Movement of Free-Ranging Koalas in Response to Male Vocalisation Playbacks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials & Methods
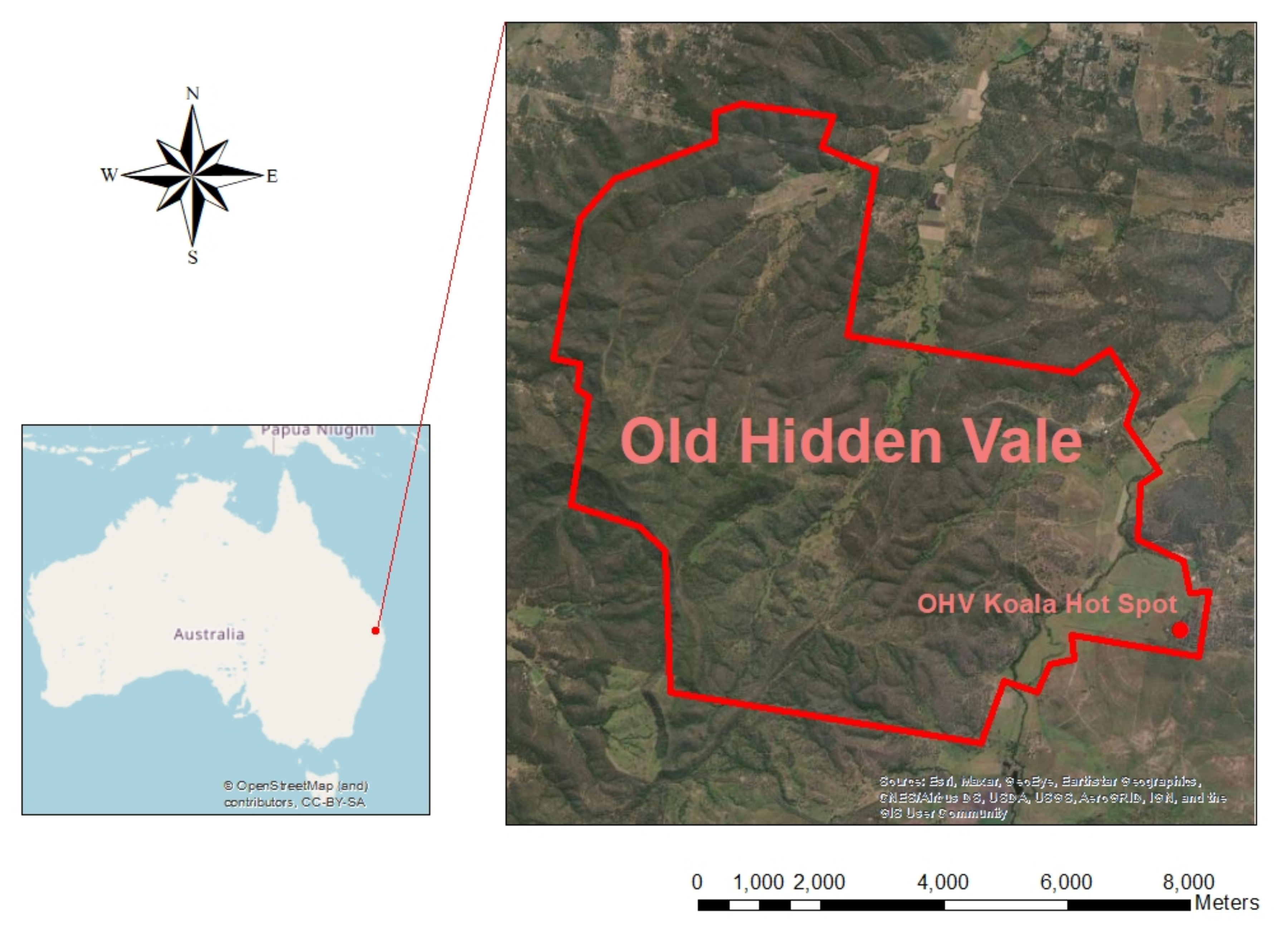
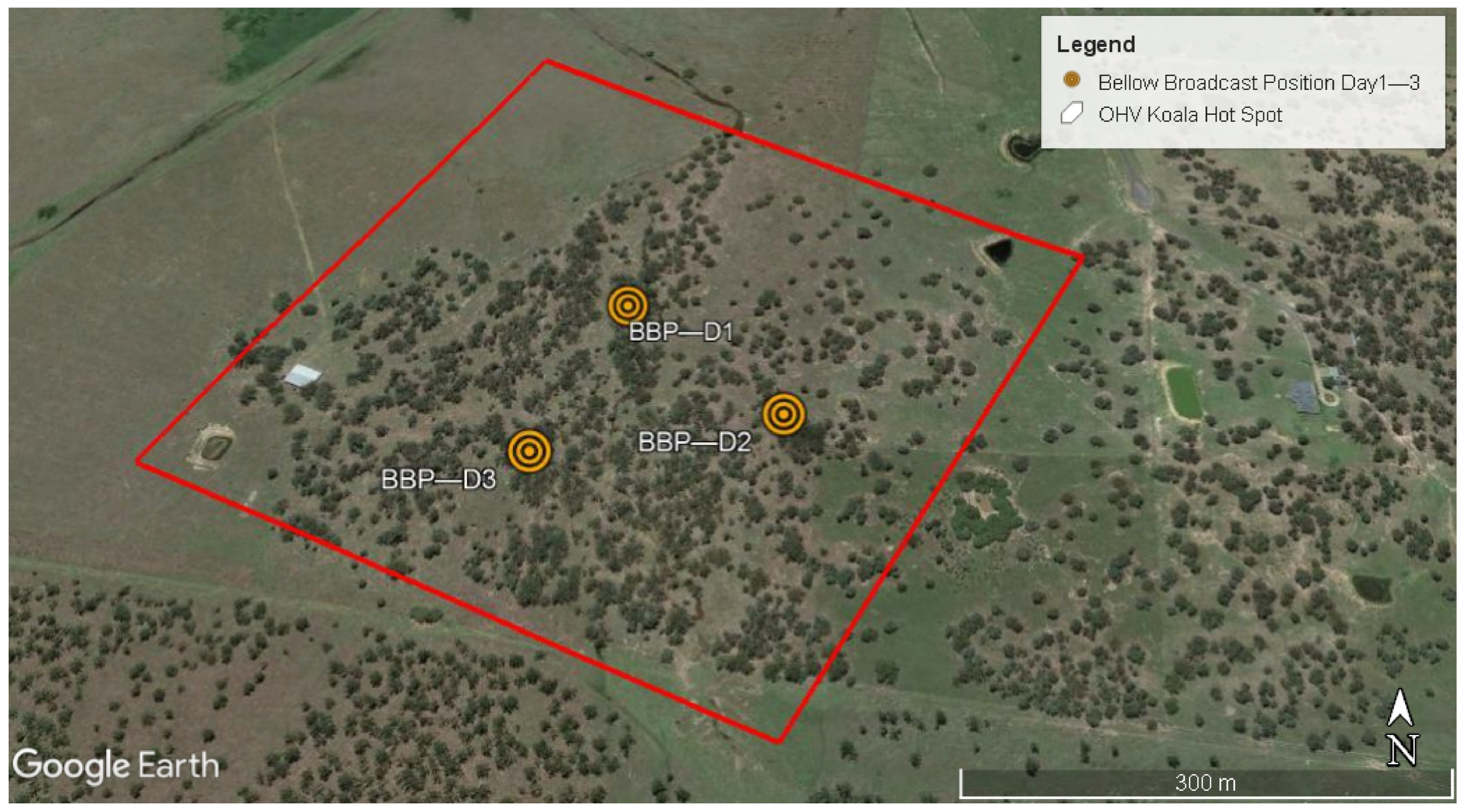
2.1. Study Site
2.2. Study Animals and Data Collection
2.3. Bellow Recordings and Broadcasting
2.4. Individual Responses to Bellows
2.5. Group Responses to Bellows
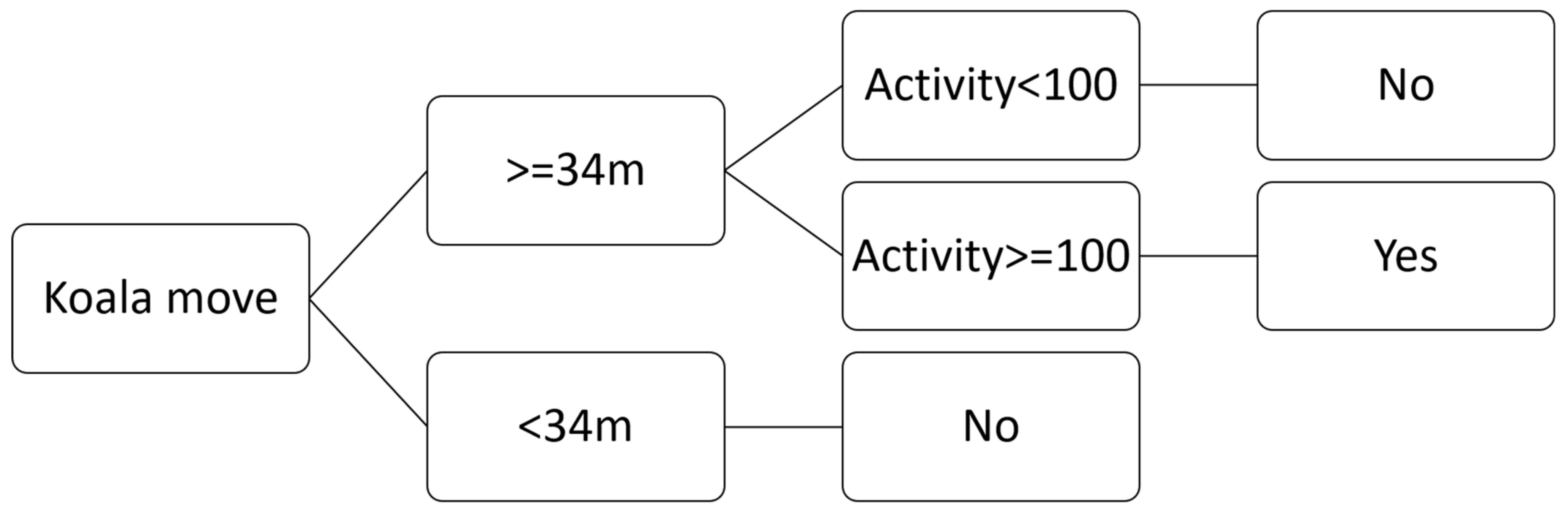
2.6. GPS Accuracy and the First Move Determination
2.7. Statistical Analyses
2.7.1. Individual Responses to Bellows
2.7.2. Group Responses to Bellows
3. Results
3.1. Individual Bellow Tests
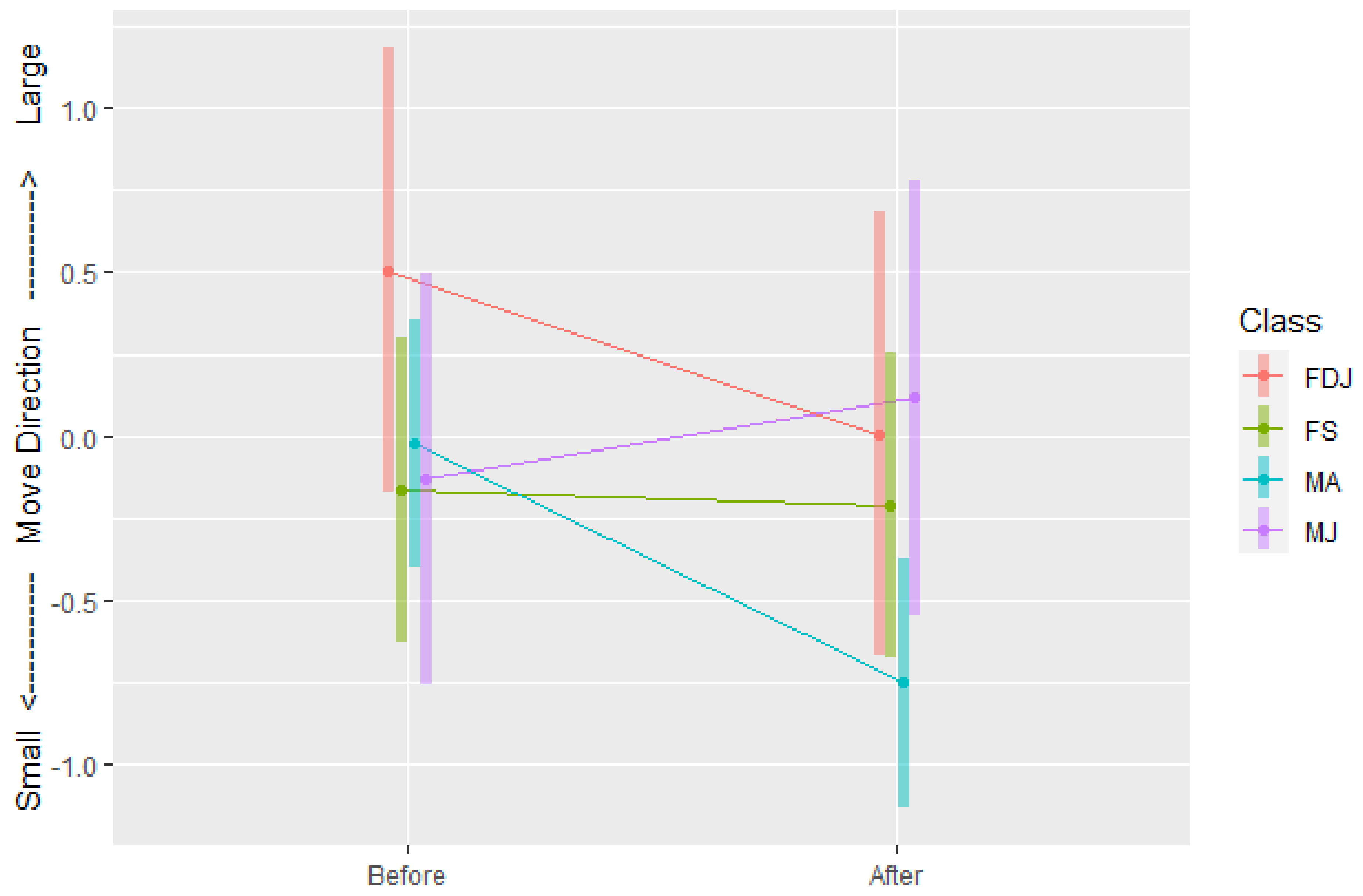
3.1.1. Move Direction
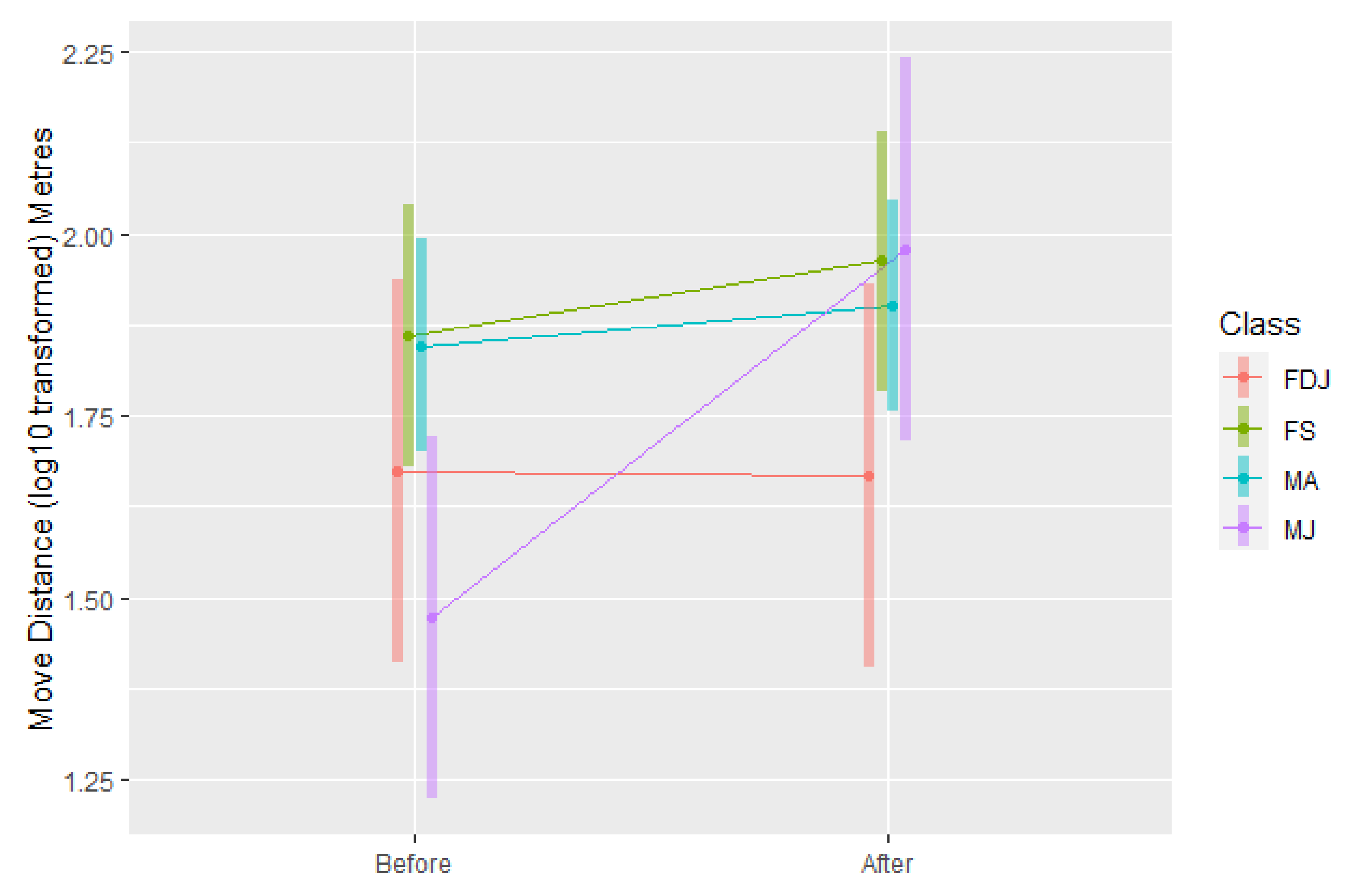
3.1.2. Move Distance
3.1.3. Stay on Site
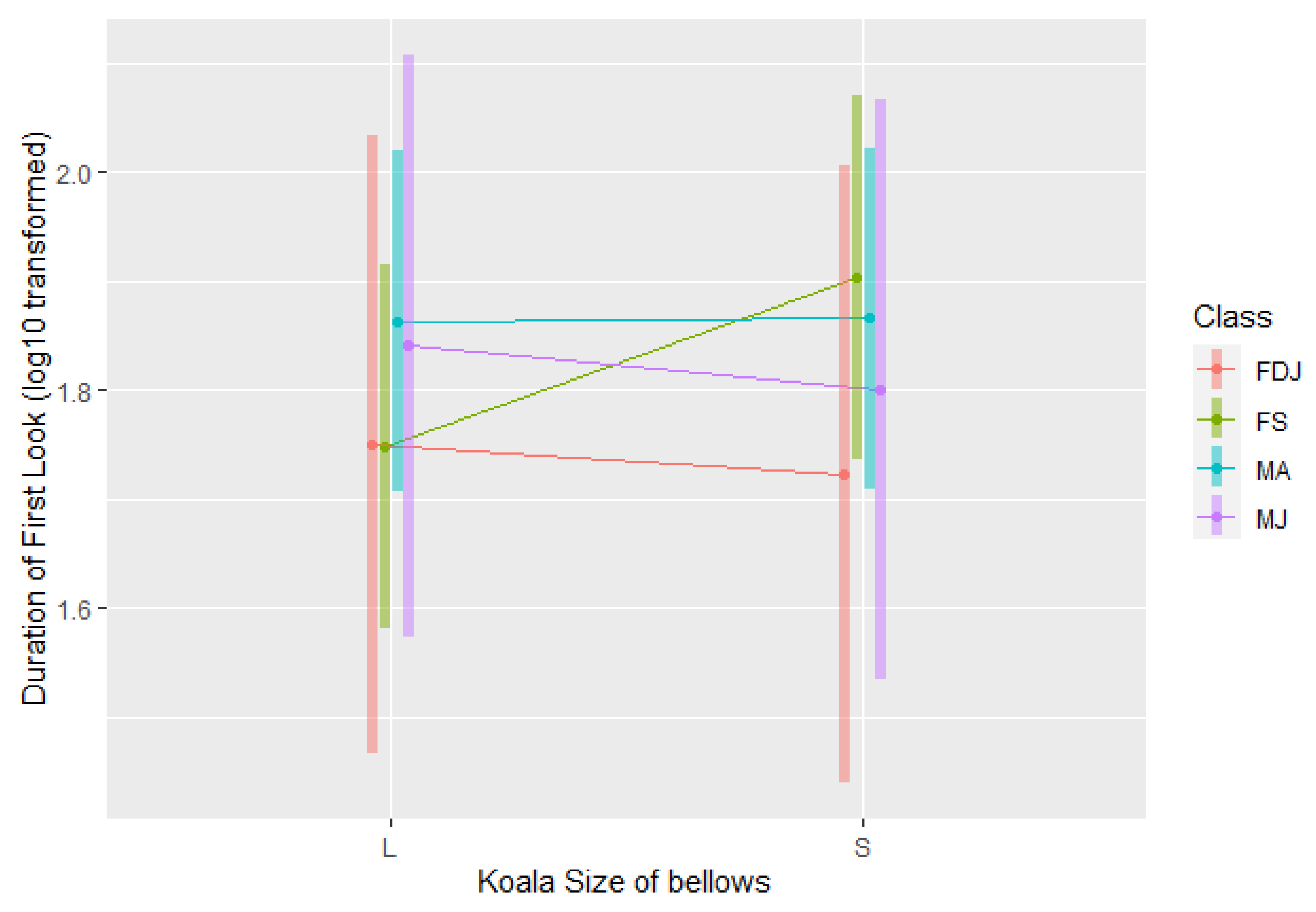
3.1.4. Look Response
3.2. Group Bellow Tests
3.2.1. Activity Levels
3.2.2. Time Stayed near Speakers Broadcasting Bellows
3.2.3. Approach and Avoidance Behaviour
4. Discussion
4.1. Adult Males
4.2. Juvenile Males
4.3. Females
4.4. First Look
4.5. Implications for Koala Breeding Dynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayigh, L.; Tyack, P.; Wells, R.; Solow, A.; Scott, M.D.; Irvine, A.B. Individual recognition in wild bottlenose dolphins: A field test using playback experiments. Anim. Behav. 1999, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- McComb, K.; Moss, C.; Sayialel, S.; Baker, L. Unusually extensive networks of vocal recognition in African elephants. Anim. Behav. 2000, 59, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Reby, D. The contribution of source–filter theory to mammal vocal communication research. J. Zool. 2010, 280, 221–236. [Google Scholar] [CrossRef]
- Rendall, D.; Rodman, P.S.; Emond, R.E. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim. Behav. 1996, 51, 1007–1015. [Google Scholar] [CrossRef]
- Charrier, I.; Mathevon, N.; Jouventin, P. Mother’s voice recognition by seal pups. Nature 2001, 412, 873. [Google Scholar] [CrossRef] [PubMed]
- Charrier, I.; Mathevon, N.; Jouventin, P. Vocal signature recognition of mothers by fur seal pups. Anim. Behav. 2003, 65, 543–550. [Google Scholar] [CrossRef]
- Torriani, M.V.G.; Vannoni, E.; McElligott, A.G. Mother-young recognition in an ungulate hider species: A unidirectional process. Am. Nat. 2006, 168, 412–420. [Google Scholar] [CrossRef]
- Hare, J. Juvenile Richardson’s ground squirrels, Spermophilus richardsonii, discriminate among individual alarm callers. Anim. Behav. 1998, 55, 451–460. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Daniel, J.C. Yellow-bellied marmots discriminate between the alarm calls of individuals and are more responsive to calls from juveniles. Anim. Behav. 2004, 68, 1257–1265. [Google Scholar] [CrossRef]
- Reby, D.; Cargnelutti, B.; Hewison, A.J.M. Contexts and possible functions of barking in roe deer. Anim. Behav. 1999, 57, 1121–1128. [Google Scholar] [CrossRef]
- Frommolt, K.-H.; Goltsman, M.E.; Macdonald, D.W. Barking foxes, Alopex lagopus: Field experiments in individual recognition in a territorial mammal. Anim. Behav. 2003, 65, 509–518. [Google Scholar] [CrossRef]
- East, M.; Hofer, H. Loud calling in a female-dominated mammalian society: II. Behavioral contexts and functions of whooping of spotted hyaenas, Crocuta crocuta. Anim. Behav. 1991, 42, 651–669. [Google Scholar] [CrossRef]
- Zimmermann, E.; Lerch, C. The Complex Acoustic Design of an Advertisement Call in Male Mouse Lemurs (Microcebus murinus, Prosimii, Primates) and Sources of its Variation. Ethology 1993, 93, 211–224. [Google Scholar] [CrossRef]
- McElligott, A.G.; O’Neill, K.; Hayden, T. Cumulative long-term investment in vocalization and mating success of fallow bucks, Dama dama. Anim. Behav. 1999, 57, 1159–1167. [Google Scholar] [CrossRef]
- Tripovich, J.S.; Charrier, I.; Rogers, T.L.; Canfield, R.; Arnould, J.P.Y. Acoustic features involved in the neighbour-stranger vocal recognition process in male Australian fur seals. Behav. Process. 2008, 79, 74–80. [Google Scholar] [CrossRef]
- McCurry, M.R.; Quayle, M.R.; Cally, J.; Adams, J.W. Velar vocal folds are present in female and immature male koalas (Phascolarctos cinereus). Aust. Mammal. 2016, 38, 232–233. [Google Scholar] [CrossRef]
- Charlton, B.D.; Ellis, W.A.H.; McKinnon, A.J.; Cowin, G.J.; Brumm, J.; Nilsson, K.; Fitch, W.T. Cues to body size in the formant spacing of male koala (Phascolarctos cinereus) bellows: Honesty in an exaggerated trait. J. Exp. Biol. 2011, 214, 3414–3422. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Handasyde, K.A. The Koala: Natural History, Conservation and Management, 2nd ed.; Krieger Publishing: Malabar, FL, USA, 1999. [Google Scholar]
- Smith, M. Behaviour of the Koala, Phascolarctos cinereus (Goldfuss), in Captivity III*. Vocalisations. Wildl. Res. 1980, 7, 13–34. [Google Scholar] [CrossRef]
- Mitchell, P. Social behaviour and communication of koalas. In Biology of the Koala; Lee, A.K., Handasyde, K.A., Sanson, G.D., Eds.; Surrey Beatty & Sons: Chipping Norton, Australia, 1990; pp. 151–170. [Google Scholar]
- Ellis, W.; FitzGibbon, S.; Pye, G.; Whipple, B.; Barth, B.; Johnston, S.; Seddon, J.; Melzer, A.; Higgins, D.; Bercovitch, F. The Role of Bioacoustic Signals in Koala Sexual Selection: Insights from Seasonal Patterns of Associations Revealed with GPS-Proximity Units. PLoS ONE 2015, 10, e0130657. [Google Scholar] [CrossRef]
- Handasyde, K.A.; Lee, A.K.; Sanson, G.D. Biology of the Koala; Surrey Beatty & Sons: Chipping Norton, Australia, 1990. [Google Scholar]
- Ellis, W.A.; Fitzgibbon, S.I.; Roe, P.; Bercovitch, F.B.; Wilson, R. Unraveling the mystery of koala vocalisations: Acoustic sensor network and GPS technology reveals males bellow to serenade females. Integr. Comp. Biol. 2010, 50, E49. [Google Scholar]
- Charlton, B.D.; Whisson, D.A.; Reby, D. Free-Ranging Male Koalas Use Size-Related Variation in Formant Frequencies to Assess Rival Males. PLoS ONE 2013, 8, e70279. [Google Scholar] [CrossRef] [PubMed]
- Charlton, B.D.; Ellis, W.A.H.; Brumm, J.; Nilsson, K.; Fitch, W.T. Female koalas prefer bellows in which lower formants indicate larger males. Anim. Behav. 2012, 84, 1565–1571. [Google Scholar] [CrossRef]
- Ellis, W.; Bercovitch, F. Body size and sexual selection in the koala. Behav. Ecol. Sociobiol. 2011, 65, 1229–1235. [Google Scholar] [CrossRef]
- Sharp, A. The Koala Book; David Bateman: Albany, Australia, 1995. [Google Scholar]
- Ellis, W.A.; Hale, P.T.; Carrick, F. Breeding dynamics of koalas in open woodlands. Wildl. Res. 2002, 29, 19–25. [Google Scholar] [CrossRef]
- Ellis, W.; Bercovitch, F.; FitzGibbon, S.; Roe, P.; Wimmer, J.; Melzer, A.; Wilson, R. Koala bellows and their association with the spatial dynamics of free-ranging koalas. Behav. Ecol. 2011, 22, 372–377. [Google Scholar] [CrossRef]
- Charlton, B.D.; Ellis, W.A.H.; Larkin, R.; Fitch, W.T. Perception of size-related formant information in male koalas (Phascolarctos cinereus). Anim. Cogn. 2012, 15, 999–1006. [Google Scholar] [CrossRef]
- Watchorn, D.J.; Whisson, D.A. Quantifying the interactions between koalas in a high-density population during the breeding period. Aust. Mammal. 2020, 42, 28–37. [Google Scholar] [CrossRef]
- Schradin, C. Intraspecific variation in social organization by genetic variation, developmental plasticity, social flexibility or entirely extrinsic factors. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120346. [Google Scholar] [CrossRef]
- Pacheco, X.P.; Madden, J.R. Does the social network structure of wild animal populations differ from that of animals in captivity? Behav. Process. 2021, 190, 104446. [Google Scholar] [CrossRef]
- Herbarium, Q. Regional Ecosystem Description Database (REDD); Queensland Department of Environment and Science: Brisbane, Australia, 2021. [Google Scholar]
- Melzer, A.; Cristescu, R.; Ellis, W.; FitzGibbon, S.; Manno, G. The habitat and diet of koalas (Phascolarctos cinereus) in Queensland. Aust. Mammal. 2014, 36, 189–199. [Google Scholar] [CrossRef]
- Jiang, A.; Tribe, A.; Murray, P. The development of an improved scat survey method for koalas (Phascolarctos cinereus). Aust. J. Zool. 2019, 67, 125. [Google Scholar] [CrossRef]
- Martin, R.W. Draft Management Plan for the Conservation of the Koala (Phascolarctos cinereus) in Victoria; Department of Conservation, Forests and Lands, Arthur Rylah Institute for Environmental Research: Heidelberg, Australia, 1989. [Google Scholar]
- Tobey, J.R.; Andrus, C.H.; Doyle, L.; Thompson, V.D.; Bercovitch, F.B. Maternal effort and joey growth in koalas (Phascolarctos cinereus). J. Zool. 2006, 268, 423–431. [Google Scholar] [CrossRef]
- Johnston, S.D.; McGowan, M.R.; O’Callaghan, P.; Cox, R.; Nicolson, V. Studies of the oestrous cycle, oestrus and pregnancy in the koala (Phascolarctos cinereus). J. Reprod. Fertil. 2000, 120, 49–57. [Google Scholar] [CrossRef][Green Version]
- Johnston, S.D.; McGowan, M.R.; O’Callaghan, P.; Cox, R.; Houlden, B.; Haig, S.; Taddeo, G. Birth of Koalas Phascolarctos cinereus at Lone Pine Koala Sanctuary following artificial insemination. Int. Zoo Yearb. 2003, 38, 160–172. [Google Scholar] [CrossRef]
- Ballantyne, K.; Lisle, A.; Mucci, A.; Johnston, S.D. Seasonal oestrous cycle activity of captive female koalas in south-east Queensland. Aust. Mammal. 2015, 37, 245–252. [Google Scholar] [CrossRef]
- Satoshi, K.; Hisashi, H.; Masato, T.; Kazuhito, T.; Hideki, I.; Yuka, O.-K.; Masako, H.; Mitsuaki, O.; Koki, M.; Sayaka, A.; et al. Noninvasive Monitoring of Reproductive Activity Based on Fecal Progestagen Profiles and Sexual Behavior in Koalas, Phascolarctos cinereus. Biol. Reprod. 2009, 81, 1033–1040. [Google Scholar] [CrossRef]
- Hanger, J.; de Villiers, D.; Forbes, N.; Nottidge, B.; Beyer, H.; Loader, J.; Timms, P. Final Technical Report: Moreton Bay Rail Koala Management Program; Department of Transport and Main Roads, Queensland: Brisbane, Australia, 2017. [Google Scholar]
- Charlton, B.D.; Ellis, W.A.H.; McKinnon, A.J.; Brumm, J.; Nilsson, K.; Fitch, W.T. Perception of Male Caller Identity in Koalas (Phascolarctos cinereus): Acoustic Analysis and Playback Experiments. PLoS ONE 2011, 6, e20329. [Google Scholar] [CrossRef]
- Charlton, B.D.; Reby, D.; Ellis, W.A.H.; Brumm, J.; Fitch, W.T. Estimating the Active Space of Male Koala Bellows: Propagation of Cues to Size and Identity in a Eucalyptus Forest. PLoS ONE 2012, 7, e45420. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; 3.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Caselli, C.B.; Mennill, D.J.; Gestich, C.C.; Setz, E.Z.F.; Bicca-Marques, J.C. Playback responses of socially monogamous black-fronted titi monkeys to simulated solitary and paired intruders. Am. J. Primatol. 2015, 77, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.A.; Michael, P.W. Using audio playback to expand the geographic breeding range of an endangered species. Divers. Distrib. 2017, 23, 1499–1508. [Google Scholar] [CrossRef]
- Tucker, G.; Melzer, A.; Ellis, W. The development of habitat selection by subadult koalas. Aust. J. Zool. 2007, 55, 285–289. [Google Scholar] [CrossRef]
- Andersson, M.B. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Espmark, Y.; Amundsen, T.; Rosenqvist, G. Animal Signals: Signalling and Signal Design in Animal Communication; Tapir Academic Press: Trondheim, Norway, 2000. [Google Scholar]
- Smith, M. Behaviour of the Koala, Phascolarctos cinereus (Goldfuss), in Captivity VI*. Aggression. Wildl. Res. 1980, 7, 177–190. [Google Scholar] [CrossRef]
- Reby, D.; McComb, K.; Cargnelutti, B.; Darwin, C.; Tecumseh Fitch, W.; Clutton-Brock, T. Red deer stags use formants as assessment cues during intrasexual agonistic interactions. Proc. R. Soc. Biol. Sci. 2005, 272, 941–947. [Google Scholar] [CrossRef]
- Charrier, I.; Ahonen, H.; Harcourt, R.G. What Makes an Australian Sea Lion (Neophoca cinerea) Male’s Bark Threatening? J. Comp. Psychol. 2011, 125, 385–392. [Google Scholar] [CrossRef]
- Charlton, B.D.; Zhihe, Z.; Snyder, R.J. The information content of giant panda, Ailuropoda melanoleuca, bleats: Acoustic cues to sex, age and size. Anim. Behav. 2009, 78, 893–898. [Google Scholar] [CrossRef]
- Vannoni, E.; McElligott, A.G. Low frequency groans indicate larger and more dominant fallow deer (Dama dama) males. PLoS ONE 2008, 3, e3113. [Google Scholar] [CrossRef]
- Sacchi, R.; Pupin, F.; Gentilli, A.; Rubolini, D.; Scali, S.; Fasola, M.; Galeotti, P. Male–male combats in a polymorphic lizard: Residency and size, but not color, affect fighting rules and contest outcome. Aggress. Behav. 2009, 35, 274–283. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Harvey, P.H. Mammals, resources and reproductive strategies. Nature 1978, 273, 191–195. [Google Scholar] [CrossRef]
- Smith, M. Behaviour of the Koala, Phascolarctos cinereus (Goldfuss), in CAptivity V*. Sexual Behaviour. Wildl. Res. 1980, 7, 41–51. [Google Scholar] [CrossRef]
- Feige, S.; Nilsson, K.; Phillips, C.J.C.; Johnston, S.D. Heterosexual and homosexual behaviour and vocalisations in captive female koalas (Phascolarctos cinereus). Appl. Anim. Behav. Sci. 2007, 103, 131–145. [Google Scholar] [CrossRef]
- Charlton, B.D. The Acoustic Structure and Information Content of Female Koala Vocal Signals. PLoS ONE 2015, 10, e0138670. [Google Scholar] [CrossRef]
- Siracusa, E.; Morandini, M.; Boutin, S.; Humphries, M.M.; Dantzer, B.; Lane, J.E.; McAdam, A.G. Red squirrel territorial vocalizations deter intrusions by conspecific rivals. Behaviour 2017, 154, 1259–1273. [Google Scholar] [CrossRef]
- Davidson, S.M.; Wilkinson, G.S. Function of male song in the greater white-lined bat, Saccopteryx bilineata. Anim. Behav. 2004, 67, 883–891. [Google Scholar] [CrossRef]
- Walcott, C.; Mager, J.N.; Piper, W. Changing territories, changing tunes: Male loons, Gavia immer, change their vocalizations when they change territories. Anim. Behav. 2006, 71, 673–683. [Google Scholar] [CrossRef]
- Goll, Y.; Demartsev, V.; Koren, L.; Geffen, E. Male hyraxes increase countersinging as strangers become ‘nasty neighbours’. Anim. Behav. 2017, 134, 9–14. [Google Scholar] [CrossRef]
- Mitchell, P.J. The Social Organization of Koalas. Ph.D. Thesis, Monash University, Melbourne, Australia, 1989. [Google Scholar]
- Martin, R.; Handasyde, K. Population dynamics of the koala (Phascolarctos cinereus) in southeastern Australia. In Biology of the Koala; Lee, A.K., Handasyde, K.A., Sanson, G.D., Eds.; Surrey Beatty & Sons: Sydney, Australia, 1990. [Google Scholar]
- Kondo, N.; Hiraiwa-Hasegawa, M. The influence of social dominance on calling rate in the Large-billed Crow (Corvus macrorhynchos). J. Ornithol. 2015, 156, 775–782. [Google Scholar] [CrossRef]
- Seddon, N.; Alvarez, A.; Tobias, J. Vocal communication in the pale-winged trumpeter (Psophia leucoptera): Repertoire, context and functional reference. Behaviour 2002, 139, 1331–1359. [Google Scholar] [CrossRef]
- Otter, K.; Chruszcz, B.; Ratcliffe, L. Honest advertisement and song output during the dawn chorus of black-capped chickadees. Behav. Ecol. 1997, 8, 167–173. [Google Scholar] [CrossRef]
- Leonard, M.L.; Horn, A.G. Crowing in relation to status in roosters. Anim. Behav. 1995, 49, 1283–1290. [Google Scholar] [CrossRef]
- Mitani, J.C.; Nishida, T. Contexts and social correlates of long-distance calling by male chimpanzees. Anim. Behav. 1993, 45, 735–746. [Google Scholar] [CrossRef]
- Ellis, B. High Society. Australas. Sci. 2016, 37, 24–25. [Google Scholar]



| Koala | Age (Years) | Weight (kg) | Category | Bellow Set | Source |
|---|---|---|---|---|---|
| Hudson | 5 | 5.6 | Small | 1 | Australia Zoo |
| Harley | 9.5 | 9.3 | Large | 1 | Australia Zoo |
| Dexter | 5.5 | 5.7 | Small | 2 | Australia Zoo |
| SA | Unknown | 9.3 | Large | 2 | RSPCA Wacol Hospital |
| Zorro | Unknown | 5.7 | Small | 3 | RSPCA Wacol Hospital |
| Marley | 5 | 8.5 | Large | 3 | Australia Zoo |
| Test Round | Koala Model | Koala Bellow | Broadcast Hours | Date | Test Location |
|---|---|---|---|---|---|
| Control for Model | None | None | n/a | 18/11/2019 | BBP D1 (virtual) |
| 19/11/2019 | BBP D2 (virtual) | ||||
| 20/11/2019 | BBP D3 (virtual) | ||||
| Model | Yes | None | n/a | 21/11/2019 | BBP D1 |
| 22/11/2019 | BBP D2 | ||||
| 23/11/2019 | BBP D3 | ||||
| Control for Evening bellow | None | None | n/a | 30/11/2019 | BBP D1 (virtual) |
| 1/12/2019 | BBP D2 (virtual) | ||||
| 2/12/2019 | BBP D3 (virtual) | ||||
| Evening bellow | Yes | Small | 1 h (18:00–19:00) | 3/12/2019 | BBP D1 |
| 4/12/2019 | BBP D2 | ||||
| 5/12/2019 | BBP D3 | ||||
| Evening bellow | Yes | Large | 1 h (18:00–19:00) | 18/12/2019 | BBP D1 |
| 19/12/2019 | BBP D2 | ||||
| 20/12/2019 | BBP D3 | ||||
| Control for Overnight bellow | None | None | n/a | 20/03/2020 | BBP D1 (virtual) |
| 21/03/2020 | BBP D2 (virtual) | ||||
| 22/03/2020 | BBP D3 (virtual) | ||||
| Overnight bellow | Yes | Small | 6 h (18:00–00:00) | 23/03/2020 | BBP D1 |
| 24/03/2020 | BBP D2 | ||||
| 25/03/2020 | BBP D3 | ||||
| Overnight bellow | Yes | Large | 6 h (18:00–00:00) | 6/04/2020 | BBP D1 |
| 7/04/2020 | BBP D2 | ||||
| 8/04/2020 | BBP D3 |
| Koala | Class | Bellow | Weight (kg) | Age | Acty | Fix in 100 m | Total fix | App | Avd |
|---|---|---|---|---|---|---|---|---|---|
| Dave | MA | none | 4.8 | 3.5 | 101 | 6 | 128 | 1 | 0 |
| Dave | MA | Small | 4.8 | 3.5 | n/a | 28 | 41 | 2 | 0 |
| Dave | MA | Large | 4.8 | 3.5 | 135 | 20 | 128 | 0 | 3 |
| Skroo | MA | none | 7.5 | 5.5 | 117 | 4 | 236 | 0 | 0 |
| Skroo | MA | Small | 7.5 | 5.5 | 147 | 61 | 120 | 3 | 0 |
| Skroo | MA | Large | 7.5 | 5.5 | 155 | 36 | 101 | 4 | 0 |
| Tom | MJ | none | 3.4 | 1.5 | 185 | 0 | 232 | 0 | 1 |
| Tom | MJ | Small | 3.4 | 1.5 | 225 | 0 | 128 | 0 | 0 |
| Tom | MJ | Large | 3.4 | 1.5 | 178 | 24 | 128 | 0 | 0 |
| Martin | MJ | none | 3.6 | 2 | n/a | 0 | 64 | 0 | 0 |
| Martin | MJ | Small | 3.6 | 2 | n/a | 1 | 42 | 0 | 1 |
| Martin | MJ | Large | 3.6 | 2 | n/a | 1 | 42 | 0 | 1 |
| Jo | FS/FDJ | none | 4.9 | 3.9 | 193 | 24 | 232 | 1 | 0 |
| Jo | FS/FDJ | Small | 4.9 | 3.9 | 186 | 43 | 109 | 1 | 4 |
| Jo | FS/FDJ | Large | 4.9 | 3.9 | 200 | 21 | 117 | 0 | 0 |
| Jude | FS | none | 4.1 | 2.9 | 175 | 0 | 256 | 0 | 0 |
| Jude | FS | Small | 4.1 | 2.9 | 181 | 1 | 128 | 1 | 0 |
| Jude | FS | Large | 4.1 | 2.9 | 189 | 18 | 128 | 1 | 0 |
| Karen | FDJ | none | 5.4 | 4.9 | 114 | 0 | 232 | 0 | 0 |
| Karen | FDJ | Small | 5.4 | 4.9 | 131 | 0 | 128 | 0 | 0 |
| Karen | FDJ | Large | 5.4 | 4.9 | 148 | 24 | 128 | 0 | 2 |
| Miriam | FS | none | 2 | 1 | n/a | 24 | 64 | 0 | 0 |
| Miriam | FS | Small | 2 | 1 | n/a | 24 | 64 | 0 | 0 |
| Miriam | FS | Large | 2 | 1 | n/a | 24 | 64 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, A.Z.; Murray, P.; Phillips, C.; Tribe, A.; Ellis, W. Movement of Free-Ranging Koalas in Response to Male Vocalisation Playbacks. Animals 2022, 12, 287. https://doi.org/10.3390/ani12030287
Jiang AZ, Murray P, Phillips C, Tribe A, Ellis W. Movement of Free-Ranging Koalas in Response to Male Vocalisation Playbacks. Animals. 2022; 12(3):287. https://doi.org/10.3390/ani12030287
Chicago/Turabian StyleJiang, Alex Zijian, Peter Murray, Clive Phillips, Andrew Tribe, and William Ellis. 2022. "Movement of Free-Ranging Koalas in Response to Male Vocalisation Playbacks" Animals 12, no. 3: 287. https://doi.org/10.3390/ani12030287
APA StyleJiang, A. Z., Murray, P., Phillips, C., Tribe, A., & Ellis, W. (2022). Movement of Free-Ranging Koalas in Response to Male Vocalisation Playbacks. Animals, 12(3), 287. https://doi.org/10.3390/ani12030287







