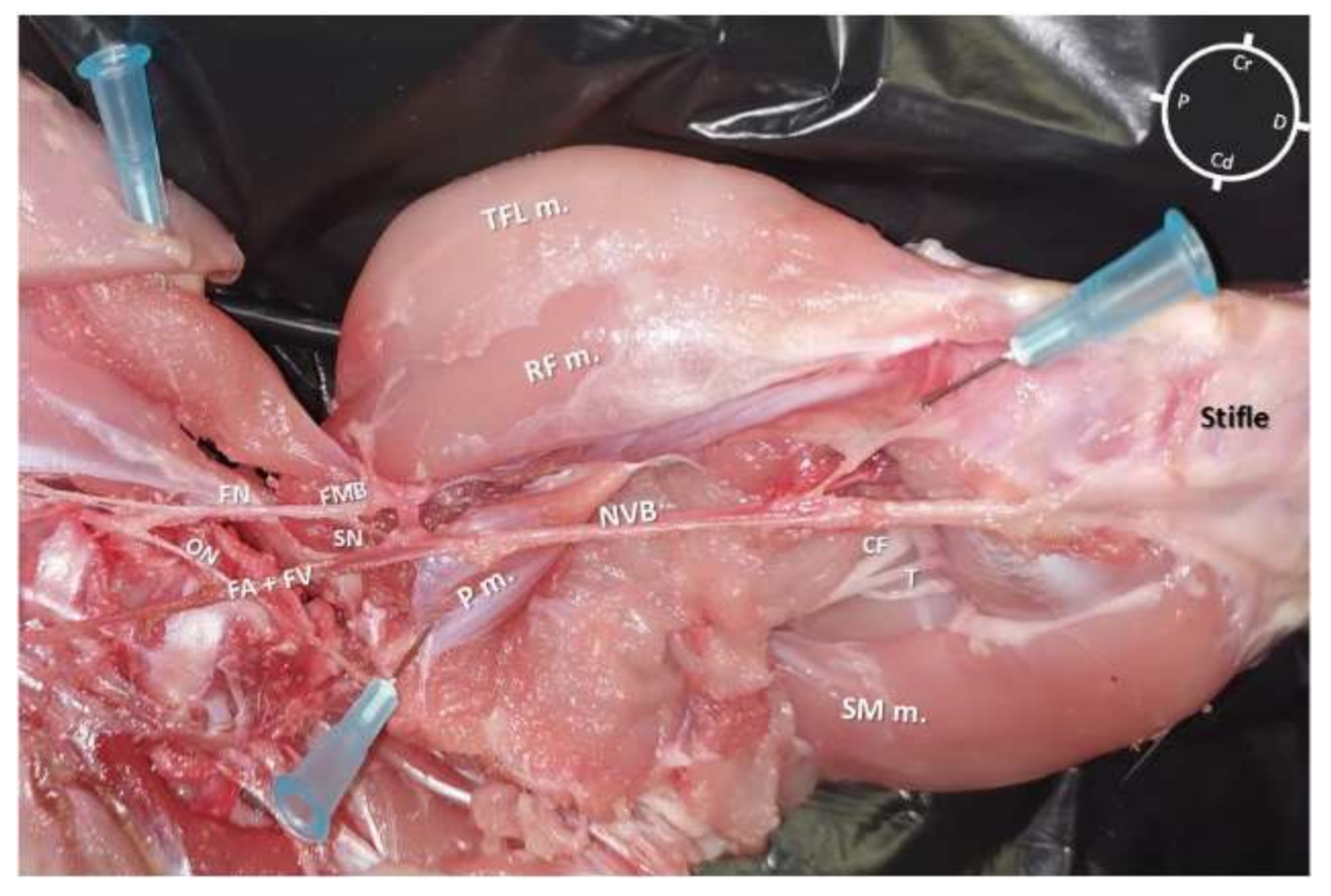
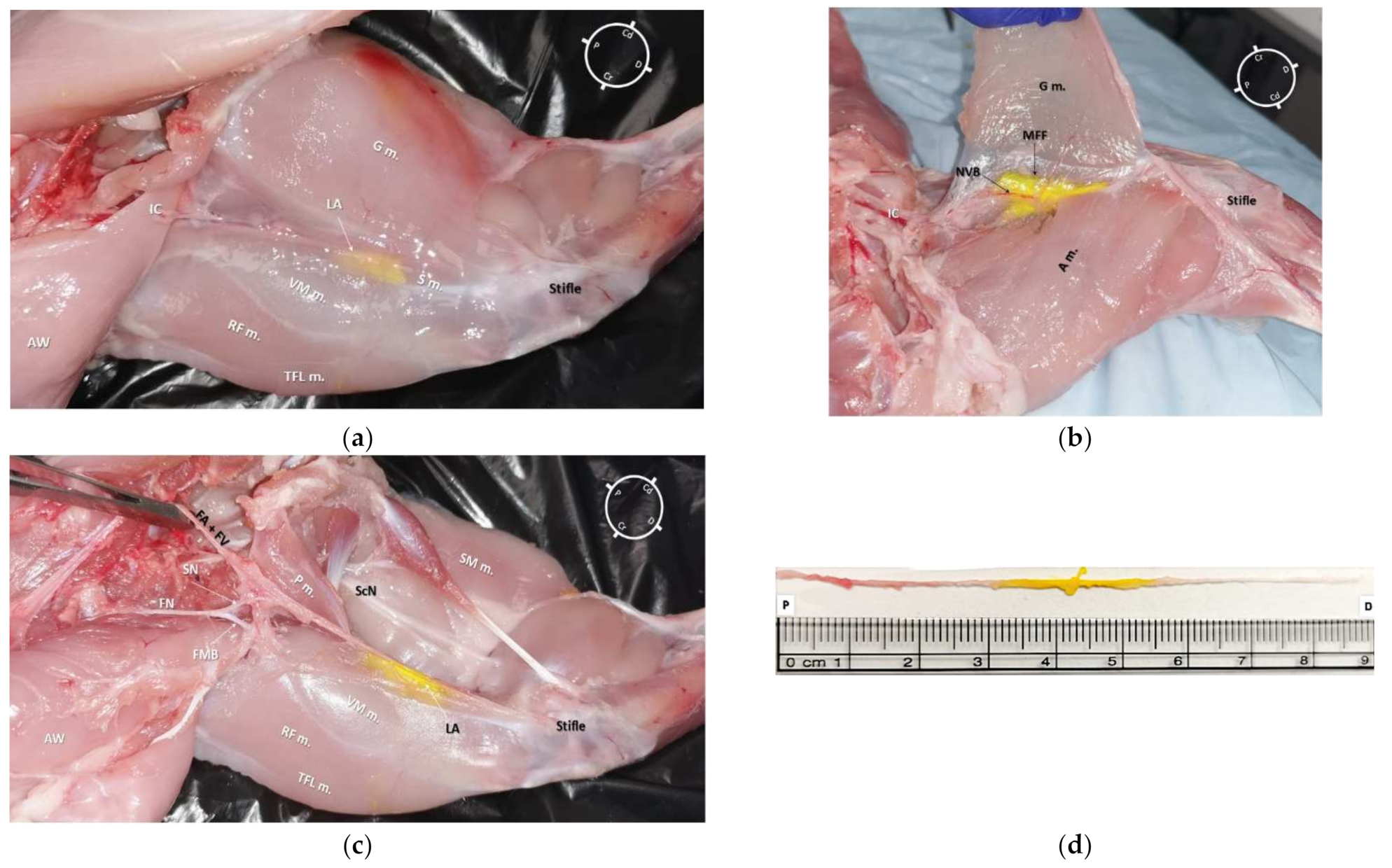
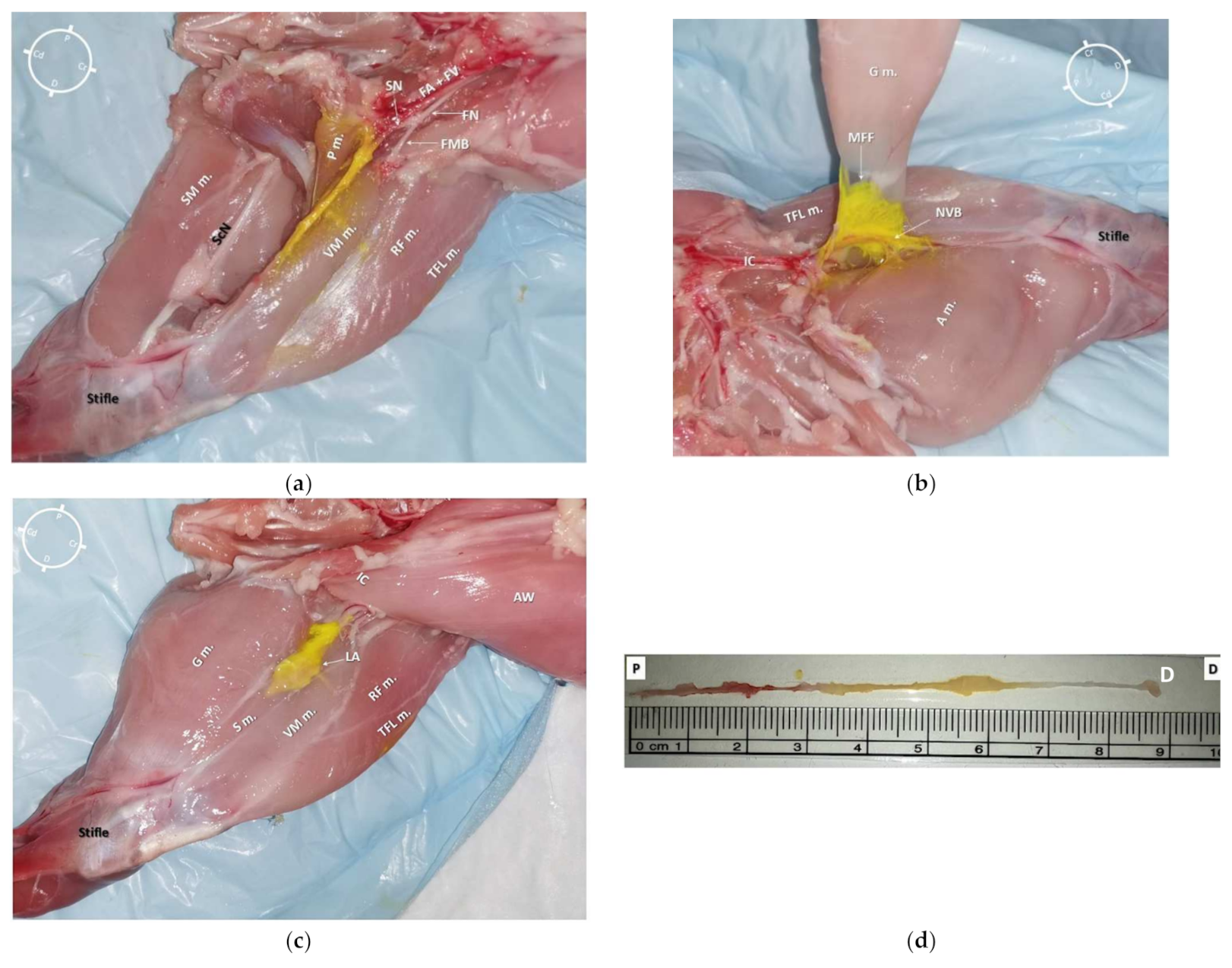
2.3. Anatomical Dissection following US-Guided Saphenous Nerve Block
2.4. Statistical Analysis
3. Results
3.1. Gross Anatomical Investigation
3.2. Sono-Anatomy Study and US-Guided Saphenous Nerve Block
3.3. Anatomical Dissection following US-Guided Saphenous Nerve Block
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Machin, H.; Adami, C. Peri-anaesthetic mortality and nonfatal gastrointestinal complications in pet rabbits: A retrospective study on 210 cases. Vet. Anaesth. Analg. 2018, 45, 520–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodbelt, D.; Blissitt, K.; Hammond, R.; Neath, P.; Young, L.; Pfeiffer, D.; Wood, J. The risk of death: The Confidential Enquiry into Perioperative Small Animal Fatalities. Vet. Anaesth. Analg. 2008, 35, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Schnellbacher, R.W.; Divers, S.J.; Comolli, J.R.; Beaufrère, H.; Maglaras, C.H.; Andrade, N.; Barbur, L.A.; Rosselli, D.D.; Stejskal, M.; Barletta, M.; et al. Effects of intravenous administration of lidocaine and buprenorphine on gastrointestinal tract motility and signs of pain in New Zealand White rabbits after ovariohysterectomy. Am. J. Vet. Res. 2017, 78, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Benato, L.; Rooney, N.; Murrell, J. Pain and analgesia in pet rabbits within the veterinary environment: A review. Vet. Anaesth. Analg. 2019, 46, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampshire, V.; Robertson, S. Using the facial grimace scale to evaluate rabbit wellness in post-procedural monitoring. Lab. Anim. 2015, 44, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Banchi, P.; Quaranta, G.; Ricci, A.; von Degerfeld, M.M. Reliability and construct validity of a composite pain scale for rabbit (CANCRS) in a clinical environment. PLoS ONE 2020, 15, e0221377. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J. Use of behaviour analysis to recognize pain in small mammals. Lab. Anim 2007, 36, 43–48. [Google Scholar] [CrossRef]
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef]
- Portela, D.A.; Verdier, N.; Otero, P.E. Regional anesthetic techniques for the pelvic limb and abdominal wall in small animals: A review of the literature and technique description. Vet. J. 2018, 238, 27–40. [Google Scholar] [CrossRef]
- Viscasillas, J.; Sanchis-Mora, S.; Burillo, P.; Esteve, V.; Del Romero, A.; Lafuente, P.; Redondo, J.I. Evaluation of Quadratus Lumborum Block as Part of an Opioid-Free Anaesthesia for Canine Ovariohysterectomy. Animals 2021, 11, 3424. [Google Scholar] [CrossRef]
- Watson, C.B. Respiratory complications associated with anesthesia. Anesthesiol. Clin. N. Am. 2002, 20, 513–537. [Google Scholar] [CrossRef]
- Portela, D.A.; Otero, P.E.; Briganti, A.; Romano, M.; Corletto, F.; Breghi, G. Femoral nerve block: A novel psoas compartment lateral pre-iliac approach in dogs. Vet. Anaesth. Analg. 2013, 40, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.E.; de Lahunta, A. Chapter 17: Spinal Nerves, Miller’s Anatomy of the Dog, 4th ed.; Saunders Elsevier: St. Louis, MO, USA, 2013; pp. 611–657. [Google Scholar]
- Rasmussen, L.M.; Lipowitz, A.J.; Graham, L.F. Development and verification of saphenous, tibial and common peroneal nerve block techniques for analgesia below the thigh in the nonchondrodystrophoid dog. Vet. Anaesth. Analg. 2006, 33, 36–48. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.L.; Woodbury, P. The primary articular nerves to the dog knee. J. Anat. 1982, 134, 563–572. [Google Scholar]
- Bensley, B.A.; Carigie, E.H. The Posterior Limb. In Bensley’s Practical Anatomy of the Rabbit: An Elementary Laboratory Textbook in Mammalian Anatomy, 8th ed.; Craigie, E.H., Ed.; The Blakiston Company: Philadelphia, PA, USA, 1948; pp. 272–291. [Google Scholar]
- d’Ovidio, D.; Rota, S.; Noviello, E.; Briganti, A.; Adami, C. Nerve Stimulator-guided sciatic and femoral block in pet rabbits (Oryctolagus cuniculus) undergoing hindlimb surgery: A case series. J. Exot. Pet. Med. 2014, 23, 91–95. [Google Scholar] [CrossRef]
- Kluge, K.; Menzies, L.; Kloeppel, H.; Pearce, S.; Bettschart-Wolfensberger, R.; Kutter, A. Femoral and sciatic nerve blockades and incision site infiltration in rabbits undergoing stifle joint arthrotomy. Lab. Anim. 2017, 51, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Costa-Farré, C.; Blanch, X.S.; Cruz, J.I.; Franch, J. Ultrasound guidance for the performance of sciatic and saphenous nerve blocks in dogs. Vet. J. 2011, 187, 221–224. [Google Scholar] [CrossRef]
- Felisberto, R.; Flaherty, D.; Tayari, H. Ultrasound- and nerve stimulation-guided sciatic and saphenous nerve blocks in a pet rabbit (Oryctolagus cuniculus) undergoing calcaneal fracture repair. Vet. Rec. Case Rep. 2022, e320. [Google Scholar] [CrossRef]
- Portela, D.A.; Castro, D.; Romano, M.; Gallastegui, A.; Garcia-Pereira, F.; Otero, P.E. Ultrasound-guided erector spinae plane block in canine cadavers: Relevant anatomy and injectate distribution. Vet. Anaesth. Analg. 2020, 47, 229–237. [Google Scholar] [CrossRef]
- Di Franco, C.; Tayari, H.; Nardi, S.; Briganti, A. Along or across the visual axis: A comparison of two ultrasound screen, needle and transducer orientation techniques. Vet. Anaesth. Analg. 2020, 48, 147–150. [Google Scholar] [CrossRef]
- Brilev, J.D.; Keenihan, E.K.; Mathews, K.G.; Chiavaccini, L. Development of an ultrasound-guided transgluteal injection of the pudendal nerve in cats: A cadaveric study. Vet. Anaesth. Analg. 2021, in press. [Google Scholar] [CrossRef]
- Hermanson, J.W. The Muscular System. In Millers and Evans’ Anatomy of the Dog, 5th ed.; Hermanson, J.W., de Lahunta, A., Evans, H.E., Eds.; Elsevier: Beijing, China, 2020; pp. 298–302. [Google Scholar]
- Tsui, B.C.H.; Dillane, D. Needle puncture site and a “Waldown” Approach for Short-Axis Alignment during ultrasound-guided blocks. Reg. Anesth. Pain Med. 2006, 31, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Tayari, H.; Tazioli, G.; Breghi, G.; Briganti, A. Ultrasound-guided femoral and obturator nerves block in the psoas compartment in dogs: Anatomical and randomized clinical study. Vet. Anaesth. Analg. 2017, 44, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Garbin, M.; Portela, D.A.; Bertolizion, G.; Gallastegui, A.; Otero, P.E. A novel ultrasound-guided lateral quadratus lumborum block in dogs: A comparative cadaveric study of two approaches. Vet. Anaesth. Analg. 2020, 47, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.M.; Whyte, M.; James, M.S.; Ferreira, T.H. Effect of contrast and local anesthetic on dye spread following transversus abdominis plane injection in dog cadavers. Vet. Anaesth. Analg. 2020, 47, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Marchina-Gonçalves, A.; Gil, F.; Laredo, F.G.; Soler, M.; Agut, A.; Belda, E. Evaluation of High-Volume Injections Using a Modified Dorsal Quadratus Lumborum Block Approach in Canine Cadavers. Animals 2022, 12, 18. [Google Scholar] [CrossRef]
- Otero, P.E.; Romano, M.; Zaccagnini, A.S.; Fuensalida, S.E.; Verdier, N.; Sanchez, F.; Portela, D.A. Transversus abdominis plane block in cat cadavers: Anatomical description and comparison of injectate spread using two-and three-point approaches. Vet Anaesth. Analg. 2021, 48, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, K.H.W.; Zegers, M.J.; Bleeker, C.P.; Tan, E.C.H.; Vissers, K.C.P.; van Geffen, G.J.; van der Wal, S.E.I. Unpredictable injectate spread of the erector spinae block in human cadavers. Anesth. Analg. 2019, 129, e163–e166. [Google Scholar] [CrossRef]
| Weight (kg) | Low Volume (0.05 mL/kg) | High Volume (0.1 mL/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SN Length (cm) | Total Dye Volume (mL) | SN Stained (cm) | SN Stained (%) | SN Length (cm) | Total Dye Volume (mL) | SN Stained (cm) | SN Stained (%) | ||
| Rabbit 1 | 1.6 | 8.8 | 0.08 | 1.5 * | 17 | 8.7 | 0.16 | 3.0 * | 34 |
| Rabbit 2 | 1.6 | 9 | 0.08 | 1.9 * | 21 | 9.2 | 0.16 | 2.4 * | 26 |
| Rabbit 3 | 1.59 | 9.8 | 0.08 | 2.0 * | 20 | 9.8 | 0.16 | 4.2 * | 43 |
| Rabbit 4 | 1.8 | 9 | 0.09 | 1.9 * | 21 | 9.3 | 0.18 | 2.9 * | 31 |
| Rabbit 5 | 1.55 | 10 | 0.07 | 1.0 * | 10 | 9.8 | 0.15 | 1.9 * | 19 |
| Mean | 1.6 | 8.9 | 0.08 | 1.6 | 17.8 | 9.2 | 0.16 | 2.9 | 31 |
| SD | 0.1 | 0.78 | 0.006 | 0.4 | 4.6 | 0.4 | 0.009 | 0.8 | 8.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felisberto, R.; Flaherty, D.; Tayari, H. Ultrasound-Guided Saphenous Nerve Block in Rabbits (Oryctolagus cuniculus): A Cadaveric Study Comparing Two Injectate Volumes. Animals 2022, 12, 624. https://doi.org/10.3390/ani12050624
Felisberto R, Flaherty D, Tayari H. Ultrasound-Guided Saphenous Nerve Block in Rabbits (Oryctolagus cuniculus): A Cadaveric Study Comparing Two Injectate Volumes. Animals. 2022; 12(5):624. https://doi.org/10.3390/ani12050624
Chicago/Turabian StyleFelisberto, Ricardo, Derek Flaherty, and Hamaseh Tayari. 2022. "Ultrasound-Guided Saphenous Nerve Block in Rabbits (Oryctolagus cuniculus): A Cadaveric Study Comparing Two Injectate Volumes" Animals 12, no. 5: 624. https://doi.org/10.3390/ani12050624
APA StyleFelisberto, R., Flaherty, D., & Tayari, H. (2022). Ultrasound-Guided Saphenous Nerve Block in Rabbits (Oryctolagus cuniculus): A Cadaveric Study Comparing Two Injectate Volumes. Animals, 12(5), 624. https://doi.org/10.3390/ani12050624