Alien or Native? How to Distinguish Feces of Fallow and Roe Deer Using Central Poland as a Case Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aulak, W.; Babińska-Werka, J. Estimation of roe deer density based on the abundance and rate of disappearance of their faeces from the forest. Acta Theriol. 1990, 35, 111–120. [Google Scholar] [CrossRef]
- Marques, F.F.C.; Buckland, S.T.; Goffin, D.; Dixon, C.; Borchers, D.L.; Mayle, B.A.; Peace, A.J. Estimating deer abundance from line transect survey of dung: Sika deer in southern Scotland. J. Appl. Ecol. 2001, 38, 349–363. [Google Scholar] [CrossRef]
- Campbell, D.; Swanson, G.; Sales, J. Comparing the precision and cost-effectiveness of faecal pellet group count methods. J. Appl. Ecol. 2004, 41, 1185–1196. [Google Scholar] [CrossRef]
- Ferretti, F.; Pisani, C.; Sforzi, A.; Lovri, S.; Fattorini, L. Estimation of roe deer and fallow deer density: The use of pellet group counts. Hystrix 2007, 250. [Google Scholar]
- Theuerkauf, J.; Rouys, S.; Jędrzejewski, W. Detectability and disappearance of ungulate and hare faeces in a European temperate forest. Ann. Zool. Fenn. 2008, 45, 73–80. [Google Scholar] [CrossRef]
- Ball, M. Faecal pellet size can be used to differentiate age-classes in caribou: Implications for non-invasive genetic studies. Conserv. Gen. Res. 2010, 2, 239–241. [Google Scholar] [CrossRef]
- Fattorini, L.; Ferretti, F.; Pisani, C.; Sforzi, A. Two-stage estimation of ungulate abundance in Mediterranean areas using pellet group count. Environ. Ecol. Stat. 2011, 8, 291–314. [Google Scholar] [CrossRef]
- Buesching, C.D.; Newman, C.; Macdonald, D.W. How dear are deer volunteers: The efficiency of monitoring deer using teams of volunteers to conduct pellet group counts. Oryx 2014, 48, 593–601. [Google Scholar] [CrossRef][Green Version]
- Mathisen, K.M.; Wójcicki, A.; Borowski, Z. Effects of forest roads on oak trees via cervid habitat use and browsing. Forest Ecol. Manag. 2018, 424, 378–386. [Google Scholar] [CrossRef]
- Borkowski, J.; Banul, R.; Jurkiewicz-Azab, J.; Hołdyński, C.; Święczkowska, J.; Nasiadko, M.; Załuski, D. There is only one winner: The negative impact of red deer density on roe deer numbers and distribution in the Słowiński National Park and its vicinity. Ecol. Evol. 2021, 11, 6889–6899. [Google Scholar] [CrossRef]
- Spitzer, R.; Churski, M.; Felton, A.; Heurich, M.; Kuijper, D.P.J.; Landman, M.; Rodriguez, E.; Singh, N.J.; Taberlet, P.; van Beeck Calkoen, S.; et al. Doubting dung: eDNA reveals high rates of misidentification in diverse European ungulate communities. Eur. J. Wildl. Res. 2019, 65, 28. [Google Scholar] [CrossRef]
- Nasiadka, P. Metody i Techniki Inwentaryzacji Zwierząt Łownych; Biblioteczka leśniczego, 38; Wydawnictwo Świat: Warsaw, Poland, 1994. [Google Scholar]
- Romanowski, J. Śladami Zwierząt; Państwowe Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 1998. [Google Scholar]
- Mayle, B.A.; Peace, A.J.; Gill, R.M.A. How Many Deer? A Field Guide to Estimating Deer Population Size; Field Book 18; Forestry Commission: Edinburgh, UK, 1999.
- Jędrzejewski, W.; Sidarowicz, W. Sztuka Tropienia Zwierząt; Zakład Badania Ssaków: Białowieża, Poland, 2010. [Google Scholar]
- Czyżewska, T. Historia pochodzenia i rozprzestrzeniania się daniela. Przegl. Zool. 1964, 3, 286–290. [Google Scholar]
- Chapman, N.; Chapman, D. The distribution of fallow deer: A worldwide review. Mamm. Rev. 1980, 10, 61–130. [Google Scholar] [CrossRef]
- Apollino, M. Dama dama (Linnaeus, 1758). In The atlas of European mammals; Mitchell-Jones, A.J., Amori, G., Bogdanowicz, W., Krystufek, B., Reijnders, P.J.H., Spitzenberger, F., Stubbe, M., Thissen, J.B.M., Vohralík, V., Zima, J., Eds.; T. & A.D. Poyser Natural History: London, UK, 1999; pp. 1–484. [Google Scholar]
- Chapman, N.; Chapman, D. Fallow Deer. Their History, Distribution and Biology; Terence Dalton Ltd.: Lavenham, UK, 1975. [Google Scholar]
- Dzięciołowski, R. Daniel; Wydawnictwo SGGW: Warsaw, Poland, 1994. [Google Scholar]
- Moriary, A. The liberation, distribution, abundance and management of wild deer in Australia. Wildl. Res. 2004, 31, 291–299. [Google Scholar] [CrossRef]
- Novillo, A.; Ojeda, R.A. The exotic mammals of Argentina. Biol. Inv. 2008, 10, 1333. [Google Scholar] [CrossRef]
- Włodek, K. Historia rozprzestrzeniania się daniela europejskiego w czasach nowożytnych i jego rozmieszczenie na świecie. Przegl. Zool. 1979, 23, 84–91. [Google Scholar]
- Pucek, Z.; Raczyński, J. (Eds.) Atlas Rozmieszczenia Ssaków w Polsce; Polish Scientific Publishers: Warsaw, Poland, 1983. [Google Scholar]
- Solarz, W. Fallow Deer. Atlas of Polish Mammals. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/124 (accessed on 10 September 2021).
- Forest Data Bank 2021. Available online: https://www.bdl.lasy.gov.pl/portal/ (accessed on 7 September 2021).
- Gryz, J. (Forest Research Institute, Sękocin Stary, Poland). Personal Communication, 2021.
- Statistics Poland. Statistical Yearbook of Forestry 2021. Available online: https://stat.gov.pl (accessed on 5 September 2021).
- Kamieniarz, R. Roe Deer. Atlas of Polish Mammals. Available online: https://www.iop.krakow.pl/Ssaki/gatunek/126 (accessed on 1 December 2021).
- Jasińska, K.D.; Jackowiak, M.; Gryz, J.; Bijak, S.; Szyc, K.; Krauze-Gryz, D. Habitat-Related Differences in Winter Presence and Spring–Summer Activity of Roe Deer in Warsaw. Forests 2021, 12, 970. [Google Scholar] [CrossRef]
- Ciach, M.; Fröhlich, A. Ungulates in the city: Light pollution and open habitats predict the probability of roe deer occurring in an urban environment. Urban Ecosyst. 2019, 22, 513–523. [Google Scholar] [CrossRef]
- Panek, M.; Budny, M. Sytuacja Zwierząt Łownych w Polsce–Wyniki Monitoringu. Rok 2020; Stacja Badawcza PZŁ: Czempiń, Polska, 2020; Available online: http://www.czempin.pzlow.pl/palio/html.wmedia?_Instance=pzl_www&_Connector=palio&_ID=6033&_CheckSum=754196868 (accessed on 1 December 2021).
- Głowaciński, Z.; Okarma, H.; Pawłowski, H.; Solarz, W. Gatunki Obce w Faunie Polski; IOP, PAN: Cracow, Poland, 2012. [Google Scholar]
- Obidziński, A.; Kiełtyk, P.; Borkowski, J.; Bolibok, L.; Remuszko, K. Autumn-winter diet overlap of fallow, red, and roe deer in forest ecosystems, Southern Poland. Centr. Eur. J. Biol. 2013, 8, 8–17. [Google Scholar] [CrossRef]
- Mierzwiński, W. Jeszcze o danielu. Low. Pol. 1952, 4, 12. [Google Scholar]
- Pinkowski, M. Daniel-niedoceniana szansa dla wielu łowisk. Low. Pol. 1988, 9, 10–13. [Google Scholar]
- Fruziński, B.; Łabudzki, L. Daniel. Low. Pol. 1998, 8, 8–10. [Google Scholar]
- Ferretti, F.; Bertodi, G.; Sforzi, A.; Fattori, L. Roe and fallow deer: Are they compatible neighbours? Eur. J. Willdl. Res. 2011, 57, 775–783. [Google Scholar] [CrossRef]
- Presnall, C.C. Present Status of Exotic Mammals in the United States. J. Wildl. Manag. 1958, 22, 45–50. [Google Scholar] [CrossRef]
- Dolman, P.M.; Wäber, K. Ecosystem and competition impacts of introduced deer. Wildl. Res. 2008, 35, 202–2014. [Google Scholar] [CrossRef]
- Gryz, J.; Krauze-Gryz, D.; Lesiński, G. Mammals in the vicinity of Rogów (central Poland). Fragm. Faun. 2011, 54, 183–197. [Google Scholar] [CrossRef]
- Dałkowski, D. (Forest Experimental Station in Rogów, Warsaw University of Life Sciences, Warsaw, Poland). Personal Communication, 2021.
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Alvarez, G. Morphological variability and identification of deer pellets in Central Spain. Folia Zool. 1994, 43, 25–37. [Google Scholar]

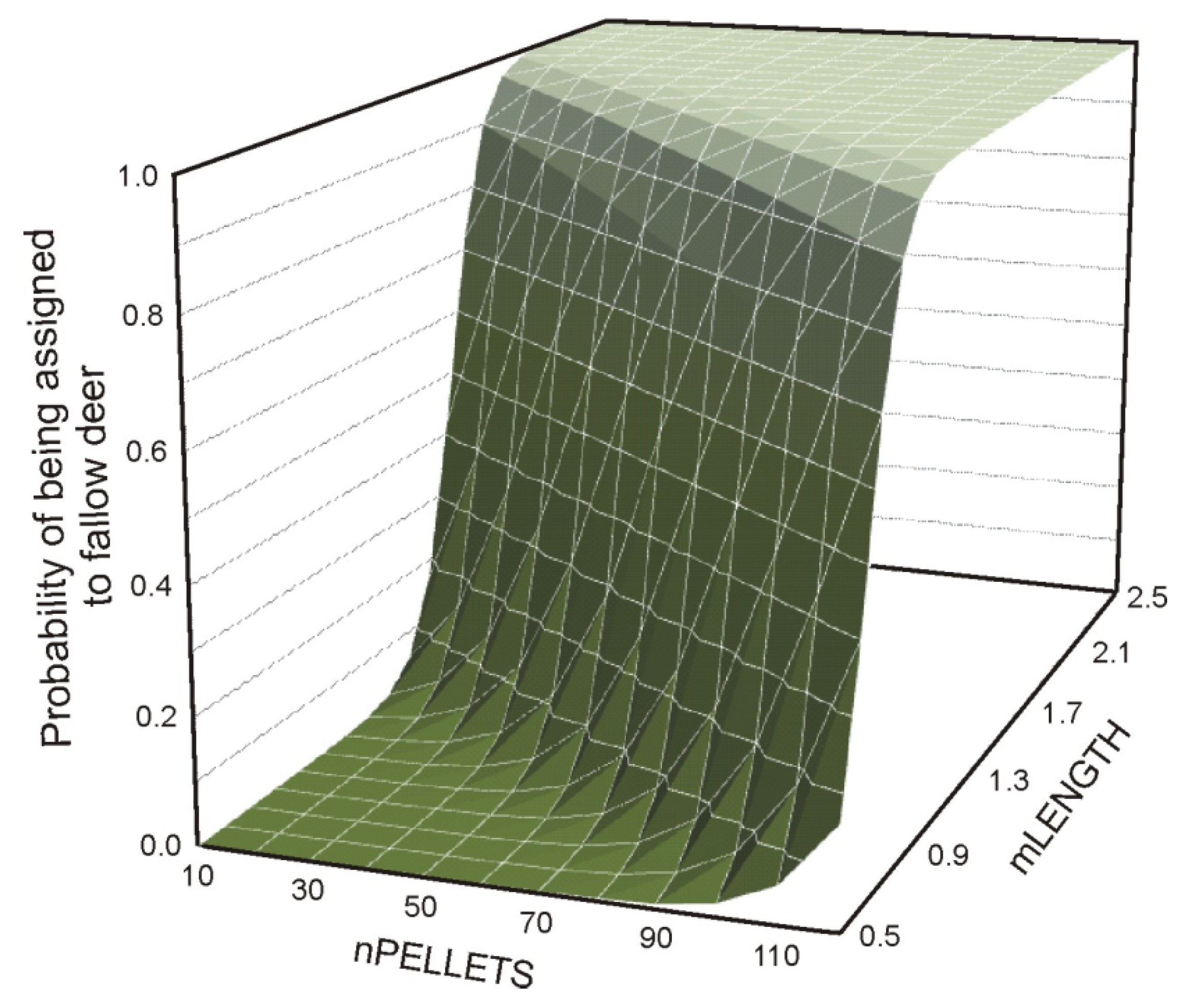

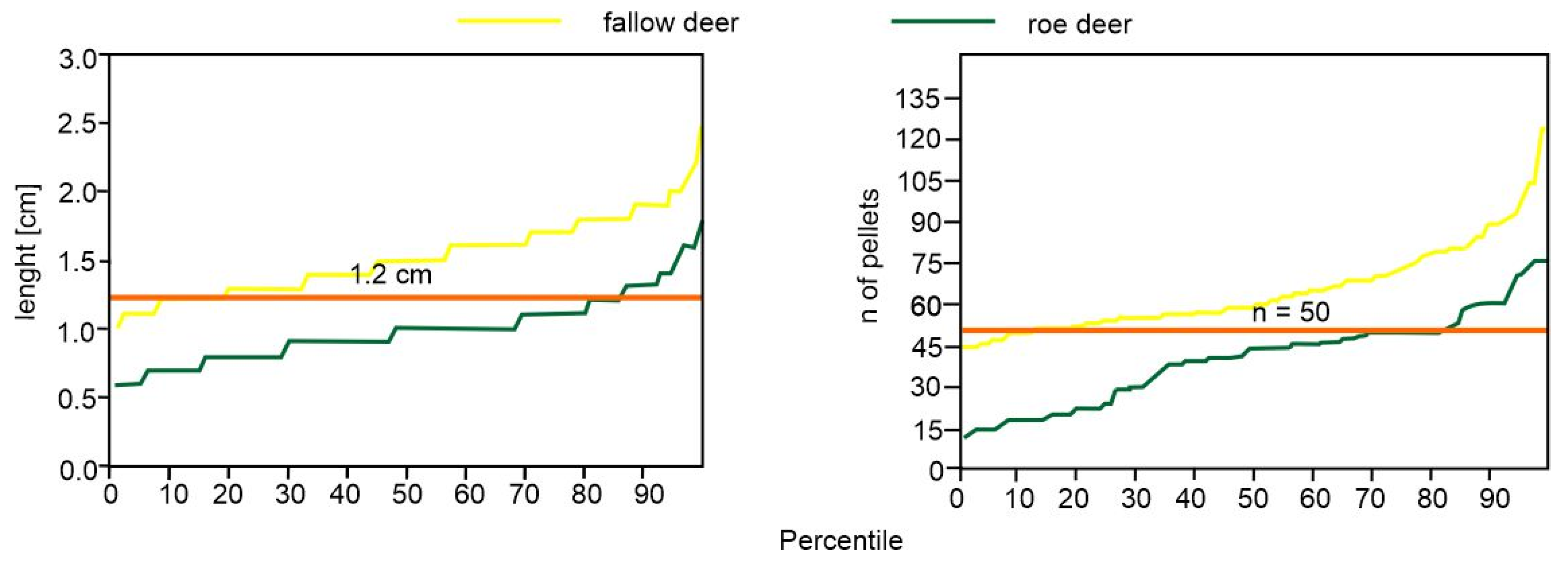
| Model No. (Dependent Variable) | Source | B | SE | p | Lower CI | Upper CI |
|---|---|---|---|---|---|---|
| 1 (LENGTH) | ||||||
| Intercept | −0.72 | 0.11 | 0.000 | −0.92 | −0.51 | |
| SPECIES | 1.03 | 0.05 | 0.000 | 0.94 | 1.13 | |
| WIDTH | 0.92 | 0.04 | 0.000 | 0.84 | 1.00 | |
| WIDTH*SPECIES | −0.81 | 0.06 | 0.000 | −0.92 | −0,69 | |
| 2 (WIDTH) | ||||||
| Intercept | −0.94 | 0.12 | 0.000 | −1.17 | −0.71 | |
| SPECIES | 0.47 | 0.07 | 0.000 | 0.34 | 0.60 | |
| LENGTH | 0.63 | 0.03 | 0.000 | 0.57 | 0.69 | |
| LENGTH*SPECIES | −0.45 | 0.05 | 0.000 | −0.54 | −0.35 | |
| 3 (nPELLETS) | ||||||
| Intercept | 3.63 | 0.26 | 0.000 | 3.12 | 4.14 | |
| SPECIES | 0.54 | 0.02 | 0.000 | 0.5 | 0.57 |
| Model No. (Name) | Source | B | SE | p | OR |
|---|---|---|---|---|---|
| 4 (RAWA) | |||||
| D = 71.32% | Intercept | −13.26 | 1.06 | 0.000 | 0.00 |
| nPELLETS | 0.08 | 0.01 | 0.000 | 1.08 | |
| LENGTH | 10.61 | 0.82 | 0.000 | 40,382.14 | |
| WIDTH | −5.07 | 0.98 | 0.000 | 0.01 | |
| 5 (RAWB) | |||||
| D = 69.86% | Intercept | −15.34 | 1.01 | 0.000 | 0.00 |
| nPELLETS | 0.85 | 0.01 | 0.000 | 1.01 | |
| LENGTH | 8.60 | 0.64 | 0.000 | 5431.43 | |
| 6 (MEANA) | |||||
| D = 78.07% | Intercept | −21.61 | 3.70 | 0.000 | 0.00 |
| nPELLETS | 0.11 | 0.03 | 0.000 | 0.12 | |
| mLENGTH | 12.59 | 2.36 | 0.000 | 293,213.11 | |
| 7 (MEANB) | |||||
| D = 77.93% | Intercept | −15.48 | 2.34 | 0.000 | 0.00 |
| mLENGTH | 12.54 | 1.88 | 0.000 | 278,144.85 | |
| 8 (MEANC) | |||||
| D = 56.87% | Intercept | −6.95 | 1.10 | 0.000 | 0.00 |
| nPELLETS | 0.13 | 0.02 | 0.000 | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryz, J.; Krauze-Gryz, D.; Klich, D. Alien or Native? How to Distinguish Feces of Fallow and Roe Deer Using Central Poland as a Case Study. Animals 2022, 12, 290. https://doi.org/10.3390/ani12030290
Gryz J, Krauze-Gryz D, Klich D. Alien or Native? How to Distinguish Feces of Fallow and Roe Deer Using Central Poland as a Case Study. Animals. 2022; 12(3):290. https://doi.org/10.3390/ani12030290
Chicago/Turabian StyleGryz, Jakub, Dagny Krauze-Gryz, and Daniel Klich. 2022. "Alien or Native? How to Distinguish Feces of Fallow and Roe Deer Using Central Poland as a Case Study" Animals 12, no. 3: 290. https://doi.org/10.3390/ani12030290
APA StyleGryz, J., Krauze-Gryz, D., & Klich, D. (2022). Alien or Native? How to Distinguish Feces of Fallow and Roe Deer Using Central Poland as a Case Study. Animals, 12(3), 290. https://doi.org/10.3390/ani12030290






