Modelling the Relative Abundance of Roe Deer (Capreolus capreolus L.) along a Climate and Land-Use Gradient
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
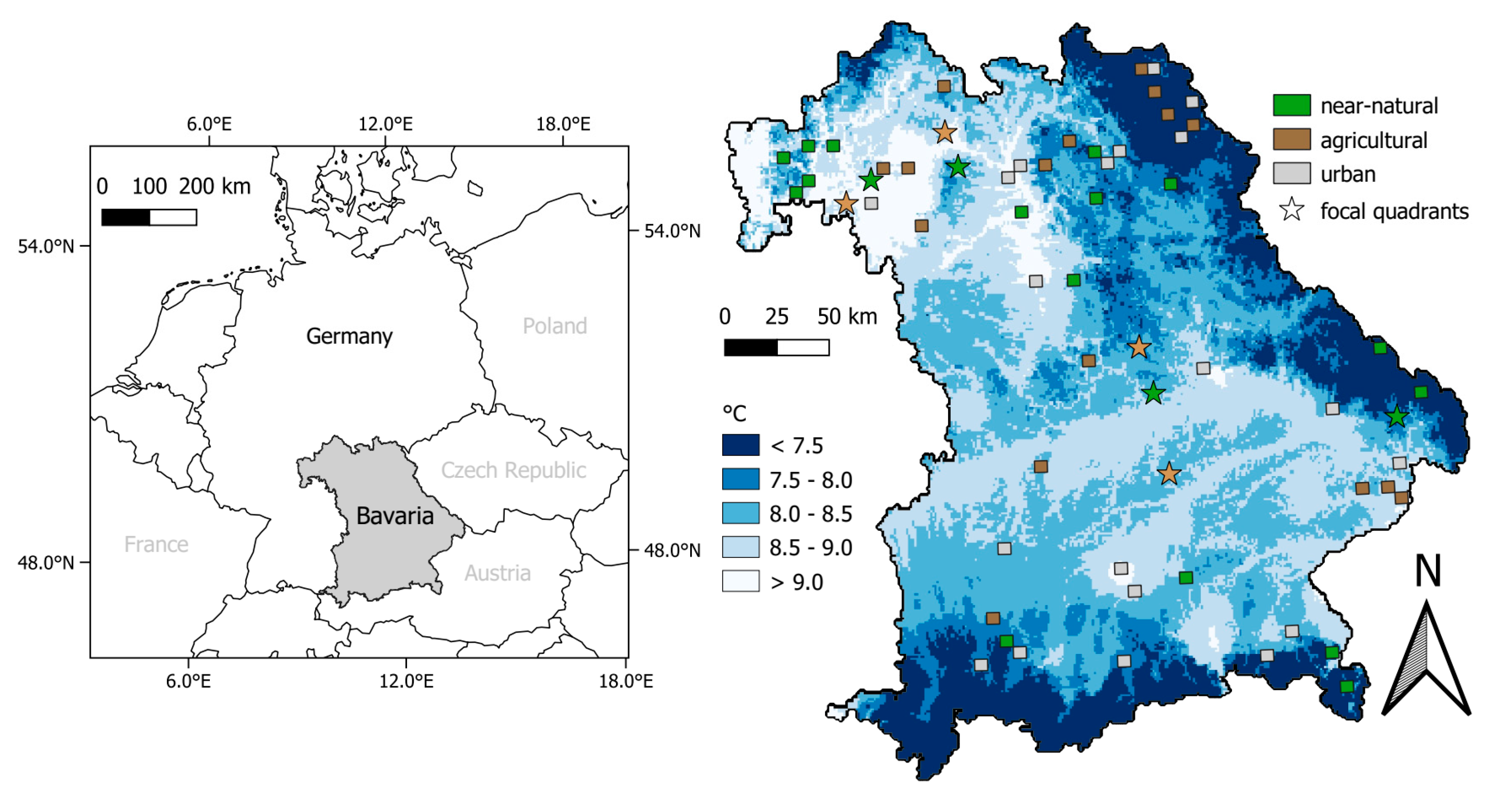
2.1. Survey Region
2.2. Field Survey
2.3. Model Development
2.4. Prediction and Uncertainty Analysis
2.5. Relative Abundance in a Climate and Land Use Gradient
3. Results
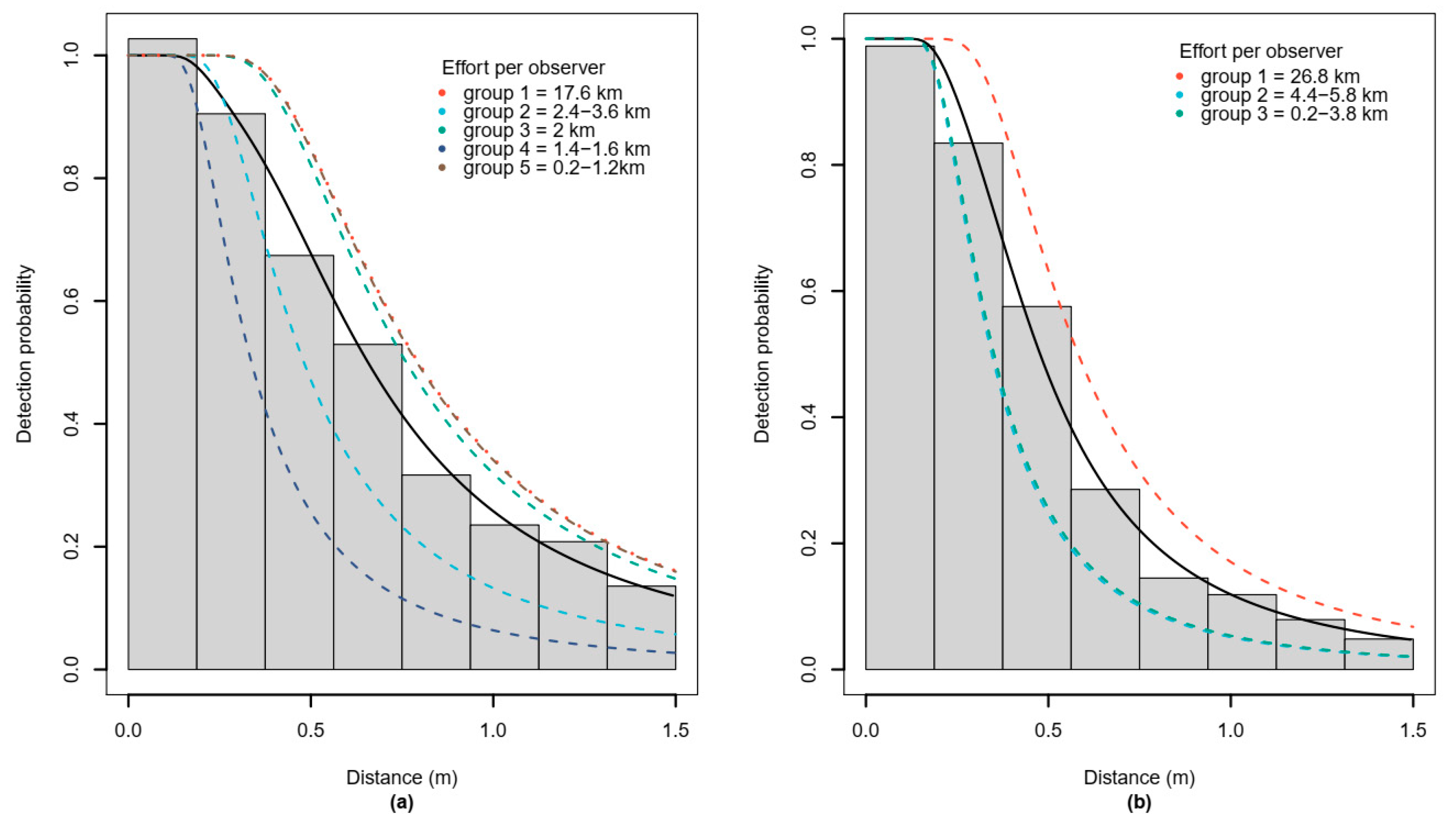
3.1. Detection Function
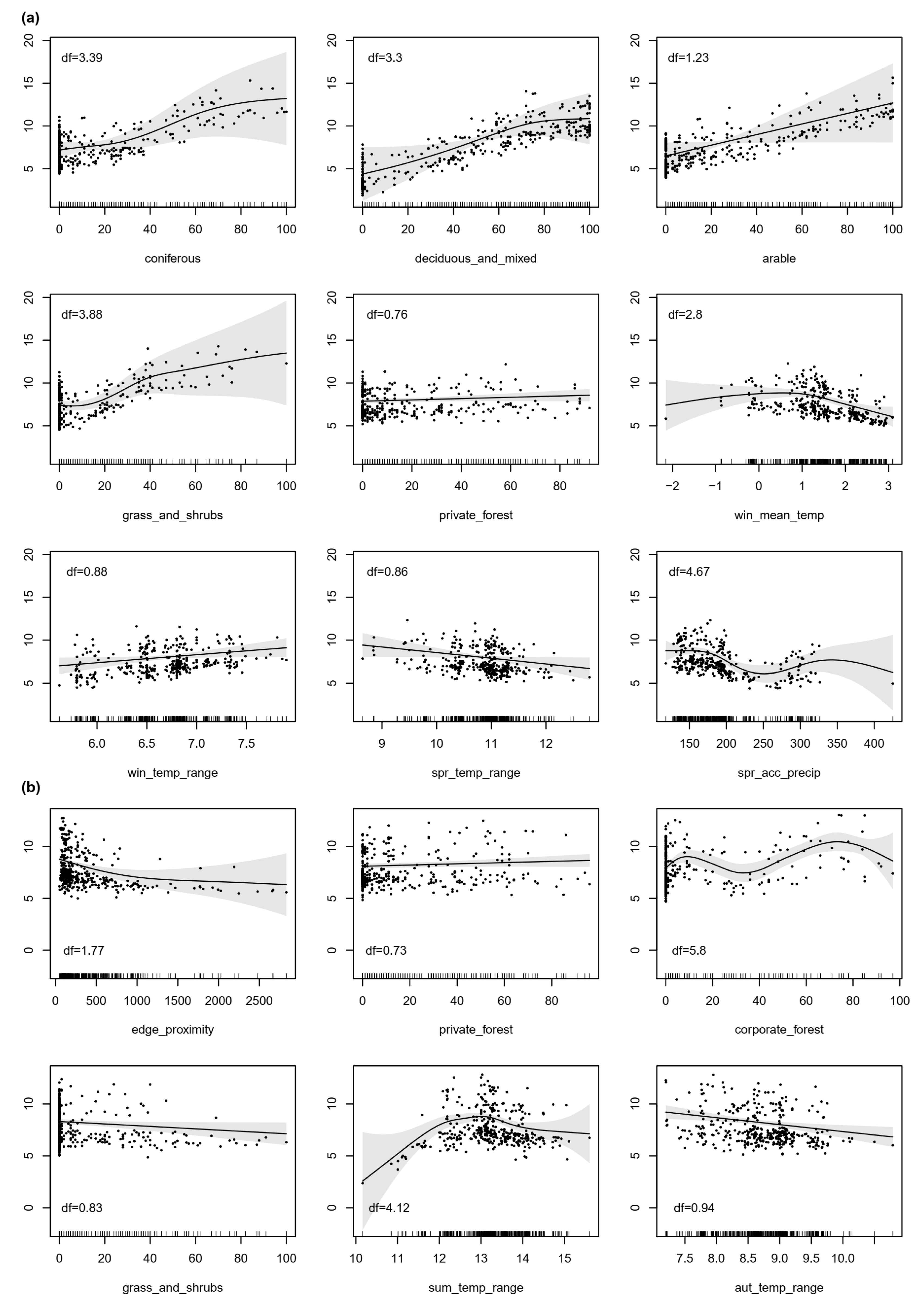
3.2. Density Surface Models
3.3. Prediction, Uncertainty and Validation
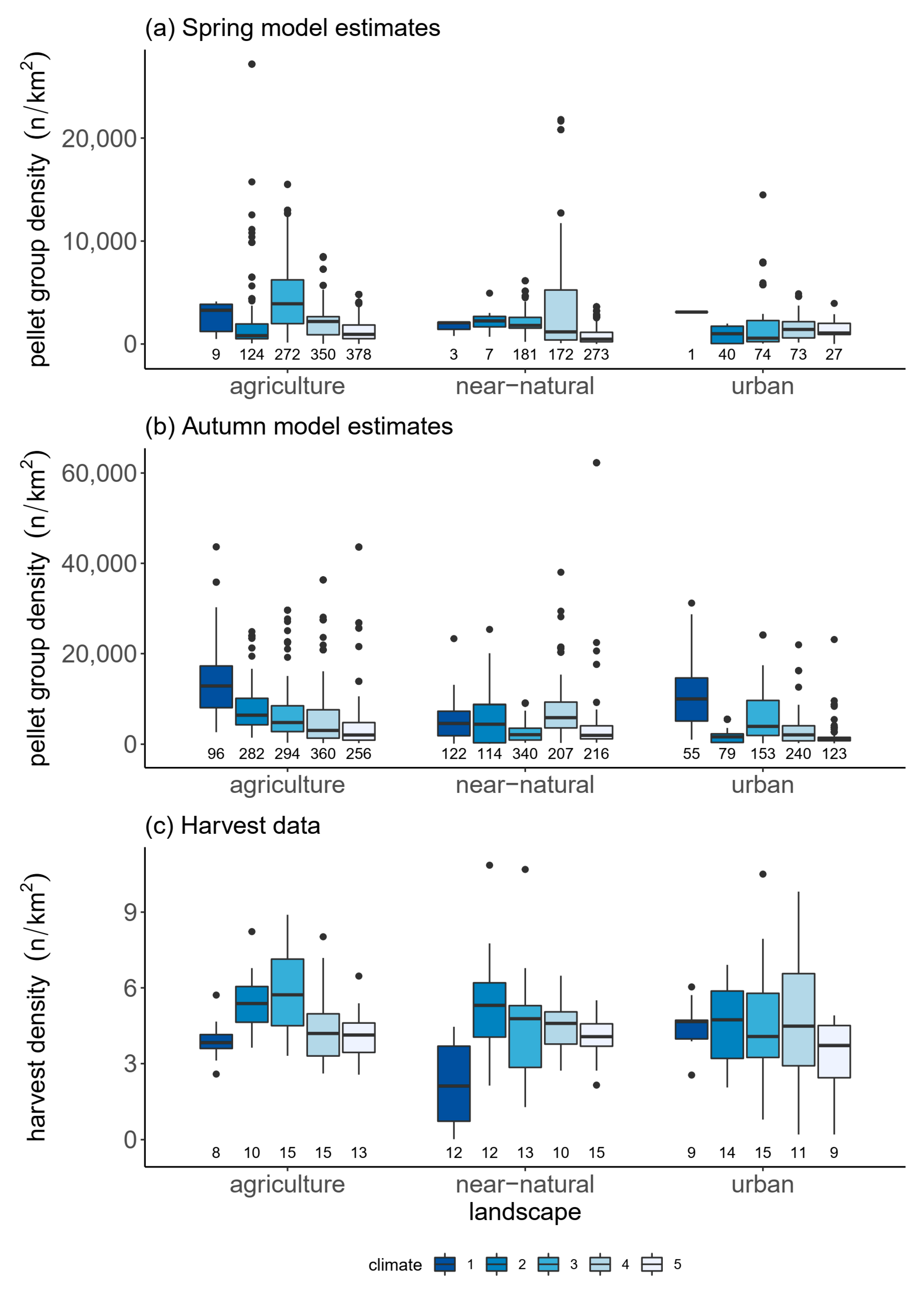
3.4. Relative Abundance in a Climate and Land-Use Gradient
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burbaiteė, L.; Csányi, S. Roe deer population and harvest changes in Europe. Est. J. Ecol. 2009, 58, 169–180. [Google Scholar] [CrossRef]
- Ferron, E.S.; Verheyden, H.; Hummel, J.; Cargnelutti, B.; Lourtet, B.; Merlet, J.; Gonzalez-Candela, M.; Angibault, J.M.; Hewison, A.J.M.; Clauss, M. Digestive plasticity as a response to woodland fragmentation in roe deer. Ecol. Res. 2012, 27, 77–82. [Google Scholar] [CrossRef]
- Hewison, A.J.M.; Vincent, J.P.; Joachim, J.; Angibault, J.M.; Cargnelutti, B.; Cibien, C. The effects of woodland fragmentation and human activity on roe deer distribution in agricultural landscapes. Can. J. Zool. 2001, 79, 679–689. [Google Scholar] [CrossRef]
- Cederlund, G.; Bergqvist, J.; Kjellander, P.; Gill, R.; Gaillard, J.M.; Boisaubert, B.; Ballon, P.; Duncan, P. Managing roe deer and their impact on the environment: Maximising the net benefits to society. In The European Roe Deer: The Biology of Success; Andersen, R., Duncan, P., Linnell, J.D.C., Eds.; Scandinavian University Press: Oslo, Norway, 1998; pp. 337–372. [Google Scholar]
- Bruinderink, G.G.; Hazebroek, E. Ungulate traffic collisions in Europe. Conserv. Biol. 1996, 10, 1059–1067. [Google Scholar] [CrossRef]
- Motta, R. Impact of wild ungulates on forest regeneration and tree composition of mountain forests in the western Italian alps. For. Ecol. Manag. 1996, 88, 93–98. [Google Scholar] [CrossRef]
- Putman, R.J. Foraging by roe deer in agricultural areas and impact on arable crops. J. Appl. Ecol. 1986, 23, 91–99. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Petersson, E.H.; Jaenson, D.G.E.; Kindberg, J.; Pettersson, J.H.-O.; Hjertqvist, M.; Medlock, J.M.; Bengtsson, H. The importance of wildlife in the ecology and epidemiology of the TBE virus in Sweden: Incidence of human TBE correlates with abundance of deer and hares. Parasites Vectors 2018, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.M.; Wierzbowska, I.A.; Gajda, A.; Czarnoleski, M.; Lesiak, M.; Widera, E. Human–wildlife conflicts in Krakow city, southern Poland. Animals 2020, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.E.; Runge, M.C.; Laskowski, H.P.; Kendall, W.L. Monitoring in the context of structured decision-making and adaptive management. J. Wildl. Manag. 2008, 72, 1683–1692. [Google Scholar] [CrossRef]
- Anderson, C.W.; Nielsen, C.K.; Hester, C.M.; Hubbard, R.D.; Stroud, J.K.; Schauber, E.M. Comparison of indirect and direct methods of distance sampling for estimating density of white-tailed deer. Wildl. Soc. Bull. 2013, 37, 146–154. [Google Scholar] [CrossRef]
- Amos, M.; Baxter, G.; Finch, N.; Lisle, A.; Murray, P. I just want to count them! Considerations when choosing a deer population monitoring method. Wildl. Biol. 2014, 20, 362–370. [Google Scholar] [CrossRef]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Focardi, S.; Isotti, R.; Tinelli, A. Line transect estimates of ungulate populations in a Mediterranean forest. J. Wildl. Manag. 2002, 66, 48–58. [Google Scholar] [CrossRef]
- Acevedo, P.; Ruiz-Fons, F.; Vicente, J.; Reyes-García, A.R.; Alzaga, V.; Gortázar, C. Estimating red deer abundance in a wide range of management situations in Mediterranean habitats. J. Zool. 2008, 276, 37–47. [Google Scholar] [CrossRef]
- Gill, R.M.A.; Thomas, M.L.; Stocker, D. The use of portable thermal imaging for estimating deer population density in forest habitats. J. Appl. Ecol. 1997, 34, 1273–1286. [Google Scholar] [CrossRef]
- Hemami, M.R.; Watkinson, A.R.; Gill, R.M.; Dolman, P.M. Estimating abundance of introduced Chinese muntjac Muntiacus reevesi and native roe deer Capreolus capreolus using portable thermal imaging equipment. Mammal Rev. 2007, 37, 246–254. [Google Scholar] [CrossRef]
- Morimando, F.; Focardi, S.; Andreev, R.; Capriotti, S.; Ahmed, A.; Lombardi, S.; Genov, P. A Method for evaluating density of roe deer, Capreolus capreolus (Linnaeus, 1758), in a forested area in Bulgaria based on camera trapping and independent photo screening. Acta Zool. Bulg. 2016, 68, 367–373. [Google Scholar]
- Romani, T.; Giannone, C.; Mori, E.; Filacorda, S. Use of track counts and camera traps to estimate the abundance of roe deer in north-eastern Italy: Are they effective methods? Mammal Res. 2018, 63, 477–484. [Google Scholar] [CrossRef]
- Acevedo, P.; Ferreres, J.; Jaroso, R.; Durán, M.; Escudero, M.A.; Marco, J.; Gortázar, C. Estimating roe deer abundance from pellet group counts in Spain: An assessment of methods suitable for Mediterranean woodlands. Ecol. Indic. 2010, 10, 1226–1230. [Google Scholar] [CrossRef]
- Alberto, M.; Francesca, S.; Paolo, L.; Nicola, G. A Review of the methods for monitoring roe deer European populations with particular reference to Italy. Hystrix Ital. J. Mammal. 2009, 19, 103–120. [Google Scholar]
- Melis, C.; Jedrzejewska, B.; Apollonio, M.; Bartoń, K.; Jedrzejewski, W.; Linnell, J.; Kojola, I.; Kusak, J.; Adamič, M.; Ciuti, S.; et al. Predation has a greater impact in less productive environments: Variation in roe deer, Capreolus capreolus, population density across Europe. Glob. Ecol. Biogeogr. 2009, 18, 724–734. [Google Scholar] [CrossRef]
- Morellet, N.; Gaillard, J.M.; Hewison, A.J.M.; Ballon, P.; Boscardin, Y.; Duncan, P.; Klein, F.; Maillard, D. Indicators of ecological change: New tools for managing populations of large herbivores. J. Appl. Ecol. 2007, 44, 634–643. [Google Scholar] [CrossRef]
- Borkowski, J.; Ukalska, J. Winter habitat use by red and roe deer in pine-dominated forest. For. Ecol. Manag. 2008, 255, 468–475. [Google Scholar] [CrossRef]
- Mysterud, A.; Østbye, E. Cover as a habitat element for temperate ungulates: Effects on habitat selection and demography. Wildl. Soc. Bull. 1999, 27, 385–394. [Google Scholar]
- Nugent, G.; McShea, W.J.; Parkes, J.; Woodley, S.; Waithaka, J.; Moro, J.; Gutierrez, R.; Azorit, C.; Guerrero, F.M.; Flueck, W.T. Policies and management of overabundant deer (native or exotic) in protected areas. Anim. Prod. Sci. 2011, 51, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Baur, S.; Peters, W.; Oettenheym, T.; Menzel, A. Weather conditions during hunting season affect the number of harvested roe deer (Capreolus capreolus). Ecol. Evol. 2021, 11, 10178–10191. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, J.M.; Hewison, A.J.M.; Klein, F.; Plard, F.; Douhard, M.; Davison, R.; Bonenfant, C. How does climate change influence demographic processes of widespread species? Lessons from the comparative analysis of contrasted populations of roe deer. Ecol. Lett. 2013, 16, 48–57. [Google Scholar] [CrossRef]
- Heurich, M.; Möst, L.; Schauberger, G.; Reulen, H.; Sustr, P.; Hothorn, T. Survival and causes of death of European roe deer before and after Eurasian lynx reintroduction in the Bavarian forest national park. Eur. J. Wildl. Res. 2012, 58, 567–578. [Google Scholar] [CrossRef]
- Toïgo, C.; Gaillard, J.M.; Van Laere, G.; Hewison, A.J.M.; Morellet, N. How does environmental variation influence body mass, body size, and body condition? Roe deer as a case study. Ecography 2006, 29, 301–308. [Google Scholar] [CrossRef]
- Miller, D.L.; Burt, M.L.; Rexstad, E.A.; Thomas, L. Spatial models for distance sampling data: Recent developments and future directions. Methods Ecol. Evol. 2013, 4, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Valente, A.M.; Marques, T.A.; Fonseca, C.; Torres, R.T. A new insight for monitoring ungulates: Density surface modelling of roe deer in a Mediterranean habitat. Eur. J. Wildl. Res. 2016, 62, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Camp, R.J.; Miller, D.L.; Thomas, L.; Buckland, S.T.; Kendall, S.J. Using density surface models to estimate spatio-temporal changes in population densities and trend. Ecography 2020, 43, 1079–1089. [Google Scholar] [CrossRef]
- Karamitros, G.; Gkafas, G.A.; Giantsis, I.A.; Martsikalis, P.; Kavouras, M.; Exadactylos, A. Model-based distribution and abundance of three Delphinidae in the Mediterranean. Animals 2020, 10, 260. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, K.; Kumar, U.; Banerjee, K.; Jhala, Y.V. Spatially explicit density and its determinants for Asiatic lions in the Gir forests. PLoS ONE 2020, 15, e0228374. [Google Scholar] [CrossRef] [Green Version]
- Bouchet, P.J.; Miller, D.L.; Roberts, J.J.; Mannocci, L.; Harris, C.M.; Thomas, L. dsmextra: Extrapolation assessment tools for density surface models. Methods Ecol. Evol. 2020, 11, 1464–1469. [Google Scholar] [CrossRef]
- Bayerisches Landesamt für Statistik. Statistics. Available online: https://www.statistik.bayern.de (accessed on 23 June 2020).
- European Environment Agency. CORINE Land Cover (CLC) 2012. 2018. Available online: https://land.copernicus.eu/pan-european/corine-land-cover/clc-2012 (accessed on 15 November 2018).
- German Meteorological Service DWD. Climate Data Center (CDC): Multi-Annual Means of 515 Grids of Precipitation and Air Temperature (2m) over Germany from 1981–2010, 516 Version v1.0. 2020. Available online: https://opendata.dwd.de/climate_environment/CDC/grids_germany/multi_annual/air_temperature_mean/ (accessed on 23 June 2020).
- Redlich, S.; Zhang, J.; Benjamin, C.; Dhillon, M.S.; Englmeier, J.; Ewald, J.; Fricke, U.; Ganuza, C.; Haensel, M.; Hovestadt, T.; et al. Disentangling effects of climate and land use on biodiversity and ecosystem services—A multi-scale experimental design. Methods Ecol. Evol. 2021, 1–14. [Google Scholar] [CrossRef]
- Buckland, S.T.; Rexstad, E.A.; Marques, T.A.; Oedekoven, C.S. Distance Sampling: Methods and Applications; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- European Environment Agency CORINE Land Cover (CLC) 2018. 2018. Available online: https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 (accessed on 28 January 2019).
- Hedley, S.L.; Buckland, S.T. Spatial models for line transect sampling. J. Agric. Biol. Environ. Stat. 2004, 9, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Miller, D.L.; Rexstad, E.; Thomas, L.; Marshall, L.; Laake, J.L. Distance sampling in R. J. Stat. Softw. 2019, 89, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Marques, T.A.; Thomas, L.; Fancy, S.G.; Buckland, S.T. Improving estimates of bird density using multiple-covariate distance sampling. Auk 2007, 124, 1229–1243. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.; Burnham, K.; Laake, J.; Borchers, D. Introduction to Distance Sampling; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Morellet, N.; Bonenfant, C.; Börger, L.; Ossi, F.; Cagnacci, F.; Heurich, M.; Kjellander, P.; Linnell, J.D.C.; Nicoloso, S.; Sustr, P.; et al. Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. J. Anim. Ecol. 2013, 82, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency EU-DEM v.1.0 and Derived Products. Available online: https://land.copernicus.eu/imagery-in-situ/eu-dem/eu-dem-v1-0-and-derived-products (accessed on 8 March 2021).
- Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006. NASA EOSDIS Land Processes DAAC 2015, 10. Available online: https://lpdaac.usgs.gov/products/mod13q1v006/ (accessed on 9 March 2021).
- German Meteorological Service DWD Climate Data Center (CDC): Seasonal Grids of Monthly Averaged Daily Air Temperature (2m) over Germany, Version v1.0. 2018. Available online: https://opendata.dwd.de/climate_environment/CDC/grids_germany/seasonal/air_temperature_mean/ (accessed on 9 March 2021).
- German Meteorological Service DWD Climate Data Center (CDC): Seasonal Grids of Monthly Averaged Daily Maximum Air Temperature (2m) over Germany, Version v1.0. 2018. Available online: https://opendata.dwd.de/climate_environment/CDC/grids_germany/seasonal/air_temperature_max/ (accessed on 9 March 2021).
- German Meteorological Service DWD Climate Data Center (CDC): Seasonal Grids of Monthly Averaged Daily Minimum Air Temperature (2m) over Germany, Version v1.0. 2018. Available online: https://opendata.dwd.de/climate_environment/CDC/grids_germany/seasonal/air_temperature_min/ (accessed on 9 March 2021).
- German Meteorological Service DWD Climate Data Center (CDC): Seasonal Grids of Sum of Precipitation over Germany, Version v1.0. 2018. Available online: https://opendata.dwd.de/climate_environment/CDC/grids_germany/seasonal/precipitation/ (accessed on 8 March 2021).
- Bavarian State Institute of Forestry (LWF). FÜK Forstliche Übersichtskarte (Forest Overview Map). 2012. Available online: https://www.lwf.bayern.de/service/lwf/007786/index.php (accessed on 23 June 2021).
- Marra, G.; Wood, S.N. Practical variable selection for generalized additive models. Comput. Stat. Data Anal. 2011, 55, 2372–2387. [Google Scholar] [CrossRef]
- Ver Hoef, J.M. Who invented the delta method? Am. Stat. 2012, 66, 124–127. [Google Scholar] [CrossRef]
- Mesgaran, M.B.; Cousens, R.D.; Webber, B.L. Here be dragons: A tool for quantifying novelty due to covariate range and correlation change when projecting species distribution models. Divers. Distrib. 2014, 20, 1147–1159. [Google Scholar] [CrossRef]
- King, G.; Zeng, L. When can history be our guide? The pitfalls of counterfactual inference. Int. Stud. Q. 2007, 51, 183–210. [Google Scholar] [CrossRef]
- Virgili, A.; Racine, M.; Authier, M.; Monestiez, P.; Ridoux, V. Comparison of habitat models for scarcely detected species. Ecol. Model. 2017, 346, 88–98. [Google Scholar] [CrossRef]
- Mannocci, L.; Roberts, J.J.; Halpin, P.N.; Authier, M.; Boisseau, O.; Bradai, M.N.; Cañadas, A.; Chicote, C.; David, L.; Di-Méglio, N. Assessing cetacean surveys throughout the Mediterranean sea: A gap analysis in environmental space. Sci. Rep. 2018, 8, 3126. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Franzetti, B.; Calabrese, A.; Cappellini, S.; Focardi, S. Response to human presence during nocturnal line transect surveys in fallow deer (Dama dama) and wild boar (Sus scrofa). Eur. J. Wildl. Res. 2009, 55, 107–115. [Google Scholar] [CrossRef]
- Pérez, J.M.; Serrano, E.; Alpízar-Jara, R.; Granados, J.E.; Soriguer, R.C. The potential of distance sampling methods to estimate abundance of mountain ungulates: Review of usefulness and limitations. Pirineos 2002, 157, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Valente, A.M.; Fonseca, C.; Marques, T.A.; Santos, J.P.; Rodrigues, R.; Torres, R.T. Living on the edge: Roe deer (Capreolus capreolus) density in the margins of its geographical range. PLoS ONE 2014, 9, e88459. [Google Scholar] [CrossRef] [Green Version]
- Focardi, S.; Montanaro, P.; Isotti, R.; Ronchi, F.; Scacco, M.; Calmanti, R. Distance sampling effectively monitored a declining population of Italian roe deer Capreolus capreolus italicus. Oryx 2005, 39, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Latham, J.; Staines, B.W.; Gorman, M.L. Correlations of red (Cervus elaphus) and roe (Capreolus capreolus) deer densities in Scottish forests with environmental variables. J. Zool. 1997, 242, 681–704. [Google Scholar] [CrossRef]
- Hothorn, T.; Brandl, R.; Müller, J. Large-scale model-based assessment of deer-vehicle collision risk. PLoS ONE 2012, 7, e29510. [Google Scholar] [CrossRef] [Green Version]
- Aulak, W.; Babińska-Werka, J. Estimation of roe deer density based on the abundance and rate of disappearance of their faeces from the forest. Acta Theriol. 1990, 35, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Mysterud, A.; Lian, L.-B.; Hjermann, D.Ø. Scale-dependent trade-offs in foraging by European roe deer (Capreolus capreolus) during winter. Can. J. Zool. 1999, 77, 1486–1493. [Google Scholar] [CrossRef] [Green Version]
- Raganella-Pelliccioni, E.; Scremin, M.; Toso, S. Phenology and synchrony of roe deer breeding in northern Italy. Acta Theriol. 2007, 52, 95–100. [Google Scholar] [CrossRef]
- Verordnung zur Ausführung des Bayerischen Jagdgesetzes AVBayJG (Ordinance on the Implementation of the Bavarian Hunting Act). §19 Jagdzeiten (Hunting Seasons). Available online: https://www.gesetze-bayern.de/Content/Document/BayAVJG-19 (accessed on 15 July 2021).
- Dzięciolowski, R. Structure and spatial organization of deer populations. Acta Theriol. 1979, 24, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Lovari, S.; San José, C. Wood dispersion affects home range size of female roe deer. Behav. Processes 1997, 40, 239–241. [Google Scholar] [CrossRef]
- Bonnot, N.; Morellet, N.; Verheyden, H.; Cargnelutti, B.; Lourtet, B.; Klein, F.; Hewison, A.J.M. Habitat use under predation risk: Hunting, roads and human dwellings influence the spatial behaviour of roe deer. Eur. J. Wildl. Res. 2013, 59, 185–193. [Google Scholar] [CrossRef]
- Courbin, N.; Dussault, C.; Veillette, A.; Giroux, M.A.; Côté, S.D. Coping with strong variations in winter severity: Plastic habitat selection of deer at high density. Behav. Ecol. 2017, 28, 1037–1046. [Google Scholar] [CrossRef]
- Abbas, F.; Morellet, N.; Hewison, A.J.M.; Merlet, J.; Cargnelutti, B.; Lourtet, B.; Angibault, J.-M.; Daufresne, T.; Aulagnier, S.; Helene, V. Landscape fragmentation generates spatial variation of diet composition and quality in a generalist herbivore. Oecologia 2011, 167, 401–411. [Google Scholar] [CrossRef]
- Tixier, H.; Duncan, P. Are european roe deer browsers? A review of variations in the composition of their diets. Rev. D’ecologie 1996, 51, 3–17. [Google Scholar]
- Taber, R.; Strandgaard, H. The roe deer (Capreolus capreolus) population at Kalo and the factors regulating its size. JSTOR 1974, 378–379. [Google Scholar] [CrossRef]
- Benhaiem, S.; Delon, M.; Lourtet, B.; Cargnelutti, B.; Aulagnier, S.; Hewison, A.J.M.; Morellet, N.; Verheyden, H. Hunting Increases Vigilance Levels in Roe Deer and Modifies Feeding Site Selection. Anim. Behav. 2008, 76, 611–618. [Google Scholar] [CrossRef]
- Linnell, J.D.C.; Andersen, R. Timing and Synchrony of Birth in a Hider Species, the Roe Deer Capreolus Capreolus. J. Zool. 1998, 244, 497–504. [Google Scholar] [CrossRef]
- Schaller, M.J. Forests and Wildlife Managementi in Germany: A Mini-Review. Eurasian J. For. Res. 2007, 10, 59–70. [Google Scholar]
- Wotschikowsky, U. Ungulates and their management in Germany. In European Ungulates and their Management in the 21st Century; Apollonio, M., Andersen, R., Putman, R., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 201–222. [Google Scholar]
- Mysterud, A.; Bjørnsen, B.H.; Østbye, E. Effects of snow depth on food and habitat selection by roe deer Capreolus capreolus along an altitudinal gradient in south-central Norway. Wildl. Biol. 1997, 3, 27–33. [Google Scholar] [CrossRef]
- Grøtan, V.; SÆther, B.E.; Engen, S.; Solberg, E.J.; Linnell, J.D.C.; Andersen, R.; Brøseth, H.; Lund, E. Climate causes large-scale spatial synchrony in population fluctuations of a temperate herbivore. Ecology 2005, 86, 1472–1482. [Google Scholar] [CrossRef]
- Mysterud, A.; Tryjanowski, P.; Panek, M.; Pettorelli, N.; Stenseth, N.C. Inter-specific synchrony of two contrasting ungulates: Wild boar (Sus scrofa) and roe deer (Capreolus capreolus). Oecologia 2007, 151, 232–239. [Google Scholar] [CrossRef]
- Plard, F.; Gaillard, J.M.; Coulson, T.; Hewison, A.J.M.; Delorme, D.; Warnant, C.; Bonenfant, C. Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biol. 2014, 12, e1001828. [Google Scholar] [CrossRef] [PubMed]
- Mysterud, A.; Østbye, E. Bed-site selection by european roe deer (Capreolus capreolus) in southern Norway during winter. Can. J. Zool. 1995, 73, 924–932. [Google Scholar] [CrossRef]
- Ossi, F.; Gaillard, J.M.; Hebblewhite, M.; Cagnacci, F. Snow sinking depth and forest canopy drive winter resource selection more than supplemental feeding in an alpine population of roe deer. Eur. J. Wildl. Res. 2015, 61, 111–124. [Google Scholar] [CrossRef]
- Peters, W.; Hebblewhite, M.; Mysterud, A.; Eacker, D.; Hewison, A.J.M.; Linnell, J.D.; Focardi, S.; Urbano, F.; De Groeve, J.; Gehr, B. Large herbivore migration plasticity along environmental gradients in Europe: Life-history traits modulate forage effects. Oikos 2019, 128, 416–429. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, J.; Topping, C. Modelling roe deer (Capreolus capreolus) in a gradient of forest fragmentation: Behavioural plasticity and choice of cover. Can. J. Zool. 2004, 82, 1528–1541. [Google Scholar] [CrossRef]
- Stüwe, M.; Hendrichs, H. Organization of roe deer (Capreolus capreolus) in an open field habitat. Z. Für Säugetierkunde 1984, 49, 359–367. [Google Scholar]
- Coulon, A.; Morellet, N.; Goulard, M.; Cargnelutti, B.; Angibault, J.M.; Hewison, A.J.M. Inferring the effects of landscape structure on roe deer (Capreolus capreolus) movements using a step selection function. Landsc. Ecol. 2008, 23, 603–614. [Google Scholar] [CrossRef]
- Torres, R.T.; Virgós, E.; Santos, J.; Linnell, J.D.; Fonseca, C. Habitat use by sympatric red and roe deer in a Mediterranean ecosystem. Anim. Biol. 2012, 62, 351–366. [Google Scholar] [CrossRef]
- Laing, S.E.; Buckland, S.T.; Burn, R.W.; Lambie, D.; Amphlett, A. Dung and nest surveys: Estimating decay rates. J. Appl. Ecol. 2003, 40, 1102–1111. [Google Scholar] [CrossRef]
- Tsaparis, D.; Katsanevakis, S.; Ntolka, E.; Legakis, A. Estimating dung decay rates of roe deer (Capreolus capreolus) in different habitat types of a mediterranean ecosystem: An information theory approach. Eur. J. Wildl. Res. 2009, 55, 167–172. [Google Scholar] [CrossRef]
- Wade, T.G.; Riitters, K.H.; Wickham, J.D.; Jones, K.B. Distribution and causes of global forest fragmentation. Conserv. Ecol. 2003, 7, 7. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Seagle, S.W. Can ungulates foraging in a multiple-use landscape alter forest nitrogen budgets? Oikos 2003, 103, 230–234. [Google Scholar] [CrossRef]
- Imperio, S.; Ferrante, M.; Grignetti, A.; Santini, G.; Focardi, S. Investigating population dynamics in ungulates: Do hunting statistics make up a good index of population abundance? Wildl. Biol. 2010, 16, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Morellet, N.; Champely, S.; Gaillard, J.-M.; Ballon, P.; Boscardin, Y. The browsing index: New tool uses browsing pressure to monitor deer populations. Wildl. Soc. Bull. 2001, 1243–1252. [Google Scholar]
- MacKenzie, D.I.; Nichols, J.D. Occupancy as a surrogate for abundance estimation. Anim. Biodivers. Conserv. 2004, 27, 461–467. [Google Scholar]

| Variable (Model Abbreviation) | Units | Data Source | Spring Model | Autumn Model |
|---|---|---|---|---|
| Coniferous forest (coniferous) | % cover | CORINE land cover 2018 [42] | ✔ | ✔ |
| Deciduous and mixed forest (deciduous_and_mixed) Arable land (arable) | % cover | ✔ | ✔ | |
| Grass and shrublands (grass_and_shrubs) | % cover | ✔ | ✔ | |
| Water and other unsuitable areas (water) | % cover | ✔ | ✔ | |
| Artificial surfaces (artificial) | % cover | ✔ | ✔ | |
| Winter mean temperature (win_mean_temp) | °C | German Meteorological Service (DWD) seasonal gridded data 2019 [52,53,54,55] | ✔ | - |
| Spring mean temperature (spr_mean_temp) | °C | x1 | - | |
| Summer mean temperature (sum_mean_temp) | °C | - | ✔ | |
| Autumn mean temperature (aut_mean_temp) | °C | - | x2 | |
| Winter temperature range (win_temp_range) | °C | ✔ | - | |
| Spring temperature range (spr_temp_range) | °C | ✔ | - | |
| Summer temperature range (sum_temp_range) | °C | - | ✔ | |
| Autumn temperature range (aut_temp_range) | °C | - | ✔ | |
| Winter accumulated precipitation (win_acc_precip) | mm | x1 | - | |
| Spring accumulated precipitation (spr_acc_precip) | mm | ✔ | - | |
| Summer accumulated precipitation (sum_acc_precip) | mm | - | ✔ | |
| Autumn accumulated precipitation (aut_acc_precip) | mm | - | ✔ | |
| Private forest (private_forest) | % cover | Forest overview map [56] | ✔ | ✔ |
| State forest | % cover | ✔ | ✔ | |
| Corporate forest (corporate_forest) | % cover | ✔ | ✔ | |
| NDVI seasonal mean (ndvi) * | - | MODIS/Terra Vegetation Index [51] | ✔ | x3 |
| Elevation (elev) | degrees | EU-DEM v.1.0 [50] | x1 | x4 |
| Slope (slope) | degrees | ✔ | ✔ | |
| Aspect (aspect) | m | ✔ | ✔ | |
| Edge proximity (edge_proximity) | m | Proximity raster generated from distance to woodlands in CORINE 2018 [42] | ✔ | ✔ |
| Season | Distribution | Model Formula | AIC (∆AIC) | % Deviance Explained |
|---|---|---|---|---|
| Spring | Tweedie | s(coniferous) + s(deciduous_and_mixed) + s(arable) + s(grass_and_shrubs) + s(private_forest) + s(win_mean_temp) + s(win_temp_range) + s(spr_acc_precip) + offset | 1046.11 (0) | 32.8% |
| Spring | Negative binomial | s(artificial) + s(win_mean_temp) + s(spr_acc_precip) + offset | 1064.06 (17.95) | 14.7% |
| Autumn | Tweedie | s(edge_proximity) + s(private_forest) + s(corporate_forest) + s(grass_and_shrubs) + s(sum_temp_range) + s(aut_temp_range) + offset | 1177.12 (0) | 34.3% |
| Autumn | Negative binomial | s(edge_proximity) + s(corporate_forest) + s(grass_and_shrubs) + s(sum_temp_range) + s(aut_temp_range) + offset | 1248.35 (71.23) | 21.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjamin, C.S.; Uphus, L.; Lüpke, M.; Rojas-Botero, S.; Dhillon, M.S.; Englmeier, J.; Fricke, U.; Ganuza, C.; Haensel, M.; Redlich, S.; et al. Modelling the Relative Abundance of Roe Deer (Capreolus capreolus L.) along a Climate and Land-Use Gradient. Animals 2022, 12, 222. https://doi.org/10.3390/ani12030222
Benjamin CS, Uphus L, Lüpke M, Rojas-Botero S, Dhillon MS, Englmeier J, Fricke U, Ganuza C, Haensel M, Redlich S, et al. Modelling the Relative Abundance of Roe Deer (Capreolus capreolus L.) along a Climate and Land-Use Gradient. Animals. 2022; 12(3):222. https://doi.org/10.3390/ani12030222
Chicago/Turabian StyleBenjamin, Caryl S., Lars Uphus, Marvin Lüpke, Sandra Rojas-Botero, Maninder Singh Dhillon, Jana Englmeier, Ute Fricke, Cristina Ganuza, Maria Haensel, Sarah Redlich, and et al. 2022. "Modelling the Relative Abundance of Roe Deer (Capreolus capreolus L.) along a Climate and Land-Use Gradient" Animals 12, no. 3: 222. https://doi.org/10.3390/ani12030222
APA StyleBenjamin, C. S., Uphus, L., Lüpke, M., Rojas-Botero, S., Dhillon, M. S., Englmeier, J., Fricke, U., Ganuza, C., Haensel, M., Redlich, S., Riebl, R., Tobisch, C., Uhler, J., Zhang, J., Menzel, A., & Peters, W. (2022). Modelling the Relative Abundance of Roe Deer (Capreolus capreolus L.) along a Climate and Land-Use Gradient. Animals, 12(3), 222. https://doi.org/10.3390/ani12030222







