Practical Method for Freezing Buck Semen
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Handling
2.3. Post-Thaw Semen Analysis
2.3.1. Membrane Integrity
2.3.2. Sperm Chromatin Structure Assay
2.3.3. Sperm Morphology
2.3.4. Statistical Analysis
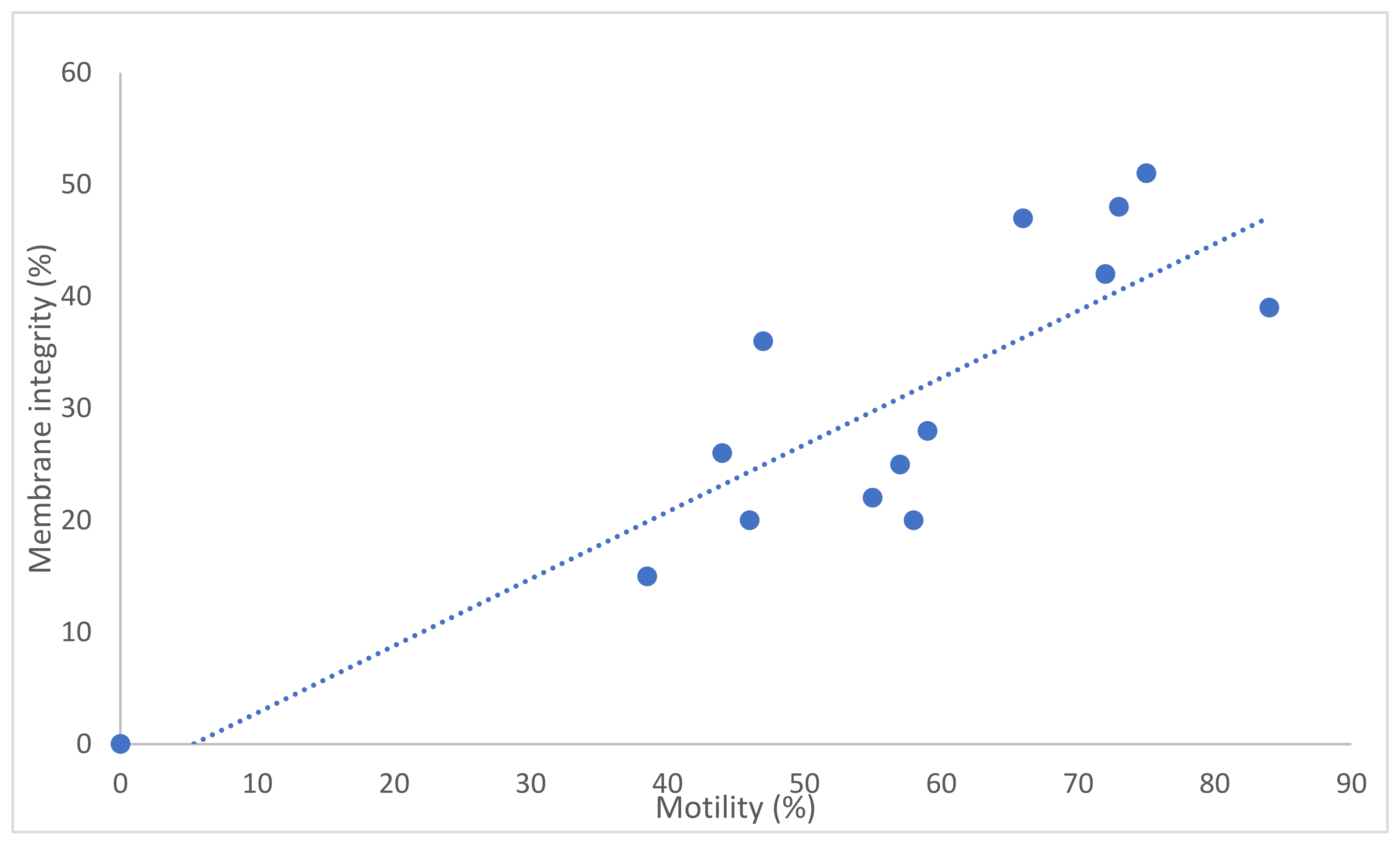
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordstoga, A.B.; Söderquist, L.; Ådnøy, T.; Farstad, W.; Paulenz, H. Vaginal deposition of frozen-thawed semen in Norwegian Dairy goats: Comparison of single and double insemination with equal total number of spermatozoa. Theriogenology 2010, 74, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Dorado, J.; Munoz-Serrano, A.; Hidalgo, M. The effect of cryopreservation on goat semen characteristics related to sperm freezability. Anim. Reprod. Sci. 2010, 121, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.; Niño, T.; Santana, M.; Alamo, D.; Castro, N.; Reyes, R.; González, F.; Cabrera, F.; Gracia, A. Influence of the preservation temperature (37, 20, 4, −196 °C) and the mixing of semen over sperm quality of Majorera bucks. Reprod. Domest. Anim. 2011, 46, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rabadán, P.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Anim. Reprod. Sci. 2012, 132, 88–95. [Google Scholar] [CrossRef]
- Salmani, H.; Towhidi, A.; Zhandi, M.; Bahreini, M.; Sharaf, M. In vitro assessment of soybean lecithin and egg yolk based diluents for cryopreservation of goat semen. Cryobiology 2014, 68, 276–280. [Google Scholar] [CrossRef]
- Chelucci, S.; Pasciu, V.; Succu, S.; Addis, D.; Leoni, G.G.; Manca, M.E.; Berlinguer, N.F. Soybean lecithin–based extender preserves spermatozoa membrane integrity and fertilizing potential during goat semen cryopreservation. Theriogenology 2015, 183, 1064–1074. [Google Scholar] [CrossRef]
- Reiten, M.R.; Malachin, G.; Kommisrud, E.; Østby, G.C.; Waterhouse, K.E.; Krogenæs, A.K.; Bjørås, M.; Jalland, M.O.; Nekså, H.; Røed, S.S.; et al. Stress resilience of spermatozoa and blood mononuclear cells without prion protein. Front. Mol. Biosci. 2018, 5, 1. [Google Scholar] [CrossRef]
- Nadir, T.; Towhidi, A.; Zeinoaldini, S.; Martinez-Pastor, F.; Moouwsavi, M.; Noei, R.; Tar, M.; Sangcheshmeh, M. Lecithin nanoparticles enhance the cryosurvival of caprine sperm. Theriogenology 2019, 133, 38–44. [Google Scholar] [CrossRef]
- Iritani, A.; Nishikawa, Y. Studies on the egg yolk coagulating factor in goat semen. III. Release of some acids accompanied by the coagulating phenomena. J. Anim. Reprod. 1963, 8, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Pellicer-Rubio, M.-T.; Thierry Magallon, T.; Combarnous, Y. Deterioration of goat sperm viability in milk extenders is due to a bulbourethral 60-kilodalton glycoprotein with triglyceride lipase activity. Biol. Reprod. 1997, 57, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Kavak, A.; Lundeheim, N.; Aidnik, M.; Einarsson, S. Sperm morphology in Estonian and Tori breed stallions. Acta Vet. Scand. 2004, 45, 11–18. [Google Scholar] [CrossRef]
- Jiménez-Rabadán, P.; Morrell, J.M.; Johannisson, A.; Ramóna, M.; Garcia-Álvarez, O.; Maroto-Morales, A.; Álvaro-Garcia, P.J.; Pérez-Guzmán, M.D.; Fernández-Santos, M.R.; Garde, J.J.; et al. Single layer centrifugation (SLC) improves sperm quality of cryopreserved Blanca-Celtibérica buck semen. Anim. Reprod. Sci. 2012, 136, 47–54. [Google Scholar] [CrossRef]
- Greyling, J.P.C.; Grobbelaar, J.A.N. Seasonal variation in semen quality of Boer and Angora goat rams using different collection techniques. S. Afr. J. Anim. Sci. 1983, 13, 250–252. [Google Scholar]
- Karagiannidis, A.; Varsakeli, S.; Karatzas, G. Characteristics and seasonal variations in the semen of Alpine, Saanen and Damascus goats bucks born and raised in Greece. Theriogenology 2000, 53, 1285–1293. [Google Scholar] [CrossRef]
- Murphy, E.M.; Eivers, B.; O’Meara, C.M.; Lonergan, P.; Fair, S. Effect of increasing equilibration time of diluted bull semen up to 72 h prior to freezing on sperm quality parameters and calving rate following artificial insemination. Theriogenology 2018, 108, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Millar, J.D.; Watson, P.F. The effect of cooling rate on the survival of cryopreserved bull, ram, and boar spermatozoa: A comparison of two controlled-rate cooling machines. Cryobiology 2003, 46, 246–253. [Google Scholar] [CrossRef]
- Purdy, P.H. A review on goat sperm cryopreservation. Small Rumin. Res. 2006, 63, 215–225. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Álvarez, M.; Guerra, C.; Chamorro, C.A.; Anel-López, L.; de Paz, P.; Anel, L.; Álvarez-Rodríguez, M. Extender osmolality, glycerol and egg yolk on the cryopreservation of epididymal spermatozoa for gamete banking of the Cantabric Chamois (Rupicapra pyrenaica parva). Theriogenology 2019, 125, 109–114. [Google Scholar] [CrossRef]
- Morrell, J.M.; Mayer, I. Reproduction biotechnologies in germplasm banking of livestock species: A review. Zygote 2017, 25, 545–557. [Google Scholar] [CrossRef]
- Zareie, K.; Farshad, A.; Rostamzadeh, J.; Azimi, G.; Ariyan, F. Freezability of goat epididymal sperm using Aloe vera extract and trehalose in Diluents. Austin J. Vet. Sci. Anim. Husb. 2021, 8, 1078–1085. [Google Scholar]
- Brito, B.F.; Pontes, K.D.S.; Leite, S.O.L.; Cabral, L.A.R.; Sousa, M.S.; Salgueiro, C.C.M.; Nunes, J.F. Extra virgin coconut oil as a cryoprotector for cryopreservation of caprine semen. Biopreserv. Biobank. 2021. [Google Scholar] [CrossRef]
- Longobardi, V.; Zullo, G.; Cotticelli, A.; Salzano, A.; Albero, G.; Navas, L.; Rufrano, D.; Clpas, S.; Neglia, G. Crocin improves the quality of cryopreserved goat semen in different breeds. Animals 2020, 10, 1101. [Google Scholar] [CrossRef]
- Zou, J.; Wei, L.; Li, D.; Zhang, Y.; Wang, G.; Zhang, L.; Cao, P.; Yang, S.; Li, G. Effect of glutathione on sperm quality in Guanzhong dairy goat sperm during cryopreservation. Front. Vet. Sci. 2021, 8, 771440. [Google Scholar] [CrossRef]
- El-Battawy, K.A. Preservation of goat semen at 5 °C with emphasis on its freezability and the impact of melatonin. Int. J. Vet. Sci. Res. 2019, 5, 035–038. [Google Scholar] [CrossRef] [Green Version]
- Al-Jeburi, N.J.; Al-Saadoon, A.A. Cryopreservation of goat epididymal sperms under freezing condition using omega 3, 6 and 9. Plant Arch. 2019, 19, 211–216. [Google Scholar]
- Silva, E.C.B.; Vieira, J.L.T.; Nery, I.H.A.V.; Araújo Silva, R.A.J.; Lima, V.F.M.H.; Guerra, M.M.P. Sorting and cryopreservation of goat sperm with or without phenolic compounds. Arq. Bras. Med. Vet. Zootec. 2020, 72, 295–304. [Google Scholar] [CrossRef]
- Alcay, S.; Ustuner, B.; Aktar, A.; Mulkiar, E.; Duman, M.; Akkasoglu, M.; Cetlnkaya, M. Goat semen cryopreservation with rainbow trout seminal plasma supplemented with lecithin-based extenders. Andrologia 2020, 52, e13555. [Google Scholar] [CrossRef]
- Morrell, J.M.; Richter, J.; Martinsson, G.; Stuhtmann, G.; Hoogewijs, M.; Roels, K.; Dalin, A.-M. Pregnancy rates are higher after artificial insemination with cooled stallion spermatozoa selected by Single Layer Centrifugation than with control semen doses. Theriogenology 2014, 82, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Martinez-Alborcia, J.-M.; Martinez-Pastor, F.; Bolarin, A. Centrifugation of boar spermatozoa through low density Porcicoll to separate them from bacteria does not affect fertility after insemination. J. Integr. OMICS 2021, 11, 12. [Google Scholar]
| Reference | Season; Breed | Collection Method | Extender | Removal of Seminal Plasma | Length of Equilibration * | Sperm Concentration | Freezing Method | Post-Thaw Motility |
|---|---|---|---|---|---|---|---|---|
| [1] | Non-BS; Norwegian Dairy goat | AV | Andromed | Yes | 2.5 h at 5 °C | 1000 × 106 (0.25 mL straws) | Controlled rate | Not given |
| [2] | Non-BS vs. BS; Florida | AV | Egg yolk extender vs. skimmed milk extender | Yes | 5 h at 5 °C | 250 × 106 rapid progressively motile spz. (0.5 mL straws) | Vapor | 48.89–60.72% |
| [3] | BS; Majorera | AV | Egg yolk | Yes | 5 h at 5 °C | 400 × 106 (0.5 mL straws) | Vapor | 32.6–45.3% |
| [4] | Non-BS vs. BS; Blanca Celtibérica | EE vs AV | Andromed vs. egg yolk vs. skimmed milk | Removal vs. non-removal | 4 h at 5 °C | 140–200 × 106 (0.25 mL straws) | Vapor | 24–5–5.3% depending on extender and season |
| [5] | Non-BS; Mahabadi | AV | Tris, citric acid, fructose, glycerol, lecithin vs. egg yolk | No | 2.5 h at 5 °C | 400 × 106 (0.25 mL straws) | Vapor | 31.6–59.8% depending on treatment |
| [6] | Non-BS; Sarda | AV | Tris, citric acid, glucose, lecithin | No | 2 h at 4 °C | 400 × 106 Pellet | Dry ice | 10–60% depending on treatment |
| [7] | Not specified: Norwegian Dairy goat | AV | Andromed | Yes | 2 h at 5 °C | 800 × 106 (0.25 mL straws) | Controlled rate | 30.6–36.0% |
| [8] | BS; Mahabadi | AV | Tris, fructose, citric acid, lecithin vs egg yolk | No | 2.5 h at 4 °C | 50 × 106 (0.25 mL straws) | Vapor | 50–70% depending on treatment |
| Buck Number | Total No. Spermatozoa | Initial Motility (%) | Post-Thaw Motility (%) | Difference between Initial and Post-Thaw Motility; % | ||||
|---|---|---|---|---|---|---|---|---|
| Ejac 1 | Ejac 2 | Ejac 1 | Ejac 2 | Ejac 1 | Ejac 2 | Ejac 1 | Ejac 2 | |
| 1 | 5540 | 1780 | 95 | 93 | 84 | 58 | −11 | −35 |
| 2 | 6970 | 4890 | 93 | 94 | 47 | 73 | −46 | −21 |
| 3 | 3500 | 4100 | 93 | 80 | 57 | 55 | −36 | −25 |
| 4 | 3610 | 4240 | 94 | 91 | 0 | 75 | −94 | −16 |
| 5 | 3640 | 2620 | 93 | 95 | 39 | 59 | −54 | −36 |
| 6 | 4270 | 6210 | 91 | 92 | 46 | 72 | −45 | −20 |
| 7 | 3610 | 5000 | 93 | 90 | 66 | 44 | −27 | −46 |
| Mean ± SD | 4449 ± 1325 | 4120 ± 1499 | 93 ± 1 | 91 ± 5 | 48 ± 26 | 62 ± 11 | ||
| LSMeans ± sem | 93 ± 0.8 | 94 ± 0.8 | 48 ± 7.6 | 62 ± 7.6 | ||||
| Overall mean ± SD (Batch 1 + Batch 2) | 4284 ± 1369 | 92 ± 4 | 55 ± 21 | |||||
| TM (%) | PM (%) | VAP µm/s | VCL µm/s | VSL µm/s | STR | LIN | WOB | ALH µm | BCF Hz | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh semen | 92 ± 3 | 85 ± 3 | 82 ± 1 | 174 ± 3 | 57 ± 2 | 0.69 ± 0.01 | 0.32 ± 0.01 | 0.46 ± 0.005 | 6.0 ± 0.2 | 24.6 ± 0.7 |
| Frozen semen | 59 ± 3 | 48 ± 3 | 77 ± 1 | 145 ± 3 | 61 ± 2 | 0.79 ± 0.01 | 0.42 ± 0.01 | 0.53 ± 0.005 | 5.1 ± 0.2 | 28.3 ± 0.7 |
| p | <0.0001 | <0.0001 | 0.02 | <0.0001 | 0.039 | <0.0001 | <0.0001 | 0.0001 | 0.0003 | <0.0001 |
| Buck Number | Membrane Intact (%) | %DFI | ||
|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 1 | Batch 2 | |
| 1 | 39 | 20 | 2.17 | 6.04 |
| 2 | 36 | 48 | 1.88 | 3.33 |
| 3 | 25 | 22 | 2.72 | 3.18 |
| 4 | 0 | 51 | 2.11 | 2.86 |
| 5 | 15 | 28 | 2.97 | 3.47 |
| 6 | 20 | 42 | 2.4 | 4.1 |
| 7 | 47 | 26 | 2.16 | 6.36 |
| Mean ± SD4 | 26 ± 16 | 34 ± 13 | 2.34 ± 0.38 * | 4.19 ± 1.42 * |
| LSMeans ± sem | 26 ± 5.5 | 34 ± 5.5 | 2.34 ± 0.2 * | 3.4 ± 0.2 * |
| Overall mean ± SD for batch 1 and 2 | 29.9 ± 14.7 | 3.27 ± 1.39 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrell, J.M.; Malaluang, P.; Ntallaris, T.; Johannisson, A. Practical Method for Freezing Buck Semen. Animals 2022, 12, 352. https://doi.org/10.3390/ani12030352
Morrell JM, Malaluang P, Ntallaris T, Johannisson A. Practical Method for Freezing Buck Semen. Animals. 2022; 12(3):352. https://doi.org/10.3390/ani12030352
Chicago/Turabian StyleMorrell, Jane M., Pongpreecha Malaluang, Theodoros Ntallaris, and Anders Johannisson. 2022. "Practical Method for Freezing Buck Semen" Animals 12, no. 3: 352. https://doi.org/10.3390/ani12030352
APA StyleMorrell, J. M., Malaluang, P., Ntallaris, T., & Johannisson, A. (2022). Practical Method for Freezing Buck Semen. Animals, 12(3), 352. https://doi.org/10.3390/ani12030352







