Effects of Dietary Supplementation with Red Yeast (Sporidiobolus pararoseus) on Productive Performance, Egg Quality, and Duodenal Cell Proliferation of Laying Hens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experiment Design
2.2. Productive Performance and Egg Quality Characteristics
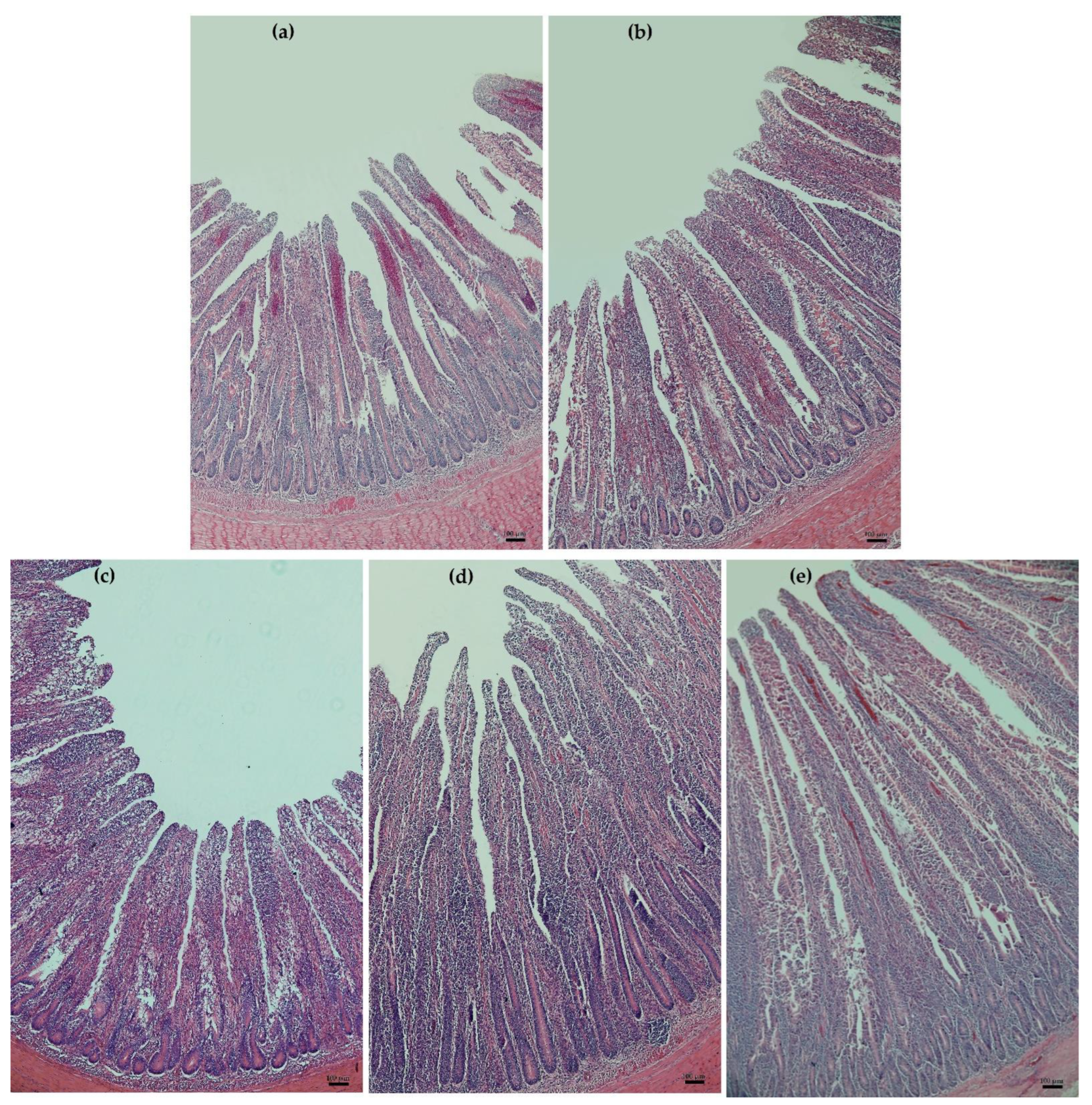
2.3. Histology of Duodenum
2.4. Duodenal Immunohistochemistry and Mitotic Index
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Egg Quality
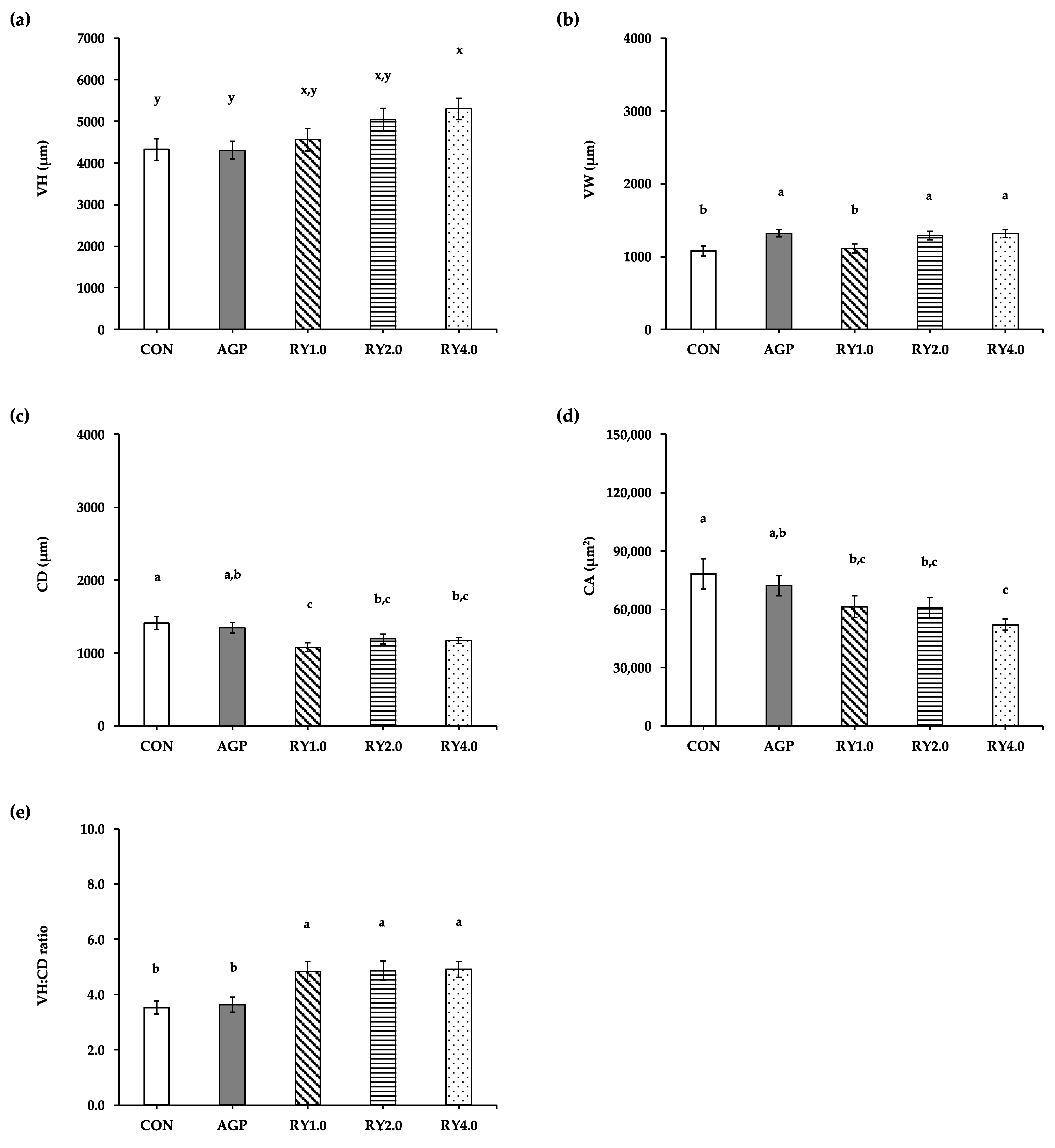
3.3. Duodenal Histology
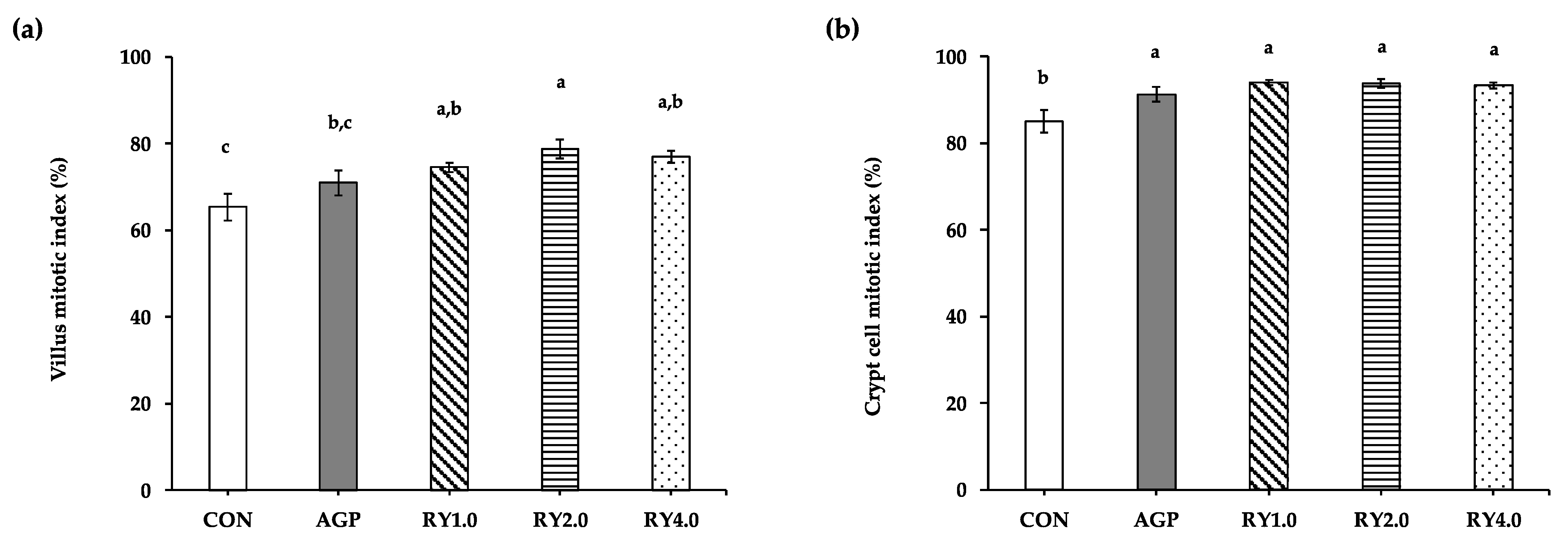
3.4. Duodenal Proliferations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- dos Santos, A.F.A.; Da Silva, A.S.; Galli, G.M.; Paglia, E.B.; Dacoreggio, M.V.; Kempka, A.P.; Souza, C.F.; Baldissera, M.D.; da Rosa, G.; Boiago, M.M.; et al. Addition of Yellow Strawberry Guava Leaf Extract in the Diet of Laying Hens Had Antimicrobial and Antioxidant Effect Capable of Improving Egg Quality. Biocatal. Agric. Biotechnol. 2020, 29, 101788. [Google Scholar] [CrossRef]
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing Persistency in Lay and Stabilising Egg Quality in Longer Laying Cycles. What Are the Challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Muaz, K.; Riaz, M.; Akhtar, S.; Park, S.; Ismail, A. Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J. Food Prot. 2018, 81, 619–627. [Google Scholar] [CrossRef]
- Samreen, A.I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, S.; Adewole, D.I. In Ovo Delivery of Bioactive Substances: An Alternative to the Use of Antibiotic Growth Promoters in Poultry Production—A Review. J. Appl. Poult. Res. 2020, 29, 744–763. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, H.; Guo, Y.; Wu, H.; Zhang, N. EEffects of Inulin Supplementation in Laying Hens Diet on the Antioxidant Capacity of Refrigerated Stored Eggs. Int. J. Biol. Macromol. 2020, 153, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Tapingkae, W.; Panyachai, K.; Yachai, M.; Doan, H.V. Effects of Dietary Red Yeast (Sporidiobolus pararoseus) on Production Performance and Egg quality of Laying Hens. J. Anim. Physiol. Anim. Nutr. 2018, 102, e337–e344. [Google Scholar] [CrossRef]
- Koiyama, N.T.G.; Utimi, N.B.P.; Santos, B.R.L.; Bonato, M.A.; Barbalho, R.; Gameiro, A.H.; Araújo, C.S.S.; Araújo, L.F. Effect of Yeast Cell Wall Supplementation in Laying Hen Feed on Economic Viability, Egg Production, and Egg Quality. J. Appl. Poult. Res. 2018, 27, 116–123. [Google Scholar] [CrossRef]
- Sun, P.; Lu, Y.; Cheng, H.; Song, D. The Effect of Grape Seed Extract and Yeast Culture on Both Cholesterol Content of Egg Yolk and Performance of Laying Hens. J. Appl. Poult. Res. 2018, 27, 564–569. [Google Scholar] [CrossRef]
- Chaiyaso, T.; Manowattana, A. Enhancement of Carotenoids and Lipids Production by Oleaginous Red Yeast Sporidiobolus pararoseus KM281507. Prep. Biochem. Biotechnol. 2018, 48, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Grashorn, M. Feed Additives for Influencing Chicken Meat and Egg Yolk Color. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R., Eds.; Woodhead Publishing Elsevier Ltd.: Oxford, UK, 2016; pp. 283–302. [Google Scholar]
- Tapingkae, W.; Yindee, P.; Moonmanee, T. Effect of Dietary Red Yeast (Sporidiobolus pararoseus) Supplementation on Small Intestinal Histomorphometry of Laying Hens. J. Anim. Plant Sci. 2016, 26, 909–915. [Google Scholar]
- Izadi, H.; Arshami, J.; Golian, A.; Reza Raji, M. Effects of chicory root powder on growth performance and histomorphometry of jejunum in broiler chicks. Vet. Res. Forum 2013, 4, 169–174. [Google Scholar]
- Prakatur, I.; Miskulin, M.; Pavic, M.; Marjanovic, K.; Blazicevic, V.; Miskulin, I.; Domacinovic, M. Intestinal Morphology in Broiler Chickens Supplemented with Propolis and Bee Pollen. Animals 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.A.; Pessotti, B.M.S.; Zanini, S.F.; Colnago, G.L.; Rodrigues, M.R.A.; Nunes, L.C.; Zanini, M.S.; Martins, I.V.F. Intestinal Mucosa Structure of Broiler Chickens Infected Experimentally with Eimeria tenella and Treated with Essential Oil of Oregano. Cienc. Rural 2009, 39, 1471–1477. [Google Scholar] [CrossRef] [Green Version]
- Samanya, M.; Yamauchi, K. Histological Alterations of Intestinal Villi in Chickens Fed Dried Bacillus subtilis var. natto. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 95–104. [Google Scholar] [CrossRef]
- Onderci, M.; Sahin, N.; Sahin, K.; Cikim, G.; Aydin, A.; Ozercan, I. Efficacy of Supplementation of Alpha-Amylase-Producing Bacterial Culture on the Performance, Nutrient Use and Gut Morphology of Broiler Chickens Fed a Corn-Based Diet. Poult. Sci. 2006, 85, 505–510. [Google Scholar] [CrossRef]
- Yamauchi, K.; Tarachai, P. Changes in Intestinal Villi, Cell Area and Intracellular Autophagic Vacuoles Related to Intestinal Function in Chickens. Br. Poult. Sci. 2000, 41, 416–423. [Google Scholar] [CrossRef]
- Torres, K.A.; Pizauro, J.M.; Soares, C.P.; Silva, T.G.; Nogueira, W.C.; Campos, D.M.; Furlan, R.L.; Macari, M. Effects of Corn Replacement by Sorghum in Broiler Diets on Performance and Intestinal Mucosa Integrity. Poult. Sci. 2013, 92, 1564–1571. [Google Scholar] [CrossRef]
- Manowattana, A.; Techapun, C.; Watanabe, M.; Chaiyaso, T. Bioconversion of Biodiesel-Derived Crude Glycerol into Lipids and Carotenoids by an Oleaginous Red Yeast Sporidiobolus pararoseus KM281507 in an Airlift Bioreactor. J. Biosci. Bioeng. 2018, 125, 59–66. [Google Scholar] [CrossRef]
- Elkin, R.G.; Yan, Z.; Zhong, Y.; Donkin, S.S.; Buhman, K.K.; Story, J.A.; Turek, J.J.; Porter, R.E., Jr.; Anderson, M.; Homan, R.; et al. Select 3-Hydroxy-3-Methylglutaryl-Coenzyme a Reductase Inhibitors Vary in Their Ability to Reduce Egg Yolk Cholesterol Levels in Laying Hens Through Alteration of Hepatic Cholesterol Biosynthesis and Plasma VLDL Composition. J. Nutr. 1999, 129, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- Awad, W.A.; Ghareeb, K.; Böhm, J. Evaluation of the Chicory Inulin Efficacy on Ameliorating the Intestinal Morphology and Modulating the Intestinal Electrophysiological Properties in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2011, 95, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Abdelqader, A.; Al-Fataftah, A.-R.; Daş, G. Effects of Dietary Bacillus subtilis and Inulin Supplementation on Performance, Eggshell Quality, Intestinal Morphology and Microflora Composition of Laying Hens in the Late Phase of Production. Anim. Feed Sci. Technol. 2013, 179, 103–111. [Google Scholar] [CrossRef]
- Marchini, C.; Silva, P.; Nascimento, M.; Beletti, M.; Silva, N.; Guimarães, E. Body Weight, Intestinal Morphometry and Cell Proliferation of Broiler Chickens Submitted to Cyclic Heat Stress. Int. J. Poult. Sci. 2011, 10, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shih, W.; Chen, S.; Wang, S. Effect of Yeast with Bacteriocin from Rumen Bacteria on Laying Performance, Blood Biochemistry, Faecal Microbiota and Egg Quality of Laying Hens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1105–1115. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacakli, P.; Ergun, A.; Koksal, B.H.; Ozsoy, B.; Cantekin, Z. Effects of Inactivated Brewer’s Yeast (Saccharomyces cereviciae) on Egg Production, Serum Antibody Titres and Cholesterol Levels in Laying Hens. Vet. Med. Zootech. 2013, 61, 53–60. [Google Scholar]
- Zhen, W.; Shao, Y.; Wu, Y.; Li, L.; Pham, V.H.; Abbas, W.; Wan, Z.; Guo, Y.; Wang, Z. Dietary Yeast β-glucan Supplementation Improves Eggshell Color and Fertile Eggs Hatchability as well as Enhances Immune Functions in Breeder Laying Hens. Int. J. Biol. Macromol. 2020, 159, 607–621. [Google Scholar] [CrossRef]
- Li, D.; Ding, X.; Zhang, K.; Bai, S.; Wang, J.; Zeng, Q.; Su, Z.; Kang, L. Effects of Dietary Xylooligosaccharides on the Performance, Egg Quality, Nutrient Digestibility and Plasma Parameters of Laying Hens. Anim. Feed Sci. Technol. 2017, 225, 20–26. [Google Scholar] [CrossRef]
- Yalçin, S.; Yalcin, S.; Şahin, A.; Duyum, H.; Çalik, A.; Gümüş, H. Effects of Dietary Inactive Yeast and Live Yeast on Performance, Egg Quality Traits, Some Blood Parameters and Antibody Production to SRBC of Laying Hens. Kafkas Uni. Vet. Fak. Derg. 2015, 21, 345–350. [Google Scholar]
- Ghasemian, M.; Jahanian, R. Dietary Mannan-Oligosaccharides Supplementation Could Affect Performance, Immunocompetence, Serum Lipid Metabolites, Intestinal Bacterial Populations, and Ileal Nutrient Digestibility in Aged Laying Hens. Anim. Feed Sci. Technol. 2016, 213, 81–89. [Google Scholar] [CrossRef]
- Gong, H.Z.; Wu, M.; Lang, W.Y.; Yang, M.; Wang, J.H.; Wang, Y.Q.; Zhang, Y.; Zheng, X. Effects of Laying Breeder Hens Dietary β-Carotene, Curcumin, Allicin, and Sodium Butyrate Supplementation on the Growth Performance, Immunity, and Jejunum Morphology of Their Offspring Chicks. Poult. Sci. 2020, 99, 151–162. [Google Scholar] [CrossRef]
- Alabi, O.J.; Shiwoya, E.; Ayanwale, B.; Mbajiorgu, C.A.; Ng’ambi, J.; Egena, S. Effects of Dried Baker’s Yeast Inclusion in Rice Husk-Based Diets on Performance and Egg Quality Parameters in Laying Hens. Indian J. Anim. Res. 2012, 46, 56–60. [Google Scholar]
- Tang, S.G.H.; Sieo, C.C.; Ramasamy, K.; Saad, W.Z.; Wong, H.K.; Ho, Y.W. Performance, Biochemical and Haematological Responses, and Relative Organ Weights of Laying Hens Fed Diets Supplemented with Prebiotic, Probiotic and Synbiotic. BMC Vet. Res. 2017, 13, 248. [Google Scholar] [CrossRef] [Green Version]
- Yalçin, S.; Onbasilar, I.; Eser, H.; Şahin, A. Effects of Dietary Yeast Cell Wall on Performance, Egg Quality and Humoral Immune Response in Laying Hens. Ankara Univ. Vet. Fak. Derg. 2014, 61, 289–294. [Google Scholar]
- Patterson, J.A.; Burkholder, K.M. Application of Prebiotics and Probiotics in Poultry Production. Poult. Sci. 2003, 82, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Şekeroğlu, A.; Yildirim, A.; Eleroğlu, H.; Camci, Ö. Relation Between Egg Shape Index and Egg Quality Characteristics. Eur. Poult. Sci. 2016, 80, 1–9. [Google Scholar]
- Prytkov, Y.N.; Kistina, A.A.; Chervyakov, M.Y. Influence of Different Dosages of Selenium Yeast in the Diets of Laying Hens Cross Lohmann Brown on Metabolic Indices and Egg Productivity. Biosci. Biotechnol. Res. Asia 2016, 13, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Lokapirnasari, W.P.; Al Arif, A.; Soeharsono, S.; Fathinah, A.; Najwan, R.; Wardhani, H.C.P.; Noorrahman, N.F.; Huda, K.; Ulfah, N.; Yulianto, A.B. Improves in External and Internal Egg Quality of Japanese Quail (Coturnix coturnix japonica) by Giving Lactic Acid Bacteria as Alternative Antibiotic Growth Promoter. Iran. J. Microbiol. 2019, 11, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef]
- Habanabashaka, M.; Sengabo, M.; Oladunjoye, I.O. Effect of Tomato Waste Meal on Lay Performance, Egg Quality, Lipid Profile and Carotene Content of Eggs in Laying Hens. Iran. J. Appl. Anim. Sci. 2014, 4, 555–559. [Google Scholar]
- Muthusamy, N.; Haldar, S.; Ghosh, T.; Bedford, M. Effects of Hydrolysed Saccharomyces cerevisiae Yeast and Yeast Cell Wall Components on Live performance, Intestinal Histo-Morphology and Humoral Immune Response of Broilers. Br. Poult. Sci. 2011, 52, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Shamoto, K.; Yamauchi, K. Recovery Responses of Chick Intestinal Villus Morphology to Different Refeeding Procedures. Poult. Sci. 2000, 79, 718–723. [Google Scholar] [CrossRef]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of Villus Height and Crypt Depth, and Enhancement of Disaccharide Digestion and Monosaccharide Absorption, in Piglets Fed on Cows’ Whole Milk after Weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, W.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of Dietary Inclusion of Probiotic and Synbiotic on Growth Performance, Organ Weights, and Intestinal Histomorphology of Broiler Chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Gao, J.; Ma, J.; Li, T.; Tan, B.; Huang, X.; Jie, Y. Opportunities of Prebiotics for the Intestinal Health of Monogastric Animals. Anim. Nutr. 2020, 6, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Uscanga, B.; François, J.M. A Study of the Yeast Cell Wall Composition and Structure in Response to Growth Conditions and Mode of Cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Tian, X.; Shao, Y.; Wang, Z.; Guo, Y. Effects of Dietary Yeast β-Glucans Supplementation on Growth Performance, Gut Morphology, Intestinal Clostridium perfringens Population and Immune Response of Broiler Chickens Challenged with Necrotic Enteritis. Anim. Feed Sci. Technol. 2016, 215, 144–155. [Google Scholar] [CrossRef]
- De Los Santos, F.S.; Donoghue, A.; Farnell, M.; Huff, G.; Huff, W.; Donoghue, D. Gastrointestinal Maturation Is Accelerated in Turkey Poults Supplemented with a Mannan-Oligosaccharide Yeast Extract (Alphamune). Poult. Sci. 2007, 86, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, M.U.; El-Hack, M.E.A.; Hassan, F.; El-Saadony, M.T.; Khafaga, A.F.; Batiha, G.E.; Yehia, N.; Elnesr, S.S.; Alagawany, M.; El-Tarabily, K.A.; et al. The Potential Mechanistic Insights and Future Implications for the Effect of Prebiotics on Poultry Performance, Gut Microbiome, and Intestinal Morphology. Poult. Sci. 2021, 100, 101143. [Google Scholar] [CrossRef] [PubMed]
- Zdunczyk, Z.; Juskiewicz, J.; Jankowski, J.; Biedrzycka, E.; Koncicki, A. Metabolic Response of the Gastrointestinal Tract of Turkeys to Diets with Different Levels of Mannan-Oligosaccharide. Poult. Sci. 2005, 84, 903–909. [Google Scholar] [CrossRef]
- Matsuki, T.; Pédron, T.; Regnault, B.; Mulet, C.; Hara, T.; Sansonetti, P.J. Epithelial Cell Proliferation Arrest Induced by Lactate and Acetate from Lactobacillus casei and Bifidobacterium breve. PLoS ONE 2013, 8, e63053. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rubio, C.; Ordóñez, C.; Abad-González, J.; Garcia-Gallego, A.; Honrubia, M.P.; Mallo, J.J.; Balaña-Fouce, R. Butyric Acid-Based Feed Additives Help Protect Broiler Chickens from Salmonella Enteritidis Infection. Poult. Sci. 2009, 88, 943–948. [Google Scholar] [CrossRef]
- Penney, J.; Lu, Y.; Pan, B.; Feng, Y.; Walk, C.; Li, J. Pure Yeast Beta-Glucan and Two Types of Yeast Cell Wall Extracts Enhance Cell Migration in Porcine Intestine Model. J. Funct. Foods 2019, 59, 129–137. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Luise, D.; Le Floc’h, N.; Tesseraud, S.; Lambert, W.; Bosi, P.; Trevisi, P.; Beaumont, M.; Corrent, E. Functional Amino Acids in Pigs and Chickens: Implication for Gut Health. Front. Vet. Sci. 2021, 8, 663727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Li, J.; Xing, T.; Jiang, Y.; Zhang, L.; Gao, F. Dietary Corn Resistant Starch Regulates Intestinal Morphology and Barrier Functions by Activating the Notch Signaling Pathway of Broilers. Asian-Australas. J. Anim. Sci. 2020, 33, 2008–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Item | g/kg |
|---|---|
| Ingredients | |
| Rice bran | 60.0 |
| Corn meal | 534.0 |
| Soy bean meal 1 | 192.0 |
| Fish meal 2 | 74.0 |
| Leucaena leaf meal | 20.0 |
| Shell flour | 81.0 |
| Dicalcium phosphate | 6.0 |
| Sodium chloride | 5.0 |
| Vegetable oil | 25.0 |
| DL-methionine | 0.5 |
| Premix 3 | 2.5 |
| Nutrient value | |
| Calculated analysis | |
| Metabolizable energy (kcal/kg) | 2753.14 |
| Calcium | 38.7 |
| Phosphorus | 5.4 |
| Sodium | 3.7 |
| Choline | 4.6 |
| Lysine | 9.9 |
| Methionine | 3.7 |
| Methionine and cysteine | 6.5 |
| Tryptophan | 2.1 |
| Linoleic | 17.0 |
| Fat | 67.1 |
| Crude fiber | 38.1 |
| Proximate analysis | |
| Dry matter | 895.4 |
| Ash | 108.9 |
| Crude fiber | 41.1 |
| Ether extract | 40.1 |
| Crude protein | 208.3 |
| Item | CON | AGP 1 | RY1.0 | RY2.0 | RY4.0 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 1814.27 | 1811.56 | 1813.54 | 1813.23 | 1815.31 | 1.18 | 0.906 |
| Final body weight (g) | 1909.36 b | 1980.73 a | 1868.53 b | 1986.51 a | 1974.89 a | 9.85 | <0.001 |
| Feed intake (g/hen/day) | 110.27 | 107.47 | 109.69 | 110.95 | 110.73 | 0.66 | 0.475 |
| Egg weight (g) | 61.94 | 60.83 | 62.07 | 62.60 | 61.60 | 0.22 | 0.128 |
| Hen day production (%) | 88.75 | 90.79 | 88.09 | 90.07 | 89.75 | 0.45 | 0.354 |
| Egg mass | 54.86 | 55.14 | 54.61 | 56.39 | 55.24 | 0.31 | 0.436 |
| Feed conversion ratio | 2.02 | 1.96 | 2.02 | 1.98 | 2.01 | 0.01 | 0.479 |
| Item | CON | AGP 1 | RY1.0 | RY2.0 | RY4.0 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Egg shape index (%) | 77.38 b | 77.62 a,b | 77.56 b | 78.27 a | 77.20 b | 0.11 | 0.025 |
| Shell weight percentage (%) | 14.35 | 14.14 | 14.17 | 13.85 | 14.30 | 0.07 | 0.232 |
| Surface area (m2) | 71.21 | 70.96 | 71.39 | 72.34 | 71.39 | 0.17 | 0.121 |
| Egg shell index (%) | 12.10 | 11.86 | 11.94 | 11.71 | 11.99 | 0.06 | 0.276 |
| Egg shell strength (kgf) | 4.40 | 4.32 | 4.30 | 4.28 | 4.27 | 0.03 | 0.518 |
| Egg shell thickness (mm) | 0.47 | 0.47 | 0.48 | 0.47 | 0.48 | 0.00 | 0.202 |
| Yolk weight percentage (%) | 24.57 | 24.34 | 24.48 | 24.12 | 24.63 | 0.08 | 0.281 |
| Albumen weight percentage (%) | 61.09 | 61.05 | 61.35 | 62.03 | 61.07 | 0.13 | 0.167 |
| Haugh unit | 91.52 a | 87.90 b | 90.59 a | 91.82 a | 90.90 a | 0.32 | <0.001 |
| Yolk color | 8.99 b | 9.08 a,b | 9.18 a | 9.22 a | 9.13 a,b | 0.02 | 0.037 |
| Yolk cholesterol (mg/g) | 18.71 a | 18.14 a | 17.17 b | 15.76 c | 15.37 c | 0.25 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanmanee, C.; Srinual, O.; Punyatong, M.; Moonmanee, T.; Lumsangkul, C.; Tangtaweewipat, S.; Van Doan, H.; Yachai, M.; Chaiyaso, T.; Tapingkae, W. Effects of Dietary Supplementation with Red Yeast (Sporidiobolus pararoseus) on Productive Performance, Egg Quality, and Duodenal Cell Proliferation of Laying Hens. Animals 2022, 12, 238. https://doi.org/10.3390/ani12030238
Kanmanee C, Srinual O, Punyatong M, Moonmanee T, Lumsangkul C, Tangtaweewipat S, Van Doan H, Yachai M, Chaiyaso T, Tapingkae W. Effects of Dietary Supplementation with Red Yeast (Sporidiobolus pararoseus) on Productive Performance, Egg Quality, and Duodenal Cell Proliferation of Laying Hens. Animals. 2022; 12(3):238. https://doi.org/10.3390/ani12030238
Chicago/Turabian StyleKanmanee, Chanidapha, Orranee Srinual, Montri Punyatong, Tossapol Moonmanee, Chompunut Lumsangkul, Suchon Tangtaweewipat, Hien Van Doan, Mongkol Yachai, Thanongsak Chaiyaso, and Wanaporn Tapingkae. 2022. "Effects of Dietary Supplementation with Red Yeast (Sporidiobolus pararoseus) on Productive Performance, Egg Quality, and Duodenal Cell Proliferation of Laying Hens" Animals 12, no. 3: 238. https://doi.org/10.3390/ani12030238
APA StyleKanmanee, C., Srinual, O., Punyatong, M., Moonmanee, T., Lumsangkul, C., Tangtaweewipat, S., Van Doan, H., Yachai, M., Chaiyaso, T., & Tapingkae, W. (2022). Effects of Dietary Supplementation with Red Yeast (Sporidiobolus pararoseus) on Productive Performance, Egg Quality, and Duodenal Cell Proliferation of Laying Hens. Animals, 12(3), 238. https://doi.org/10.3390/ani12030238












