Diversity of Volatile Compounds in Raw Milk with Different n-6 to n-3 Fatty Acid Ratio
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Statistical Analysis
3. Results
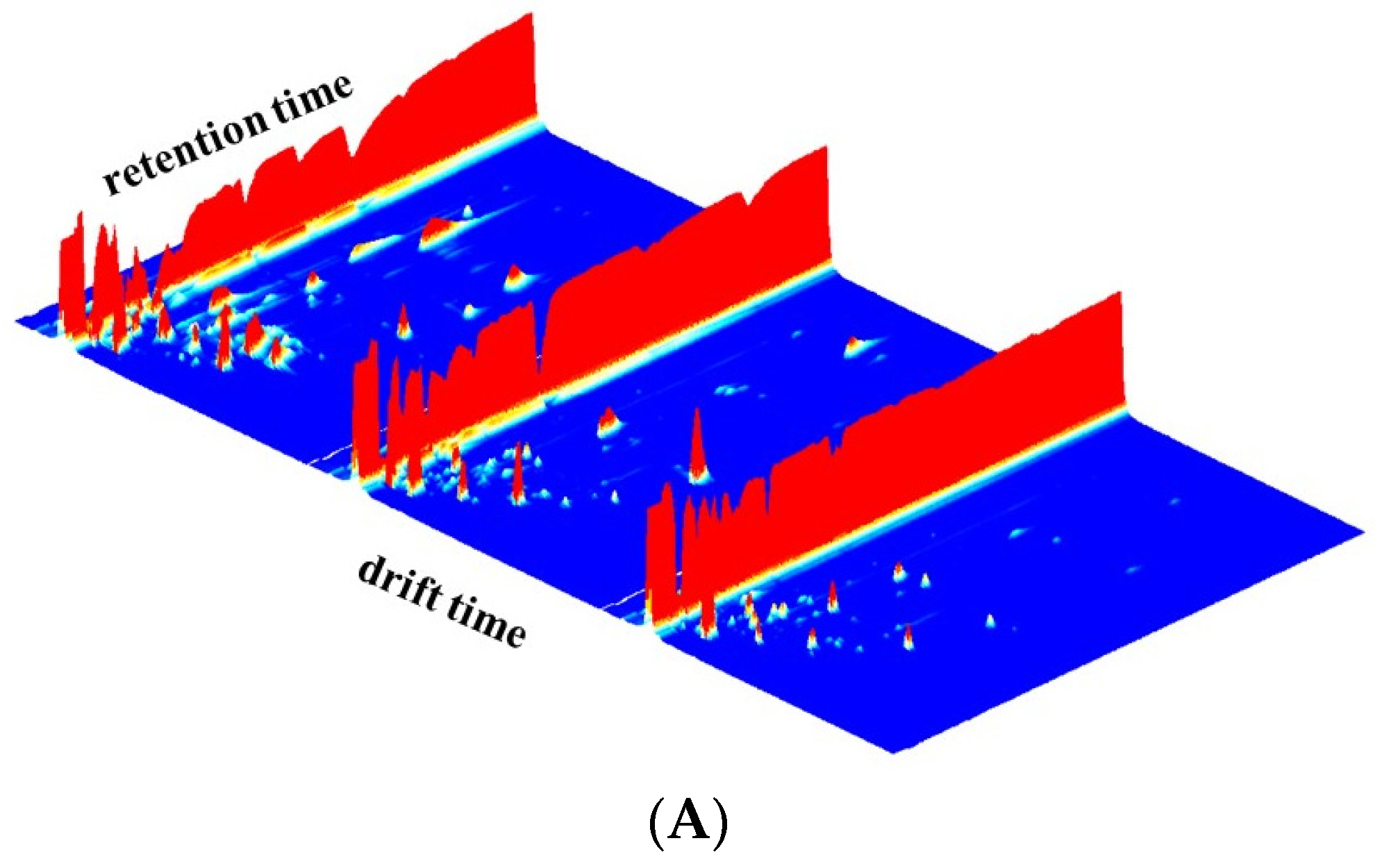
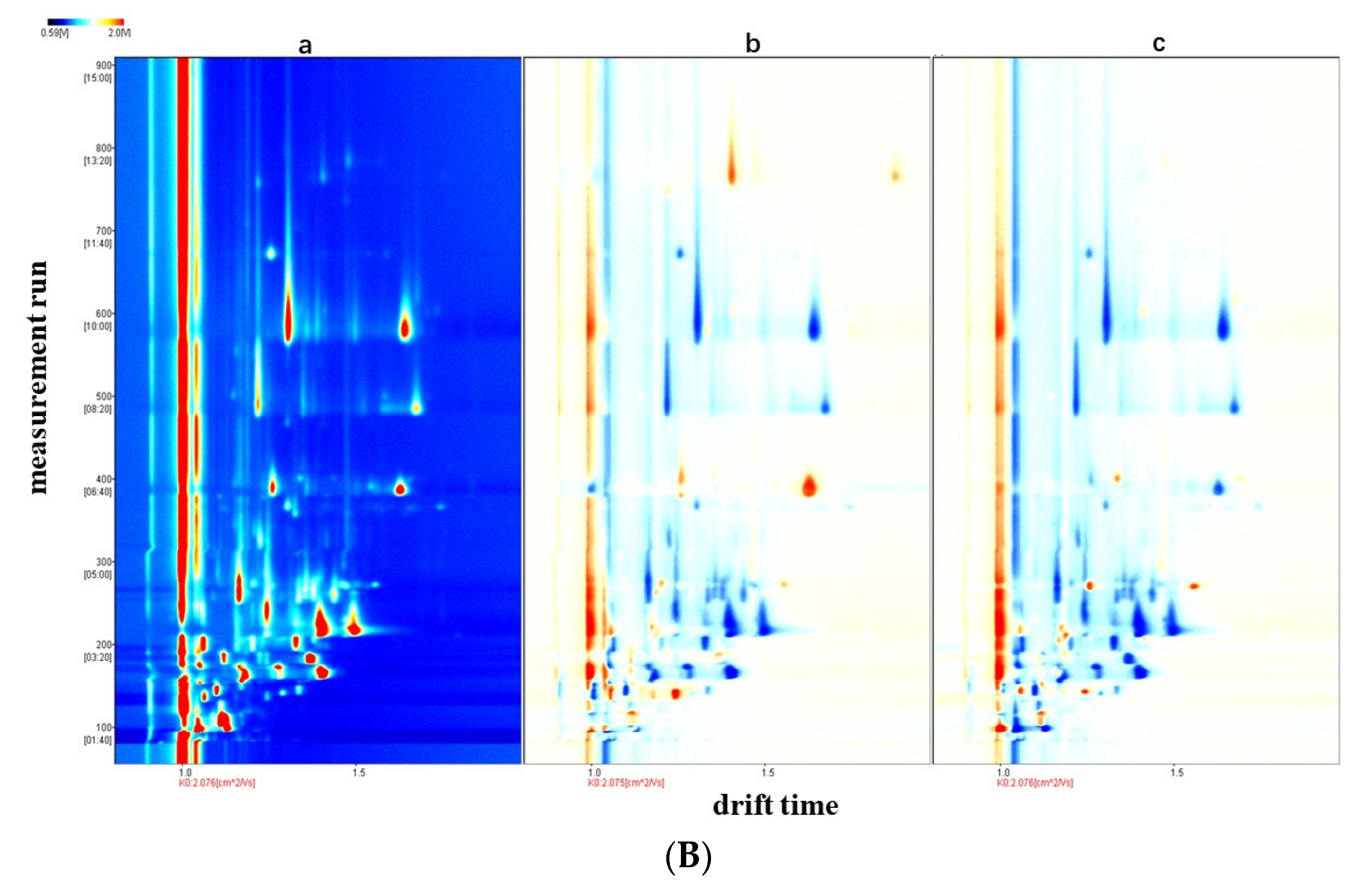
3.1. GC-IMS Topographic Plots of Different Raw Milk Samples
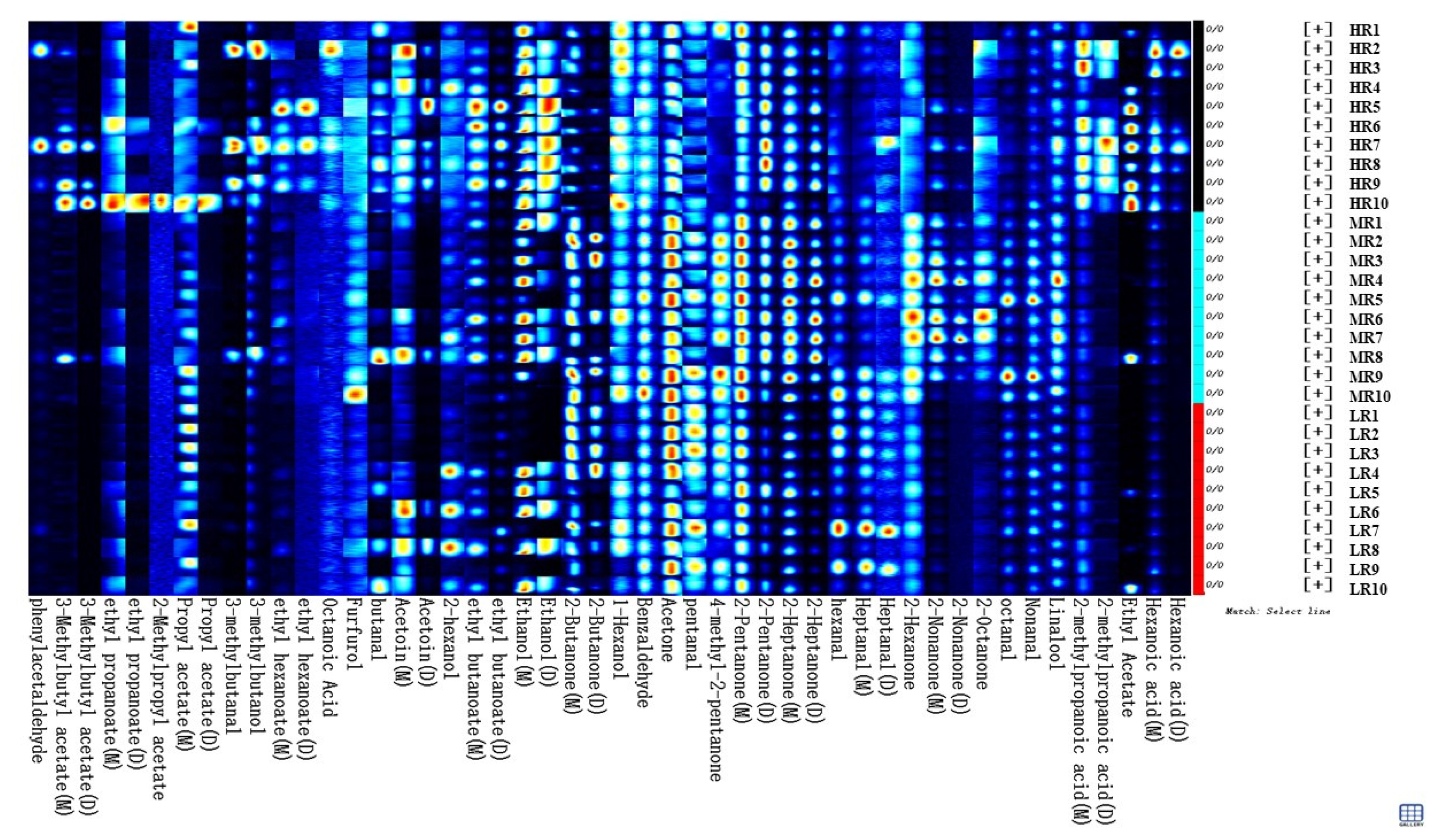
3.2. Diversities of Volatile Matter of Raw MILK samples from Different Treatments
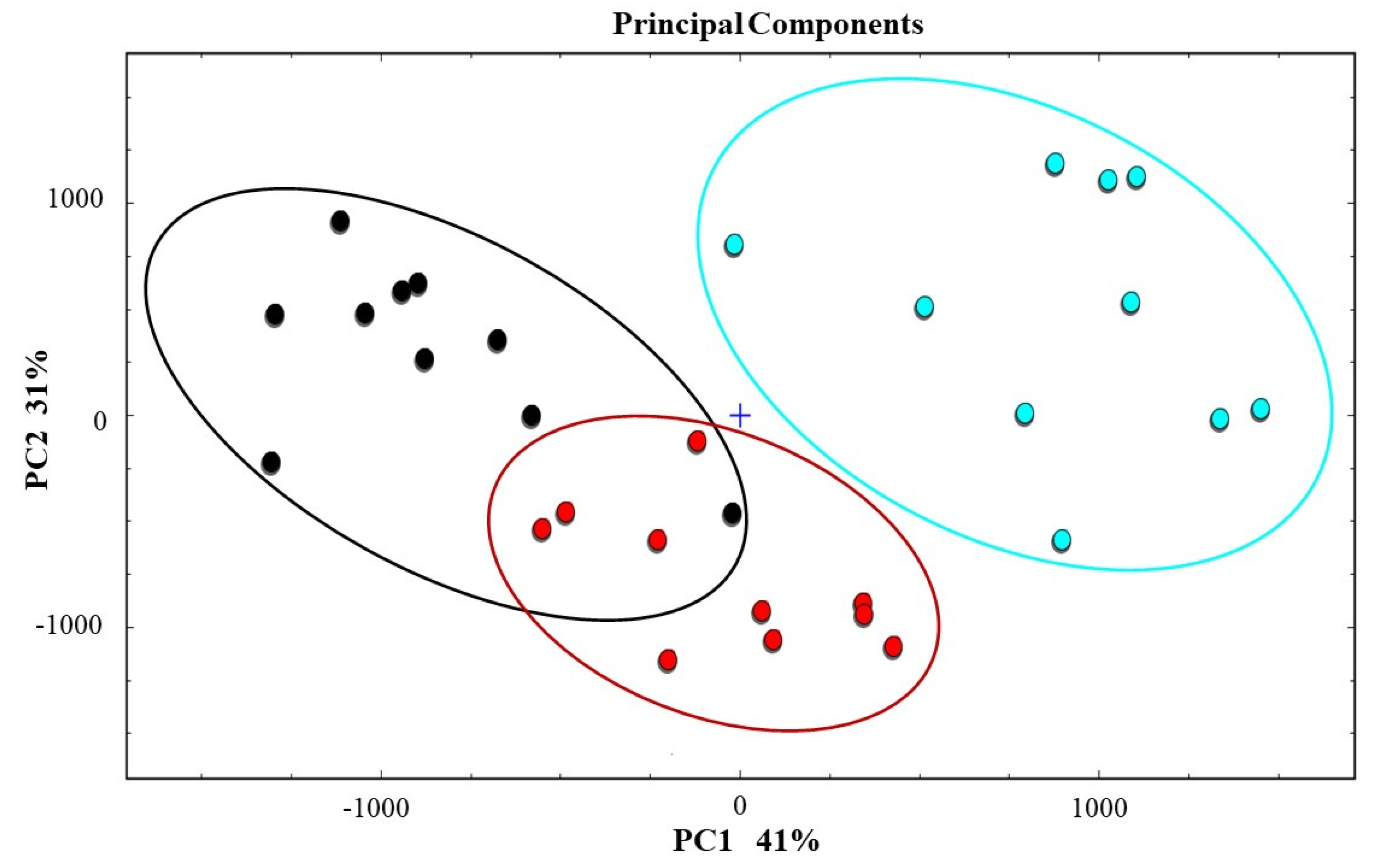
3.3. Identification of Differences among All Samples by Principal Component Analysis(PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badings, H.T. Volatile Compounds in Foods and Beverages, 1st ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 205–213. [Google Scholar]
- She, C.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Friesen, R.W.; Innis, S.M. Dietary Arachidonic Acid to EPA and DHA balance is increased among canadian pregnant women with low fish intake. J. Nutr. 2009, 139, 2344–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, A.Y.; Saffar, A.E.; Abdullah, F.K.; Bahouh, M.E.; Ragheb, G.; Mashaly, M.M. Effect of adding flaxseed in the diet of laying hens on both production of omega-3 enriched eggs and on production performance. Int. J. Poult. Sci. 2011, 10, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Roberta, F.; Felicia, C.; Marilena, M.; Laura, M.; Alba, V.; Roberto, B.; Andrea, M.; Luciano, B.; Angelo, F.; Aldo, B.; et al. α-Linolenic Acid-enriched diet prevents myocardial damage and expands longevity in cardiomyopathic hamsters. Am. J. Pathol. 2006, 169, 1913–1924. [Google Scholar] [CrossRef] [Green Version]
- Bassett, C.; Mccullough, R.S.; Edel, A.L.; Patenaude, A.; Lavallee, R.K.; Pierce, G.N. The α-linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans fat. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, 2220–2226. [Google Scholar] [CrossRef] [Green Version]
- Innesa, J.K.; Caldera, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Wahrburg, U. What are the health effects of fat? Eur. J. Nutr. 2004, 43, i6–i11. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Assaad, B.; Poston, W.C. Tissue omega-6/omega-3 fatty acid ratio and risk for coronary artery disease. Am. J. Cardiol. 2006, 98, 19–26. [Google Scholar] [CrossRef]
- WHO. FAO. Joint Consultant. Nutr. Rev. 1995, 53, 202–220. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Riediger, N.D.; Azordegan, N.; Harris-Janz, S.; Ma, W.L.; Suh, M.; Moghadasian, M.H. Designer oils’ low in n-6: N-3 fatty acid ratio beneficially modifies cardiovascular risks in mice. Eur. J. Nutr. 2009, 48, 307–314. [Google Scholar] [CrossRef]
- Cotogni, P.; Muzio, G.; Trombetta, A.; Ranieri, V.M.; Canuto, R.A. Impact of the omega-3 to omega-6 polyunsaturated fatty acid ratio on cytokine release in human alveolar cells. J. Parenter. Enteral Nutr. 2011, 35, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.B.; Huang, L.L.; Rong, R.; Tan, R.; Wang, J.D.; Kang, J.X. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2487–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagi, A.; Nakayama, M.; Shinzaki, W.; Hagi, S.; Ohyanagi, H. Effects of the omega-6: Omega-3 fatty acid ratio of fat emulsions on the fatty acid composition in cell membranes and the anti-inflammatory action. J. Parenter. Enter. Nutr. 2010, 34, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lapthorn, C.; Pullen, F.; Chowdary, B.Z. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrom. Rev. 2012, 32, 43–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallegos, J.; Arce, C.; Jordano, R.; Arce, L.; Medina, L.M. Target identification of volatile metabolites to allow the differentiation of lactic acid bacteria by gas chromatography-ion mobility spectrometry. Food Chem. 2017, 220, 362–370. [Google Scholar] [CrossRef]
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123. [Google Scholar] [CrossRef]
- Zeev, K. Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res. Int. 2013, 54, 1146–1151. [Google Scholar] [CrossRef]
- López-Feria, S.; Cárdenas, S.; Valcárcel, M. Simplifying chromatographic analysis of the volatile fraction of foods. Trends Anal. Chem. 2008, 27, 794–803. [Google Scholar] [CrossRef]
- Toso, B.; Procida, G.; Stefanon, B. Determination of volatile compounds in cows’ milk using headspace GC-MS. J. Dairy Res. 2002, 69, 569–577. [Google Scholar] [CrossRef]
- Yao, W.S.; Cai, Y.X.; Liu, D.Y. Analysis of volatile flavor compounds in smoked chicken thighs by HS-GC-IMS and HS-SPME-GC-MS. Food Ferment. Ind. 2021, 47, 1–9. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2020, 140, 109975. [Google Scholar] [CrossRef]
- Holland, R.; Liu, S.Q.; Crow, V.L.; Delabre, M.L.; Lubbers, M.; Bennett, M.; Norris, G. Esterases of lactic acid bacteria and cheese flavour: Milk fat hydrolysis, alcoholysis and esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Licón, C.C.; Mendoza, J.D.; Maggi, L.; Berruga, M.I.; Aranda, R.; Carmona, M. Optimization of headspace sorptive extraction for the analysis of volatiles in pressed ewes’ milk cheese. Int. Dairy J. 2012, 23, 53–61. [Google Scholar] [CrossRef]
- Gallegos, J.; Garrido-Delgado, R.; Aree, L.; Medina, L.M. Volatile metabolites of goat cheeses determined by ion mobility spectrometry. Potential applications in quality control. Food Anal. Method. 2014, 8, 1699–1709. [Google Scholar] [CrossRef]
- Mao, L.; Roos, Y.H.; Costas, G. Food emulsions as delivery systems for flavor compounds: A review. Crit. Rev. Food Sci. Crit. Rev. Food Sci. 2015, 57, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, A.; Cho, I.H.; Kim, Y. Changes in fatty acids and volatile components in mackerel by broiling. Eur. J. Lipid. Sci. Technol. 2015, 113, 1481–1490. [Google Scholar] [CrossRef]
- Sebzalli, Y.M.; Wang, X.Z. Knowledge discovery from process operational data using PCA and fuzzy clustering. Eng Appl. Artif. Intel. 2001, 14, 607–616. [Google Scholar] [CrossRef]
| Treatments | n-6/n-3 | Treatments | n-6/n-3 | Treatments | n-6/n-3 |
|---|---|---|---|---|---|
| HR1 | 8.604 | MR1 | 3.722 | LR1 | 3.091 |
| HR2 | 7.908 | MR2 | 5.237 | LR2 | 3.111 |
| HR3 | 8.005 | MR3 | 3.575 | LR3 | 4.509 |
| HR4 | 8.457 | MR4 | 4.599 | LR4 | 3.133 |
| HR5 | 7.961 | MR5 | 5.189 | LR5 | 3.037 |
| HR6 | 8.320 | MR6 | 4.814 | LR6 | 3.030 |
| HR7 | 7.924 | MR7 | 4.215 | LR7 | 3.113 |
| HR8 | 8.523 | MR8 | 4.260 | LR8 | 2.880 |
| HR9 | 8.670 | MR9 | 4.426 | LR9 | 3.365 |
| HR10 | 7.991 | MR10 | 4.502 | LR10 | 3.233 |
| Average | 8.236 | Average | 4.454 | Average | 3.250 |
| Time | E1 | E2 | R |
|---|---|---|---|
| 00:00 | 150 mL/min | 2 mL/min | Rec |
| 02:00 | 150 mL/min | 2 mL/min | - |
| 10:00 | 150 mL/min | 15 mL/min | - |
| 20:00 | 150 mL/min | 80 mL/min | - |
| 25:00 | 150 mL/min | 130 mL/min | Stop |
| Count | Compound | CAS No. | Formula | MW | RI | RT [sec] | DT [a.u.] | Structure |
|---|---|---|---|---|---|---|---|---|
| 1 | Nonanal | C124196 | C9H18O | 142.2 | 1104.8 | 784.727 | 1.47794 | |
| 2 | 2-Nonanone | C821556 | C9H18O | 142.2 | 1097.9 | 769.582 | 1.40903 | Monomer |
| 2-Nonanone | C821556 | C9H18O | 142.2 | 1096.1 | 765.851 | 1.88047 | Dimer | |
| 3 | Phenylacetaldehyde | C122781 | C8H8O | 120.2 | 1049.7 | 672.112 | 1.25843 | |
| 4 | ethyl hexanoate | C123660 | C8H16O2 | 144.2 | 1012.8 | 605.99 | 1.34128 | Monomer |
| ethyl hexanoate | C123660 | C8H16O2 | 144.2 | 1010.8 | 602.707 | 1.79409 | Dimer | |
| 5 | Octanal | C124130 | C8H16O | 128.2 | 1012.2 | 605.052 | 1.41127 | |
| 6 | Hexanoic acid | C142621 | C6H12O2 | 116.2 | 998.1 | 581.604 | 1.30985 | Monomer |
| Hexanoic acid | C142621 | C6H12O2 | 116.2 | 999.9 | 584.418 | 1.64411 | Dimer | |
| 7 | Benzaldehyde | C100527 | C7H6O | 106.1 | 957.9 | 501.045 | 1.14803 | |
| 8 | 2-Heptanone | C110430 | C7H14O | 114.2 | 891.5 | 390.856 | 1.26047 | Monomer |
| 2-Heptanone | C110430 | C7H14O | 114.2 | 889.0 | 387.203 | 1.63389 | Dimer | |
| 9 | 3-Methylbutyl acetate | C123922 | C7H14O2 | 130.2 | 877.8 | 371.375 | 1.30767 | Monomer |
| 3-Methylbutyl acetate | C123922 | C7H14O2 | 130.2 | 876.0 | 368.94 | 1.74911 | Dimer | |
| 10 | 1-Hexanol | C111273 | C6H14O | 102.2 | 868.8 | 359.199 | 1.32572 | |
| 11 | ethyl butanoate | C105544 | C6H12O2 | 116.2 | 797.2 | 275.188 | 1.20633 | Monomer |
| ethyl butanoate | C105544 | C6H12O2 | 116.2 | 795.4 | 273.361 | 1.55893 | Dimer | |
| 12 | Hexanal | C66251 | C6H12O | 100.2 | 793.6 | 271.535 | 1.25492 | |
| 13 | 2-Pentanone | C107879 | C5H10O | 86.1 | 685.8 | 183.262 | 1.12026 | Monomer |
| 2-Pentanone | C107879 | C5H10O | 86.1 | 683.4 | 182.044 | 1.37014 | Dimer | |
| 14 | 2-Butanone | C78933 | C4H8O | 72.1 | 584.9 | 138.821 | 1.06196 | Monomer |
| 2-Butanone | C78933 | C4H8O | 72.1 | 586.5 | 139.43 | 1.24798 | Dimer | |
| 15 | Acetone | C67641 | C3H6O | 58.1 | 503.0 | 110.817 | 1.11888 | |
| 16 | 4-methyl-2-pentanone | C108101 | C6H12O | 100.2 | 736.9 | 220.398 | 1.1744 | |
| 17 | Acetoin | C513860 | C4H8O2 | 88.1 | 714.6 | 203.091 | 1.06014 | Monomer |
| Acetoin | C513860 | C4H8O2 | 88.1 | 714.6 | 203.091 | 1.33335 | Dimer | |
| 18 | 3-methylbutanol | C123513 | C5H12O | 88.1 | 735.4 | 219.246 | 1.5004 | |
| 19 | Ethyl Acetate | C141786 | C4H8O2 | 88.1 | 607.2 | 147.615 | 1.33495 | |
| 20 | Ethanol | C64175 | C2H6O | 46.1 | 457.3 | 97.718 | 1.05001 | Monomer |
| Ethanol | C64175 | C2H6O | 46.1 | 465.7 | 100.021 | 1.13238 | Dimer | |
| 21 | 3-methylbutanal | C590863 | C5H10O | 86.1 | 645.7 | 164.119 | 1.4073 | |
| 22 | ethyl propanoate | C105373 | C5H10O2 | 102.1 | 706.4 | 197.127 | 1.15019 | Monomer |
| ethyl propanoate | C105373 | C5H10O2 | 102.1 | 709.1 | 199.046 | 1.45405 | Dimer | |
| 23 | Propyl acetate | C109604 | C5H10O2 | 102.1 | 712.7 | 201.733 | 1.16243 | Monomer |
| Propyl acetate | C109604 | C5H10O2 | 102.1 | 710.1 | 199.814 | 1.47854 | Dimer | |
| 24 | 2-Methylpropyl acetate | C110190 | C6H12O2 | 116.2 | 767.5 | 246.639 | 1.61544 | |
| 25 | 2-methylpropanoic acid | C79312 | C4H8O2 | 88.1 | 782.4 | 260.457 | 1.16243 | Monomer |
| 2-methylpropanoic acid | C79312 | C4H8O2 | 88.1 | 782.4 | 260.457 | 1.37391 | Dimer | |
| 26 | Linalool | C78706 | C10H18O | 154.3 | 1088.7 | 749.932 | 1.22063 | |
| 27 | Octanoic Acid | C124072 | C8H16O2 | 144.2 | 1184.8 | 982.302 | 1.45182 | |
| 28 | 2-Octanone | C111137 | C8H16O | 128.2 | 997.6 | 580.767 | 1.33443 | |
| 29 | Furfurol | C98011 | C5H4O2 | 96.1 | 827.9 | 308.433 | 1.08207 | |
| 30 | 2-hexanol | C626937 | C6H14O | 102.2 | 776.7 | 255.096 | 1.28405 | |
| 31 | Heptanal | C111717 | C7H14O | 114.2 | 898.3 | 400.876 | 1.33955 | Monomer |
| Heptanal | C111717 | C7H14O | 114.2 | 898.9 | 401.71 | 1.69995 | Dimer | |
| 32 | Pentanal | C110623 | C5H10O | 86.1 | 697.8 | 191.007 | 1.1889 | |
| 33 | Butanal | C123728 | C4H8O | 72.1 | 590.9 | 141.125 | 1.2903 | |
| 34 | 2-Hexanone | C591786 | C6H12O | 100.2 | 785.5 | 263.444 | 1.18811 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Huang, G.; Zhang, Y.; Zheng, N.; Zhao, S.; Wang, J. Diversity of Volatile Compounds in Raw Milk with Different n-6 to n-3 Fatty Acid Ratio. Animals 2022, 12, 252. https://doi.org/10.3390/ani12030252
Li N, Huang G, Zhang Y, Zheng N, Zhao S, Wang J. Diversity of Volatile Compounds in Raw Milk with Different n-6 to n-3 Fatty Acid Ratio. Animals. 2022; 12(3):252. https://doi.org/10.3390/ani12030252
Chicago/Turabian StyleLi, Ning, Guoxin Huang, Yangdong Zhang, Nan Zheng, Shengguo Zhao, and Jiaqi Wang. 2022. "Diversity of Volatile Compounds in Raw Milk with Different n-6 to n-3 Fatty Acid Ratio" Animals 12, no. 3: 252. https://doi.org/10.3390/ani12030252
APA StyleLi, N., Huang, G., Zhang, Y., Zheng, N., Zhao, S., & Wang, J. (2022). Diversity of Volatile Compounds in Raw Milk with Different n-6 to n-3 Fatty Acid Ratio. Animals, 12(3), 252. https://doi.org/10.3390/ani12030252








