Factors Affecting Intraoperative Gastro-Oesophageal Reflux in Dogs and Cats
Abstract
Simple Summary
Abstract
1. Introduction
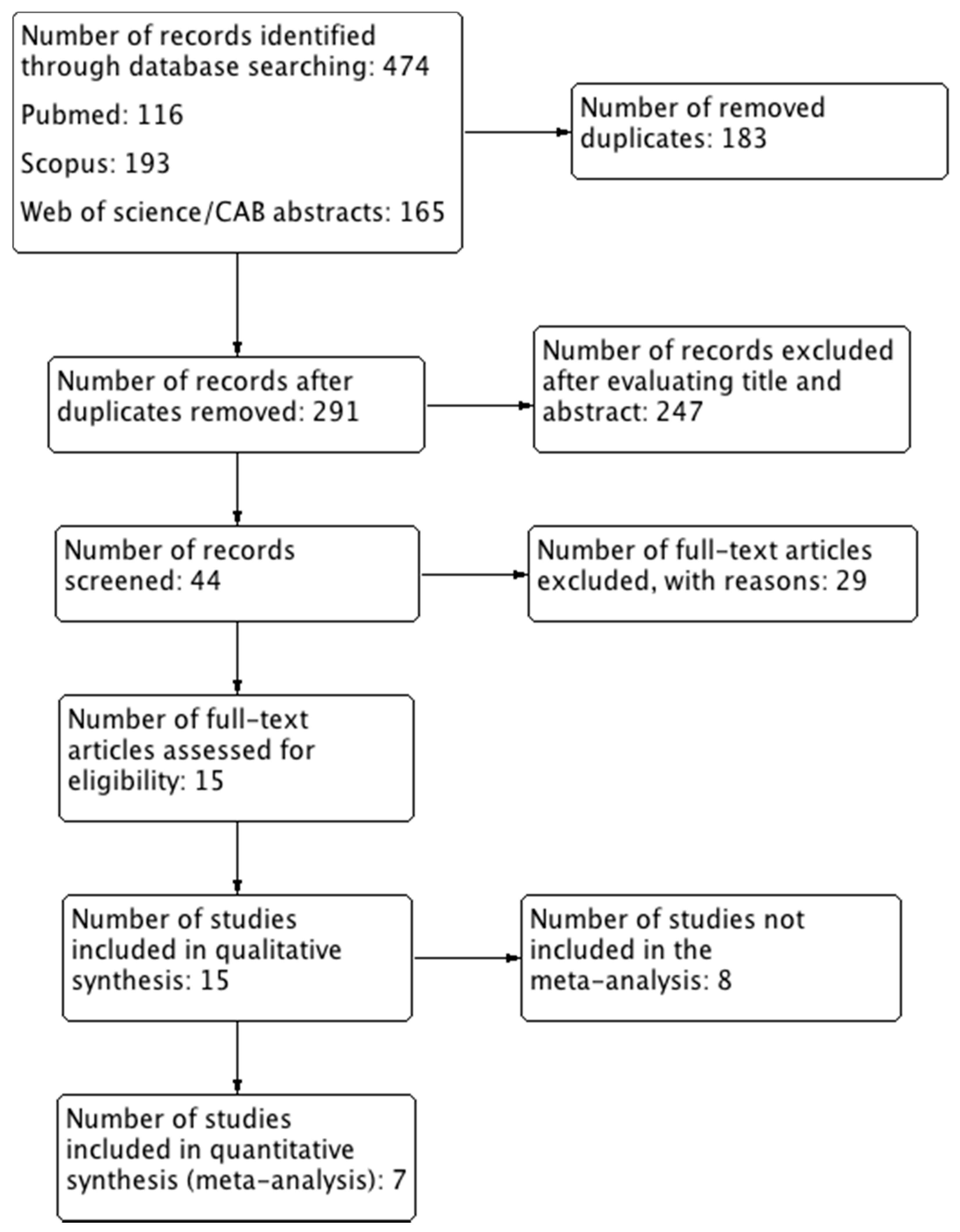
2. Methods
2.1. Type of Studies
2.2. Population/Species Studied
2.3. Interventions
- The duration of preoperative fasting (control more than 5 h, intervention less than 5 h).
- The induction agents (thiopentone vs. propofol).
- The inhalant agents for maintenance of anaesthesia (halothane vs. isoflurane or sevoflurane).
- The use of opioids (control no, intervention yes).
- The use of antacids (control no, intervention yes).
- The use of D2 receptor antagonists (control no, intervention yes)
- The use of antiemetics (control no, intervention yes).
2.4. Outcome Measures
2.5. Search Method
- MEDLINE via PubMed.
- Web of Science.
- CAB Abstracts.
- SCOPUS.
2.6. Selection of Studies
2.7. Data Extraction and Management
- Authors, title, year of publication, journal.
- Number of animals in intervention and control groups.
- Dogs/cats, age, weight, status ASA.
- Outcome measures.
- Presence of any other outcome measures.
- Excluded animals (dropouts).
2.8. Assessment of Risk of Bias in Included Studies
- In randomised studies, if the randomisation method was not mentioned, the risk of bias was set to unclear.
- Random housing of the experimental animals, the animal assessors’, and animal selection blindness were judged as low or unclear risk of bias, because we assumed that this was mostly irrelevant to our review.
2.9. Data Analysis
3. Results
3.1. Description of the Included Studies
3.2. Risk of Bias of the Included Studies
3.3. Characteristics of the Excluded Studies
- One was a retrospective study of the risk factors and prevalence of regurgitation in dogs [1].
- One was a retrospective study about the oesophageal strictures in dogs and cats [8],
- another study was about the effect of the body position (dorsal or lateral) on the incidence of GOR in dogs [17]
- One experimental, cross-over study was about the effect of food withholding duration on gastric content and pH without any incidence of GOR [18].
- One study was about the effect of opioids on GOR, there was no control group [21].
- One study was in Portuguese language [49].
- One study was a case report about the incidence of rhinitis after intraoperative GOR in a dog [50].
- One clinical study was about the incidence of GOR in kittens, comparing two airway devices [53].
- One clinical study was about the effect of maropitant on preventing postoperative GOR in dogs [56].
- One clinical study was about the correlation between the shape of the dog’s chest and GOR [58].
- One study was a prospective, observational study for intraoperative GOR but with no interventions [59].
- One study was about passive regurgitation in dogs [60].
- One was a randomized, controlled clinical study but there was no control group, without opioids [61].
- One study investigated the incidence of GOR in comparison with the type of surgical procedure [62].
- One study was about the incidence of GOR in brachycephalic dogs in comparison with nonbrachycephalic dogs [63].
- One clinical study described a noninvasive model of gastro-oesophageal reflux in dogs [64].
- One was a review article about oesophagitis and stricture formation in dogs and cats [65].
3.4. The Effect of Fasting Duration on GOR
3.5. The Effect of Induction Agent on GOR
3.6. The Effect of the Inhalant Anaesthetic Agents for the Maintenance of Anaesthesia on GOR
3.7. The Effect of Opioids on GOR
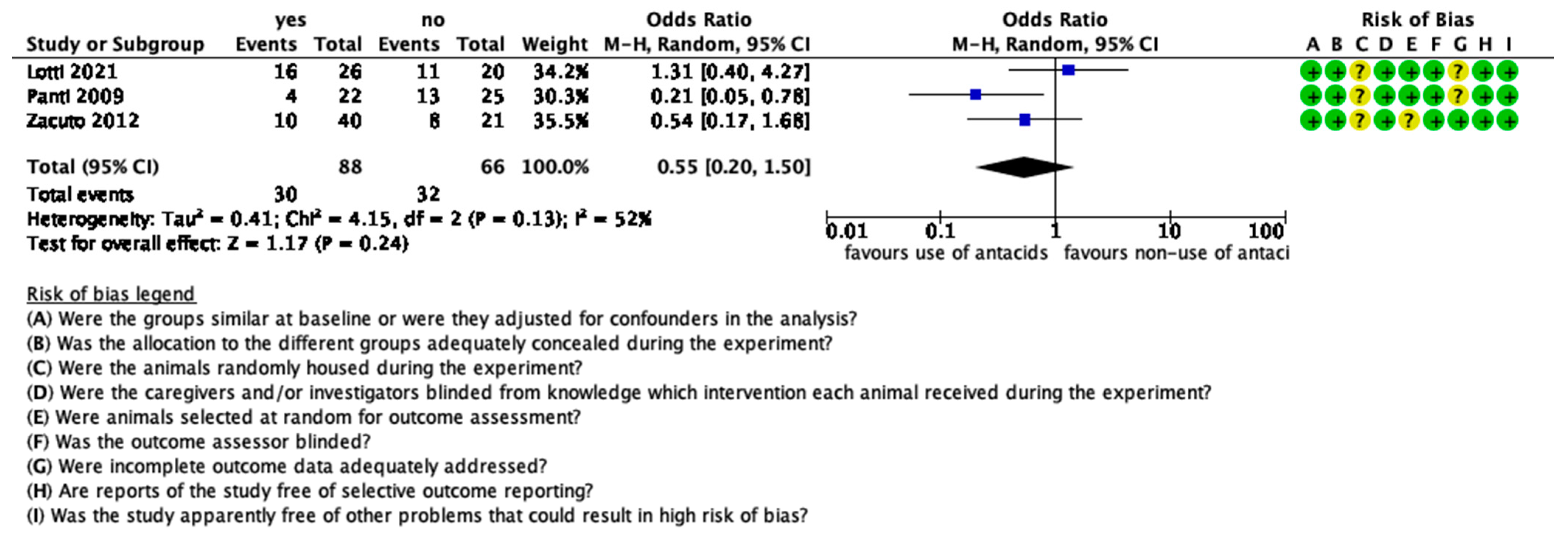
3.8. The Effect of Antacids on GOR
3.9. The Effect of D2 Receptor Antagonists on GOR
3.10. The Effect of Antiemetics on GOR
4. Discussion
5. Conclusions
- There are many factors affecting the development of GOR during anaesthesia in dogs.
- There is a limited number of studies investigating each one of these factors.
- The evidence is low-to-medium and cannot allow for the extraction of reliable conclusions on how these factors affect the development of GOR during anaesthesia.
- Evidence in cats is even more scarce.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Miguel Garcia, C.; Pinchbeck, G.; Dugdale, A.; Senior, J. Retrospective Study of the Risk Factors and Prevalence of Regurgitation in Dogs Undergoing General Anaesthesia. Open Vet. Sci. J. 2013, 7, 6–11. [Google Scholar] [CrossRef][Green Version]
- Raptopoulos, D.; Galatos, A.D. Post anaesthetic reflux oesophagitis in dogs and cats. Vet. Anaesth. Analg. 1995, 22, 6–8. [Google Scholar] [CrossRef]
- Waterman, A.E.; Hashim, M.A. Effects of thiopentone and propofol on lower oesophageal sphincter and barrier pressure in the dog. J. Small Anim. Pract. 1992, 33, 530–533. [Google Scholar] [CrossRef]
- Hashim, M.A.; Waterman, A.E. Effects of thiopentone, propofol, alphaxalone-alphadolone, ketamine and xylazine-ketamine on lower oesophageal sphincter pressure and barrier pressure in cats. Vet. Rec. 1991, 129, 137–139. [Google Scholar] [CrossRef]
- Hashim, M.A.; Waterman, A.E. Effects of acepromazine, pethidine and atropine premedication on lower oesophageal sphincter pressure and barrier pressure in anaesthetised cats. Vet. Rec. 1993, 133, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.V.; Evans, A.T.; Miller, R. Effects of preanesthetic administration of morphine on gastroesophageal reflux and regurgitation during anesthesia in dogs. Am. J. Vet. Res. 2005, 66, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Strombeck, D.R.; Harrold, D. Effects of atropine, acepromazine, meperidine, and xylazine on gastroesophageal sphincter pressure in the dog. Am. J. Vet. Res. 1985, 46, 963–965. [Google Scholar]
- Adamama-Moraitou, K.K.; Rallis, T.S.; Prassinos, N.N.; Galatos, A.D. Benign esophageal stricture in the dog and cat: A retrospective study of 20 cases. Can. J. Vet. Res. 2002, 66, 55–59. [Google Scholar] [PubMed]
- Wilson, D.V.; Walshaw, R. Postanesthetic Esophageal Dysfunction in 13 Dogs. J. Am. Anim. Hosp. Assoc. 2004, 40, 455–460. [Google Scholar] [CrossRef]
- Galatos, A.D.; Raptopoulos, D. Gastro-oesophageal reflux during anaesthesia in the dog: The effect of preoperative fasting and premedication. Vet. Rec. 1995, 137, 479–483. [Google Scholar] [CrossRef]
- Anagnostou, T.L.; Savvas, I.; Kazakos, G.M.; Ververidis, H.N.; Haritopoulou, M.R.; Rallis, T.S.; Raptopoulos, D. Effect of endogenous progesterone and oestradiol-17β on the incidence of gastro-oesophageal reflux and on the barrier pressure during general anaesthesia in the female dog. Vet. Anaesth. Analg. 2009, 36, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Panti, A.; Bennett, R.C.; Corletto, F.; Brearley, J.; Jeffrey, N.; Mellanby, R.J. The effect of omeprazole on oesophageal pH in dogs during anaesthesia. J. Small Anim. Pract. 2009, 50, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Favarato, E.S.S.; Souza, M.V.V.; Costa, P.R.S.R.S.; Favarato, L.S.C.S.C.; Nehme, R.C.C.; Monteiro, B.S.S.; Bonfá, L.P.P. Evaluation of metoclopramide and ranitidine on the prevention of gastroesophageal reflux episodes in anesthetized dogs. Res. Vet. Sci. 2012, 93, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Viskjer, S.; Sjöström, L. Effect of the duration of food withholding prior to anesthesia on gastroesophageal reflux and regurgitation in healthy dogs undergoing elective orthopedic surgery. Am. J. Vet. Res. 2017, 78, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Savvas, I.; Raptopoulos, D.; Rallis, T. A “Light Meal” Three Hours Preoperatively Decreases the Incidence of Gastro-Esophageal Reflux in Dogs. J. Am. Anim. Hosp. Assoc. 2016, 52, 357–363. [Google Scholar] [CrossRef]
- Flouraki, E. The Effect of Premedication on the Incidence of of Gastroesophageal Reflux during General Anesthesia in the Dog; Aristotle University of Thessaloniki: Thessaloniki, Greece, 2017. [Google Scholar]
- Waterman, A.E.; Hashim, M.A.; Pearson, H. Effect of body position on oesophageal and gastric pressures in the anaesthetised dog. J. Small Anim. Pract. 1995, 36, 196–200. [Google Scholar] [CrossRef]
- Savvas, I.; Rallis, T.; Raptopoulos, D. The effect of pre-anaesthetic fasting time and type of food on gastric content volume and acidity in dogs. Vet. Anaesth. Analg. 2009, 36, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Galatos, A.D.; Raptopoulos, D. Gastro-oesophageal reflux during anaesthesia in the dog: The effect of age, positioning and type of surgical procedure. Vet. Rec. 1995, 137, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Galatos, A.D.; Savas, I.; Prassinos, N.N.; Raptopoulos, D. Gastro-Oesophageal Reflux during Thiopentone or Propofol Anaesthesia in the Cat. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2001, 48, 287–294. [Google Scholar] [CrossRef]
- Wilson, D.V.; Tom Evans, A.; Mauer, W.A. Pre-anesthetic meperidine: Associated vomiting and gastroesophageal reflux during the subsequent anesthetic in dogs. Vet. Anaesth. Analg. 2007, 34, 15–22. [Google Scholar] [CrossRef]
- Wilson, D.V.; Boruta, D.T.; Evans, A.T. Influence of halothane, isoflurane, and sevoflurane on gastroesophageal reflux during anesthesia in dogs. Am. J. Vet. Res. 2006, 67, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Maclean, A.R.; Renwick, C. Audit of pre-operative starvation. Anaesthesia 1993, 48, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Pearse, R.; Rajakulendran, Y. Pre-operative fasting and administration of regular medications in adult patients presenting for elective surgery. Has the new evidence changed practice? Eur. J. Anaesthesiol. 1999, 16, 565–568. [Google Scholar] [CrossRef]
- Crenshaw, J.T.; Winslow, E.H. Preoperative fasting: Old habits die hard. Am. J. Nurs. 2002, 102, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.A.; Caplan, R.A.; Epstein, B.S.; Gibbs, C.P.; Keller, C.E.; Leak, J.A.; Maltby, R.; Nickinovich, D.G.; Schreiner, M.S.; Weinlander, C.M. Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures. Anesthesiology 2017, 126, 376–393. [Google Scholar] [CrossRef][Green Version]
- Engelhardt, T.; Webster, N.R. Pulmonary aspiration of gastric contents in anaesthesia. Br. J. Anaesth. 1999, 83, 453–460. [Google Scholar] [CrossRef]
- Candian Anaesthetist’s Society The shortened fluid fast and the Canadian Anaesthetists’ Society’s new guidelines for fasting in elective/ emergency patients. Candian J. Anaesth. 1990, 37, 905–906. [CrossRef]
- Brady, M.C.; Kinn, S.; Stuart, P.; Ness, V. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst. Rev. 2003, 1–126. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.; Kinn, S.; O’Rourke, K.; Randhawa, N.; Stuart, P. Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst. Rev. 2009, 7, 1–134. [Google Scholar] [CrossRef]
- Miller, M.; Wishart, H.Y.; Nimmo, W.S. Gastric contents at induction of anaesthesia: Is a 4-hour fast necessary? Br. J. Anaesth. 1983, 55, 1185–1188. [Google Scholar] [CrossRef]
- Lewis, M.; Crawford, J.S. Can one risk fasting the obstetric patient for less than 4 hours? Br. J. Anaesth. 1987, 59, 312–314. [Google Scholar] [CrossRef]
- Agarwal, A.; ChariI, P.; Singh, H. Fluid deprivation before operation: The effect of a small drink. Anaesthesia 1989, 44, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.; Lerman, J.; Christensen, S.; Farrow-Gillespie, A. Effects of duration of fasting on gastric fluid pH and volume in healthy children. Anesth. Analg. 1990, 71, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Powley, T.L. Gastric content volume inhibits rather than food intake nutrient. Am. J. Physiol. 1996, 271, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Soreide, E.; Hausken, T.; Soreide, J.A.; Steen, P.A. Gastric emptying of a light hospital breakfast: A study using real time ultrasonography. Acta Anaesthesiol. Scand. 1996, 40, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.F.; Lepage, Y.; Bonneville-Chouinard, N. Occurrence of gastroesophageal reflux on induction of anaesthesia does not correlate with the volume of gastric contents. Can. J. Anaesth. 1990, 37, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Mcgrady, E.M.; Macdonald, A.G. Effect of the preoperative administration of water on gastric volume and ph. Br. J. Anaesth. 1988, 60, 803–805. [Google Scholar] [CrossRef]
- Lotti, F.; Twedt, D.; Warrit, K.; Bryan, S.; Vaca, C.; Krause, L.; Fukushima, K.; Boscan, P. Effect of two different pre-anaesthetic omeprazole protocols on gastroesophageal reflux incidence and pH in dogs. J. Small Anim. Pract. 2021, 62, 677–682. [Google Scholar] [CrossRef]
- Zacuto, A.C.; Marks, S.L.; Osborn, J.; Douthitt, K.L.; Hollingshead, K.L.; Hayashi, K.; Kapatkin, A.S.; Pypendop, B.H.; Belafsky, P.C. The Influence of Esomeprazole and Cisapride on Gastroesophageal Reflux During Anesthesia in Dogs. J. Vet. Intern. Med. 2012, 26, 518–525. [Google Scholar] [CrossRef]
- Johnson, R.A. Maropitant prevented vomiting but not gastroesophageal reflux in anesthetized dogs premedicated with acepromazine-hydromorphone. Vet. Anaesth. Analg. 2014, 41, 406–410. [Google Scholar] [CrossRef]
- Raptopoulos, D.; Galatos, A.D. Gastro-oesophageal reflux during anaesthesia induced with either thiopentone or propofol in the dog. Vet. Anaesth. Analg. 1997, 24, 20–22. [Google Scholar] [CrossRef]
- Wilson, D.V.; Evans, A.T.; Mauer, W.A. Influence of metoclopramide on gastroesophageal reflux in anesthetized dogs. Am. J. Vet. Res. 2006, 67, 26–31. [Google Scholar] [CrossRef]
- Garcia, R.S.; Belafsky, P.C.; Della Maggiore, A.; Osborn, J.M.; Pypendop, B.H.; Pierce, T.; Walker, V.J.; Fulton, A.; Marks, S.L. Prevalence of Gastroesophageal Reflux in Cats during Anesthesia and Effect of Omeprazole on Gastric pH. J. Vet. Intern. Med. 2017, 31, 734–742. [Google Scholar] [CrossRef]
- de Vries, R.B.M.; Hooijmans, C.R.; Langendam, M.W.; van Luijk, J.; Leenaars, M.; Ritskes-Hoitinga, M.; Wever, K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence-based Preclin. Med. 2015, 2, e00007. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Tsompanidou, P.; Robben, J.H.; Savvas, I.; Anagnostou, T.; Prassinos, N.N.; Kazakos, G.M. The Effect of the Preoperative Fasting Regimen on the Incidence of Gastro-Oesophageal Reflux in 90 Dogs. Animals 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.A.; Waterman, A.E.; Pearson, H. A comparison of the effects of halothane and isoflurane in combination with nitrous oxide on lower oesophageal sphincter pressure and barrier pressure in anaesthetised dogs. Vet. Rec. 1995, 137, 658–661. [Google Scholar] [CrossRef]
- Hartmann, H.F.; Feranti, J.P.S.; Oliveira, M.T.; Linhares, M.T.; Correa, L.F.D.; Coradini, G.P.; Abati, S.L.; Brun, M.V. Gastroesophageal reflux in dogs undergoing convencional or video-assisted ovariohysterectomy. Arq. Bras. Med. Vet. e Zootec. 2018, 70, 101–108. [Google Scholar] [CrossRef]
- Flouraki, E.; Kazakos, G.; Savvas, I.; Pardali, D.; Adamama-Moraitou, K. Rhinitis following intraoperative gastro-oesophageal reflux in a dog. Vet. Rec. Case Rep. 2019, 7, e000792. [Google Scholar] [CrossRef]
- Favarato, E.S.; de Souza, M.V.; dos Santos Costa, P.R. Gastroesophageal reflux in anesthetized dogs: Physiopathology, clinic, diagnosis and therapeutics. Cienc. Rural 2010, 40, 2427–2434. [Google Scholar] [CrossRef]
- Rodríguez-Alarcón, C.A.; Beristain-Ruiz, D.M.; Rivera-Barreno, R.; Díaz, G.; Usón-Casaús, J.M.; García-Herrera, R.; Pérez-Merino, E.M. Gastroesophageal reflux in anesthetized dogs: A review. Rev. Colomb. Ciencias Pecu. 2015, 28, 144–155. [Google Scholar] [CrossRef]
- Sideri, A.I.; Galatos, A.D.; Kazakos, G.M.; Gouletsou, P.G. Gastro-oesophageal reflux during anaesthesia in the kitten: Comparison between use of a laryngeal mask airway or an endotracheal tube. Vet. Anaesth. Analg. 2009, 36, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A.; Fransson, B.A.; Davis, A.M.; Gilbertsen, A.M.; Gay, J.M. Incidence of and risk factors for postoperative regurgitation and vomiting in dogs: 244 cases (2000–2012). J. Am. Vet. Med. Assoc. 2015, 246, 327–335. [Google Scholar] [CrossRef]
- Costa, R.S.; Abelson, A.L.; Lindsey, J.C.; Wetmore, L.A. Postoperative regurgitation and respiratory complications in brachycephalic dogs undergoing airway surgery before and after implementation of a standardized perianesthetic protocol. J. Am. Vet. Med. Assoc. 2020, 256, 899–905. [Google Scholar] [CrossRef]
- Jones, C.T.; Fransson, B.A. Evaluation of the effectiveness of preoperative administration of maropitant citrate and metoclopramide hydrochloride in preventing postoperative clinical gastroesophageal reflux in dogs. J. Am. Vet. Med. Assoc. 2019, 255, 437–445. [Google Scholar] [CrossRef]
- Anagnostou, T.L.; Savvas, I.; Kazakos, G.M.; Ververidis, H.N.; Psalla, D.; Kostakis, C.; Skepastianos, P.; Raptopoulos, D. The effect of the stage of the ovarian cycle (anoestrus or dioestrus) and of pregnancy on the incidence of gastro-oesophageal reflux in dogs undergoing ovariohysterectomy. Vet. Anaesth. Analg. 2015, 42, 502–511. [Google Scholar] [CrossRef]
- Anagnostou, T.L.; Kazakos, G.M.; Savvas, I.; Kostakis, C.; Papadopoulou, P. Gastro-oesophageal reflux in large-sized, deep-chested versus small-sized, barrel-chested dogs undergoing spinal surgery in sternal recumbency. Vet. Anaesth. Analg. 2017, 44, 35–41. [Google Scholar] [CrossRef]
- Torrente, C.; Vigueras, I.; Manzanilla, E.G.; Villaverde, C.; Fresno, L.; Carvajal, B.; Fiñana, M.; Costa-Farré, C. Prevalence of and risk factors for intraoperative gastroesophageal reflux and postanesthetic vomiting and diarrhea in dogs undergoing general anesthesia. J. Vet. Emerg. Crit. Care 2017, 27, 397–408. [Google Scholar] [CrossRef]
- Lamata, C.; Loughton, V.; Jones, M.; Alibhai, H.; Armitage-Chan, E.; Walsh, K.; Brodbelt, D. The risk of passive regurgitation during general anaesthesia in a population of referred dogs in the UK. Vet. Anaesth. Analg. 2012, 39, 266–274. [Google Scholar] [CrossRef]
- Costa, R.S.; Wetmore, L.A.; Stein, A. Randomized, blinded, controlled clinical trial to assess gastroesophageal reflux and regurgitation in dogs undergoing general anesthesia after hydromorphone premedication with or without acepromazine or dexmedetomidine. Am. J. Vet. Res. 2021, 82, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, C.; Pietra, M.; Galiazzo, G.; Torresan, F.; Pinna, S.; Pisoni, L.; Romagnoli, N. Incidence of gastroesophageal reflux in dogs undergoing orthopaedic surgery or endoscopic evaluation of the upper gastrointestinal tract. Vet. Sci. 2020, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Shaver, S.L.; Barbur, L.A.; Jimenez, D.A.; Brainard, B.M.; Cornell, K.K.; Radlinsky, M.A.G.; Schmiedt, C.W. Evaluation of gastroesophageal reflux in anesthetized dogs with brachycephalic syndrome. J. Am. Anim. Hosp. Assoc. 2017, 53, 24–31. [Google Scholar] [CrossRef]
- Carvalho, P.J.P.C.; Miidla, I.; Donahue, P.E.; Vaz, O.; Sugitani, A.; Nyhus, L.M. A Noninvasive Model of Gastroesophageal Reflux in Dogs. Dig. Surg. 1992, 9, 89–94. [Google Scholar] [CrossRef]
- Pearson, H.; Darke, P.G.G.G.; Gibbs, C.; Kelly, D.F.; Orr, C.M. Reflux oesophagitis and stricture formation after anaesthesia: A review of seven cases in dogs and cats. J. Small Anim. Pract. 1978, 19, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Søreide, E.; Eriksson, L.I.; Hirlekar, G.; Eriksson, H.; Henneberg, S.W.; Sandin, R.; Raeder, J. Pre-operative fasting guidelines: An update. Acta Anaesthesiol. Scand. 2005, 49, 1041–1047. [Google Scholar] [CrossRef]
- Stuart, P.C. The evidence base behind modern fasting guidelines. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 457–469. [Google Scholar] [CrossRef]
- Fernandez Alasia, A.C.; Levionnois, O.; Raillard, M. A Systematic Review of the Methods of Assessment of Gastro-Oesophageal Reflux in Anaesthetized Dogs. Animals 2021, 11, 852. [Google Scholar] [CrossRef]



| Reference | Type of Study/Design | Animals (Dogs) | Control/ Intervention | Type of Drugs Used | Procedure |
|---|---|---|---|---|---|
| Galatos et al., 1995 [10] | prospective cohort clinical study | 240 (125 females, 115 males), age 6 months–9 years, weight 2.5 to 46 kg | 2–4 h/12–18 h | propionylpromazine, atropine, xylazine, pethidine, diazepam, thiopentone, halothane | nonabdominal, nonthoracic |
| Savvas et al., 2016 [15] | randomized clinical trial | 120, age 1–8 years, weight 4.6–42 kg | 3 h/10 h | propionyl promazine, thiopentone, halothane | nonabdominal, nonthoracic, no head tilt |
| Viskjer 2017 et al. [14] | prospective, randomized, controlled clinical trial | 82, age 48.39 ± 35.31 months, weight 27.37 ± 13.14 kg | 3 h/18 h | acepromazine, methadone, morphine, propofol, isoflurane, morphine epidurally in some dogs | orthopaedic surgery |
| Tsompanidou et al., 2022 [47] | prospective, randomized, controlled clinical trial | 90 (37 females, 53 males), age 1–10 years, weight 5–39 kg | 3 h/12 h | acepromazine, pethidine, propofol, isoflurane | nonabdominal, nonthoracic |
| Reference | Type of Study/Design | Animals (Dogs) | Control/Intervention | Fasting Duration | Procedure |
|---|---|---|---|---|---|
| Raptopoulos et al., 1997 [42] | randomized clinical trial | 68 (35 females, 33 males), age 6 months–9 years, weight 2.5–50 kg | thiopentone/propofol (maintenance with halothane) | 12–18 h | soft tissue, orthopaedic, imaging |
| Reference | Type of Study/Design | Animals (Dogs) | Control/Intervention | Fasting Duration | Procedure |
|---|---|---|---|---|---|
| Wilson et al., 2006 [22] | randomized, prospective clinical trial | 90 dogs, various ages and weights | halothane/isoflurane or sevoflurane | overnight | orthopaedic surgery |
| Reference | Type of Study/Design | Animals (Dogs) | Control/Intervention | Fasting Duration | Procedure |
|---|---|---|---|---|---|
| Wilson et al., 2005 [6] | randomized, prospective clinical trial | 90, age 4.8 ± 2.4 years, weight 32 ± 2.7 kg | no morphine/morphine at 0.22 mg/kg or 1.10 mg/kg | overnight | orthopaedic surgery |
| Reference | Type of Study/Design | Animals (Dogs) | Control/Intervention | Fasting Duration | Procedure |
|---|---|---|---|---|---|
| Panti et al., 2009 [12] | randomized, blinded, controlled clinical trial | 47, weight 32.7 ± 14.3 kg | no omeprazole/omeprazole 1 mg/kg | >12 h | pelvic limb surgery |
| Zacuto et al., 2012 [40] | prospective, randomized, blinded, placebo-controlled study | 61, age 4.9 ± 3.4 years, weight 25.0 ± 12.9 kg | saline/esomeprazole 1 mg/kg or esomeprazole 1 mg/kg and cisapride 1 mg/kg | >12 h | orthopaedic surgery |
| Lotti et al., 2021 [39] | prospective, randomised, blinded, clinical trial | 55, age 5–60 months, weight 21 ± 6.9 kg | no omeprazole/omeprazole 1 mg/kg or omeprazole two doses of 1 mg/kg each | >12 h | ovariectomy |
| Reference | Type of Study/Design | Animals (Dogs) | Control/Intervention | Fasting Duration | Procedure |
|---|---|---|---|---|---|
| Wilson et al., 2006 [43] | prospective, randomised, clinical trial | 52, ages and weights not mentioned | saline/metoclopramide 0.4 mg/kg or 1.0 mg/kg | overnight | orthopaedic surgery |
| Favarato et al., 2012 [13] | randomized, controlled clinical study | 90 (female), age 0.5–9 years, weight 1.5–34 kg | no metoclopramide/metoclopramide 1 mg/kg or ranitidine 2 mg/kg | 12 h | ovariohysterectomy |
| Reference | Type of Study/Design | Animals (Dogs) | Control/Intervention | Fasting Duration | Procedure |
|---|---|---|---|---|---|
| Johnson et al., 2014 [41] | randomized, blinded, prospective clinical study | 26 (18 females, 8 males), age 3.1 ± 3.1 years, weight 20.5 ± 11.4 kg | saline/maropitant 1.0 mg/kg | >12 h | soft tissue and orthopaedic surgery |
| Reference | Type of Study/Design | Animals (Cats) | Control/Intervention | Fasting Duration | Procedure |
|---|---|---|---|---|---|
| Galatos et al., 2001 [20] | randomized, clinical trial | 50 (2 females, 48 males), age 6 months–8 years, weight 2.9–6.3 kg | thiopentone/propofol | overnight | castration, skin tumour excision, orthopaedic surgery |
| Garcia et al., 2017 [44] | prospective, blinded, placebo-controlled, randomized clinical trial | 27, age 8.6 ± 3.8 years, weight 5.5 ± 0.8 kg | placebo/two different doses of omeprazole | 12 h | dental procedures |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvas, I.; Pavlidou, K.; Anagnostou, T.; Flouraki, E.; Kazakos, G.; Raptopoulos, D. Factors Affecting Intraoperative Gastro-Oesophageal Reflux in Dogs and Cats. Animals 2022, 12, 247. https://doi.org/10.3390/ani12030247
Savvas I, Pavlidou K, Anagnostou T, Flouraki E, Kazakos G, Raptopoulos D. Factors Affecting Intraoperative Gastro-Oesophageal Reflux in Dogs and Cats. Animals. 2022; 12(3):247. https://doi.org/10.3390/ani12030247
Chicago/Turabian StyleSavvas, Ioannis, Kiriaki Pavlidou, Tilemachos Anagnostou, Eugenia Flouraki, George Kazakos, and Dimitrios Raptopoulos. 2022. "Factors Affecting Intraoperative Gastro-Oesophageal Reflux in Dogs and Cats" Animals 12, no. 3: 247. https://doi.org/10.3390/ani12030247
APA StyleSavvas, I., Pavlidou, K., Anagnostou, T., Flouraki, E., Kazakos, G., & Raptopoulos, D. (2022). Factors Affecting Intraoperative Gastro-Oesophageal Reflux in Dogs and Cats. Animals, 12(3), 247. https://doi.org/10.3390/ani12030247