Protective Application of Morus and Its Extracts in Animal Production
Abstract
Simple Summary
Abstract
1. Introduction
2. Nutrients Derived from the Mulberry Tree and Its Extracts
2.1. Leaves
| Authors | Species | Source | Country | Season | DM, % | CP, % | Fat 1, % | CF, % | NFE, % | NDF, % | ADF, % | Ash, % | Carb, % | GE, kcal/100 g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yao et al., 2000 | Morus alba L. | Fresh | China | Spring and Autumn | 23.6–30.4 | 19.6–21.9 | - | - | - | 37.5–43.4 | - | - | - | - |
| Srivastava et al., 2006 | Morus alba L. | Fresh | India | Spring | 23.32–28.87 | 4.72–9.96 | 0.64–1.35 | - | - | 8.15–11.32 | - | 4.26–5.32 | 8.01–13.42 | 69–79 |
| Dried leaf powder | 92.76–94.89 | 15.31–30.91 | 2.09–4.93 | - | - | 27.6–36.66 | - | 14.59–17.24 | 9.7–29.64 | 113–224 | ||||
| Todaro et al., 2009 | Morus latifolia | Fresh | Italy | Summer | 25.98 | 21.05 | 4.36 | - | - | 22.88 | 19.72 | 13.31 | - | - |
| Adeduntan et al., 2009 | Morus alba L. | Dried leaf powder | Nigeria | - | 20.65–27.84 | 21.24–21.66 | 5.31–8.02 | 8.74–13.7 | - | - | - | 8.19–12.63 | 47.27–56.42 | - |
| Kandylis et al., 2009 | Morus alba L. | Dried leaf powder | Greece | - | 89.4 | 15.1 | 2 | 18.9 | 54 | - | - | 10 | - | - |
| Al-Kirshi et al., 2009 | Morus alba L. | Dried leaf powder | Malaysia | - | 89.3 | 29.8 | 11.1 | 32.3 | - | 22.8 | 22.8 | 11.8 | - | 422 |
| Vu et al., 2011 | Morus alba L. | Fresh | The Netherlands | - | 19.8 | 22.3 | 3.5 | 15.9 | - | 31.1 | 18.3 | 14.5 | - | - |
| Sahoo et al., 2011 | Morus alba L. | Dried leaf powder | India | - | 27.8 | 19.4 | 4.1 | - | - | 36.1 | 26.8 | 13.3 | - | - |
| Guven, 2012 | Morus nigra | Dried leaf powder | Turkey | Summer | 42.2 | 16.06 | - | - | - | 22.08 | 19.46 | 17.5 | - | - |
| Morus alba | 46.27 | 18.73 | - | - | - | 19.38 | 17.33 | 15.4 | - | - | ||||
| Morus rubra | 37.36 | 11.75 | - | - | - | 33.33 | 24.06 | 22.36 | - | - | ||||
| Morus alba pendula | 25.97 | 23.72 | - | - | - | 29.53 | 26.06 | 17.7 | - | - | ||||
| Wang et al., 2012 | Morus atropurpurea Roxb | Dried leaf powder | China | Summer | - | 25.17 | 2.85 | - | - | 27.88 | 16.49 | - | - | - |
| Morus alba L. | - | 25.9 | 4.21 | - | - | 26.25 | 17.07 | - | - | - | ||||
| Morus multicaulis Perr | - | 25.18 | 4.91 | - | - | 27.54 | 17.66 | - | - | - | ||||
| Iqbal et al., 2012 | Morus alba L. | Dried leaf powder | Pakistan | - | 94.7 | 18.41 | 6.57 | 10.11 | - | - | - | 8.91 | - | - |
| Morus nigra L. | 93.3 | 19.76 | 5.13 | 12.32 | - | - | - | 9.12 | - | - | ||||
| Morus rubra L. | 95.5 | 24.63 | 4.24 | 8.17 | - | - | - | 11.73 | - | - | ||||
| Flaczyk et al., 2013 | Morus alba L. | Aqueous extracts | Poland | - | 94.6 | 12.7 | 0.15 | - | - | - | - | 22.7 | - | - |
| Dolis et al., 2017 | M. multicaulis | Fresh | Romania | Summer | - | 6.2 | 1.04 | 5.26 | 12.77 | - | - | 4.1 | - | - |
| Dried leaf powder | - | 21.16 | 3.54 | 17.88 | 43.46 | - | - | 13.96 | - | - | ||||
| Yu et al., 2018 | Morus alba L. | Dried leaf powder | China | Summer | - | 29.02–37.36 | - | 13.01–16.61 | - | - | - | - | - | - |
| M. multicaulis Perr. | - | 27.63–36.42 | - | 11.46–15.27 | - | - | - | - | - | - | ||||
| M.atropurpurea Roxb. | - | 28.29–34.19 | - | 12.41–15.50 | - | - | - | - | - | - | ||||
| Cai et al., 2019 | Morus alba L. | Dried leaf powder | China | - | 18–27 | 17–19.4 | - | - | - | 21.8–27.8 | 10.2–13 | 10.8–12.8 | - | - |
| Kang et al., 2020 | - | Dried leaf powder | China | Spring and Autumn | - | 24.8 | 5.4 | - | 42.2 | 33.6 | 25.8 | - | - | - |
| - | 20.9 | 7 | - | 47.2 | 26.6 | 18.1 | - | - | - | |||||
| - | 26.9 | 6.3 | - | 43.1 | 25.2 | 29.7 | - | - | - | |||||
| - | 22.4 | 7.9 | - | 44.3 | 31.8 | 29.7 | - | - | - | |||||
| Ouyang et al., 2019 | Morus alba var. multicaulis | Dried leaf powder | China | Spring | 89.54 | 20.3 | 8.15 | - | - | 34.3 | 16.28 | 7.56 | - | - |
2.2. Fruits
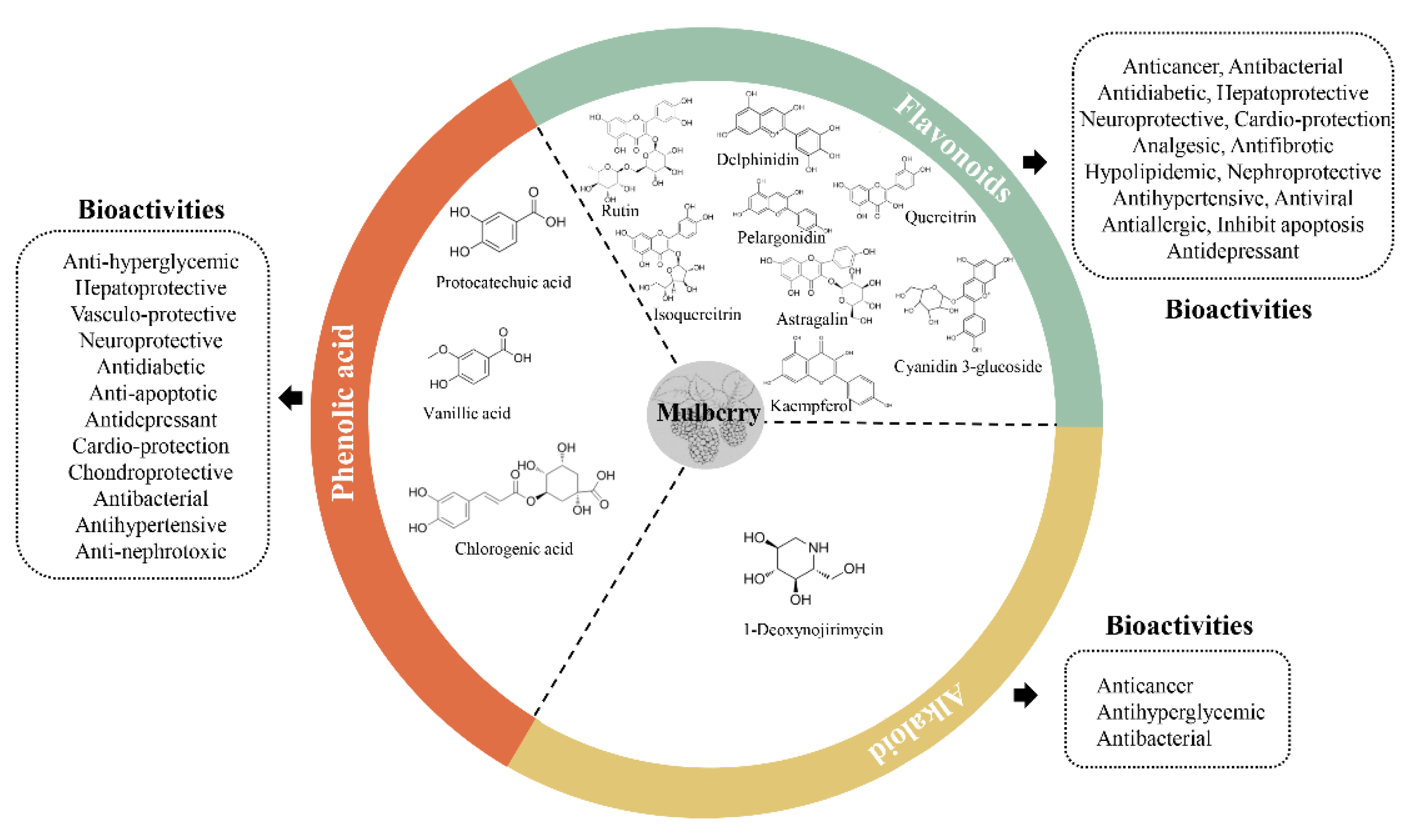
3. Bioactive Compounds in the Mulberry and Their Bioactivities
4. Application of Mulberry Tree Extracts in Animal Production
4.1. Poultry
4.2. Swine
4.3. Ruminants
4.3.1. Effects on Calf Health
4.3.2. Effects on the Rumen Development and Rumen Microbiota
4.3.3. Effects on the Growth Performance and Immune Function
4.3.4. Effects on Animal Products
4.4. Fishes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- FAO. Transforming the livestock sector through the sustainable development goals; World Livestock: Rome, Italy, 2018. [Google Scholar]
- Rauw, W.M.; Rydhmer, L.; Kyriazakis, I.; Øverland, M.; Gilbert, H.; Dekkers, J.C.; Hermesch, S.; Bouquet, A.; Gómez Izquierdo, E.; Louveau, I.; et al. Prospects for sustainability of pig production in relation to climate change and novel feed resources. J. Sci. Food Agric. 2020, 100, 3575–3586. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Holman, B.W.B. Chapter 22—Sustainability II: Sustainable animal production and meat processing. In Lawrie’s Meat Science (Ninth Edition); Toldrá, F., Ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 727–798. [Google Scholar] [CrossRef]
- Abadi, N.A. Major non-conventional feed resources of livestock. Int. J. Eng. Dev. Res. 2018, 6, 786–789. [Google Scholar]
- Torma, S.; Vilček, J.; Lošák, T.; Kužel, S.; Martensson, A. Residual plant nutrients in crop residues—An important resource. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 68, 358–366. [Google Scholar] [CrossRef]
- Machii, H.; Koyama, A.; Yamanouchi, H. Mulberry breeding, cultivation and utilization in Japan. FAO Anim. Prod. Health Pap. 2000, 147, 63. [Google Scholar]
- Huo, Y. Mulberry cultivation and utilization in China. FAO Anim. Prod. Health Pap. 2000, 1, 11–44. [Google Scholar]
- Sánchez, M.D. World distribution and utilization of mulberry, potential for animal feeding. FAO Anim. Prod. Health Pap. 2000, 111, 1–11. [Google Scholar]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Ba, N.X.; Giang, V.D.; NgoanJan, L.D. Ensiling of mulberry foliage (Morus alba) and the nutritive value of mulberry foliage silage for goats in central Vietnam. Livest. Res. Rural. Dev. 2005, 17, 23–25. [Google Scholar]
- Simbaya, J.; Chibinga, O.; Salem, A.Z.M. Nutritional evaluation of selected fodder trees: Mulberry (Molus alba Lam.), Leucaena (Leucaena luecocephala Lam de Wit.) and Moringa (Moringa oleifera Lam.) as dry season protein supplements for grazing animals. Agrofor. Syst. 2020, 94, 1189–1197. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, Y.H.; Peng, Y.L.; He, J.H.; Xiao, D.F.; Chen, C.; Li, F.N.; Huang, R.L.; Yin, Y.L. Dietary mulberry leaf powder affects growth performance, carcass traits and meat quality in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1934–1945. [Google Scholar] [CrossRef]
- Al-kirshi, R.; Alimon, A.; Idrus, Z.; Mohamed, W.Z.; Michel, I. Utilization of mulberry leaf meal (Morus alba) as protein supplement in diets for laying hens. Ital. J. Anim. Sci. 2010, 9, 265–267. [Google Scholar] [CrossRef]
- Mengistu, G.; Assefa, G.; Tilahun, S. Noug seed (Guizotia abyssinica) cake substituted with dried mulberry (Morus indica) and Vernonia amygdalina mixed leaves’ meal on growth performances of Bonga Sheep at Teppi, Ethiopia. J. Nutr. Metab. 2020, 2020, 9308761. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Kim, K.H.; Jeon, B.; Park, P.J.; Hwang, I.; Choi, N.; Kim, E.; Hong, S.; Park, J.; Sung, S.; et al. Effect of mulberry silage supplementation during late fattening stage of Hanwoo (Bos taurus coreanae) steer on antioxidative enzyme activity within the longissimus muscle. Anim. Prod. Sci. 2012, 52, 240–247. [Google Scholar] [CrossRef]
- Zhang, S.D.; Soltis, D.E.; Yang, Y.; Li, D.Z.; Yi, T.S. Multi-gene analysis provides a well-supported phylogeny of Rosales. Mol. Phylogenetics Evol. 2011, 60, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tutin, G.T.; Morus, L. Psilotaceae to Platanaceae; Cambridge University Press: Melbourne VIC, Australia, 1996. [Google Scholar]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, R. Biochemical constituents of different parts of mulberry genotypes. Int. J. Agric. Sci. 2011, 3, 90–96. [Google Scholar] [CrossRef]
- Srivastava, S.; Kapoor, R.; Thathola, A.; Srivastava, R.P. Nutritional quality of leaves of some genotypes of mulberry (Morus alba). Int. J. Food Sci. Nutr. 2006, 57, 305–313. [Google Scholar] [CrossRef]
- Todaro, M.; Bonanno, A.; Tornambè, G.; Di Grigoli, A.; Luisa Scatassa, M.; Giaccone, P. Utilization of mulberry leaves (Morus latifolia cv. Kokusou 21) in diets for dairy ewes. Ital. J. Anim. Sci. 2009, 8, 438–440. [Google Scholar] [CrossRef]
- Vu, C.C.; Verstegen, M.W.A.; Hendriks, W.H.; Pham, K. The Nutritive value of mulberry leaves (Morus Alba) and partial replacement of cottonseed in rations on the performance of growing Vietnamese cattle. Asian Australas. J. Anim. Sci. 2011, 24, 1233–1242. [Google Scholar] [CrossRef]
- Dolis, M.G.; Simeanu, C.; Usturoi, A.; Simeanu, D. Research regarding chemical composition and the digestibility of the mulberry leaves from eforie variety. Rev. De Chim. 2017, 68, 151–156. [Google Scholar] [CrossRef]
- Yao, J.; Yan, B.; Wang, X.Q.; Liu, J.X. Nutritional evaluation of mulberry leaves as feeds for ruminants. Livest. Res. Rural. Dev. 2000, 12, 9–16. [Google Scholar]
- Adeduntan, S.; Oyerinde, A. Evaluation of chemical and antinutritional characteristics of obeche (Triplochition scleroxylon) and some mulberry (Morus alba) leaves. Int. J. Biol. Chem. Sci. 2009, 3, 681–687. [Google Scholar] [CrossRef]
- Kandylis, K.; Hadjigeorgiou, I.; Harizanis, P. The nutritive value of mulberry leaves (Morus alba) as a feed supplement for sheep. Trop. Anim. Health Prod. 2009, 41, 17–24. [Google Scholar] [CrossRef]
- Al-kirshi, R.; Alimon, A.; Zulkifli, R.; Sazili, I.; Zahari, A. The chemical composition and nutritive value of mulberry leaf meal as a protein source in poultry diets. In International Seminar on Animal Industry; Atlantis Press: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sahoo, A.; Singh, B.; Sharma, O.P. Evaluation of feeding value of Eupatorium adenophorum in combination with mulberry leaves. Livest. Sci. 2011, 136, 175–183. [Google Scholar] [CrossRef]
- Guven, I. Effect of species on nutritive value of mulberry leaves. Kafkas Univ. Veter Fak. Derg. 2012, 18, 865–869. [Google Scholar] [CrossRef]
- Wang, W.X.; Yang, H.J.; Bo, Y.K.; Ding, S.; Cao, B.H. Nutrient composition, polyphenolic contents, and in situ protein degradation kinetics of leaves from three mulberry species. Livest. Sci. 2012, 146, 203–206. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Sirajuddin; Chan, K.W.; Sarfraz, R.A.; Uddin, K. Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef]
- Flaczyk, E.; Kobus-Cisowska, J.; Przeor, M.; Korczak, J.; Remiszewski, M.; Korbas, E.; Buchowski, M. Chemical characterization and antioxidative properties of polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes. Agric. Sci. 2013, 4, 141–147. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.; Yang, J.; Zhang, Y.; Deng, Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]
- Cai, M.; Mu, L.; Wang, Z.L.; Liu, J.Y.; Liu, T.L.; Wanapat, M.; Huang, B.Z. Assessment of mulberry leaf as a potential feed supplement for animal feeding in P.R. China. Asian Australas. J. Anim. Sci. 2019, 32, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, R.; Tang, S.; Wang, M.; Tan, Z.; Bernard, L.A. Chemical composition and in vitro ruminal fermentation of pigeonpea and mulberry leaves. Agrofor. Syst. 2020, 94, 1521–1528. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, M.; Hou, Q.; Feng, D.; Pi, Y.; Zhao, W. Effects of dietary mulberry leaf powder in concentrate on the rumen fermentation and ruminal epithelium in Fattening Hu Sheep. Animals 2019, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Hussain, A.; Tazeddinova, D.; Abylgazinova, A.; Xu, B. Assessing the nutritional-value-based therapeutic potentials and non-destructive approaches for mulberry fruit assessment: An overview. Comput. Intell. Neurosci. 2022, 2022, 6531483–6531499. [Google Scholar] [CrossRef] [PubMed]
- Koca, I.; Ustun, S.; Koca, A.; Karadeniz, B. Chemical composition, antioxidant activity and anthocyanin profiles of purple mulberry (Morus rubra) fruits. J. Food Agric. Environ. 2008, 6, 39–42. [Google Scholar]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Martínez, J.J.; Hernández, F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods 2015, 12, 399–408. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, W.J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Owon, M.A.; Gafar, A.M.; Saleh, S.M.; Shaheen, M.M. Identification of bioactive compounds from egyptian mulberry fruits and their uses in improvement the quality of some foods. J. Sustain. Agric. Sci. 2016, 42, 33–52. [Google Scholar] [CrossRef]
- Munir, A.; Khera, R.A.; Rehman, R.; Nisar, S. Multipurpose white mulberry: A review. Int. J. Biol. Chem. Sci. 2018, 13, 31–35. [Google Scholar]
- Sun, R.; Sun, L.; Han, C. Partial-least-squares and canonical-correlation analysis of chemical constituents and active ingredients of new types of Chinese mulberries. Food Sci. Nutr. 2018, 6, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.; Ozcan, M.M. Comparison of mineral contents of mulberry (Morus spp.) fruits and their pekmez (boiled mulberry juice) samples. Int. J. Food Sci. Nutr. 2009, 60, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.P.; Liu, C.; Shen, L. Analysis of 14 minerals of mulberry fruit in different mature stage by ICP-OES method. Spectscopy Spectr. Anal. 2009, 29, 2574–2576. [Google Scholar]
- Altundag, H.; Tuzen, M. Comparison of dry, wet and microwave digestion methods for the multi element determination in some dried fruit samples by ICP-OES. Food Chem. Toxicol. 2011, 49, 2800–2807. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.; Dhumal, N.; Khyade, V. The mulberry, Morus alba (L.): The medicinal herbal source for human health. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2941–2964. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- He, X.Y.; Chen, X.; Ou, X.Q.; Ma, L.Y.; Xu, W.T.; Huang, K.L. Evaluation of flavonoid and polyphenol constituents in mulberry leaves using HPLC fingerprint analysis. Int. J. Food Sci. Technol. 2020, 55, 526–533. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, J.; Pradhan, A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Res. 2012, 16, 130–134. [Google Scholar]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Jia, Z.; Battino, M.; Miron, A.; Yu, Z.; Cao, H.; Xiao, J. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Crit. Rev. Food Sci. Nutr. 2018, 58, 2908–2924. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Chen, J.J.; Li, Q.Q.; Zeng, G.Y.; Chen, Q.Y.; Chen, J.L.; Liao, Z.M.; Jin, P.; Wang, K.S.; Yang, Z.C. Alpha-glucosidase inhibitor 1-Deoxynojirimycin promotes beige remodeling of 3T3-L1 preadipocytes via activating AMPK. Biochem. Biophys. Res. Commun. 2019, 509, 1001–1007. [Google Scholar] [CrossRef]
- Hu, X.Q.; Thakur, K.; Chen, G.H.; Hu, F.; Zhang, J.G.; Zhang, H.B.; Wei, Z.J. Metabolic effect of 1-deoxynojirimycin from mulberry leaves on db/db diabetic mice using liquid chromatography-mass spectrometry based metabolomics. J. Agric. Food Chem. 2017, 65, 4658–4667. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.H.; Li, S.D.; Sui, X.D.; Guo, L.Y.; Liu, X.M.; Li, H.M.; Gao, L.M.; Cai, S.S.; Li, Y.R.; Wang, T.T.; et al. 1-Deoxynojirimycin (DNJ) ameliorates indomethacin-induced gastric ulcer in mice by affecting NF-kappaB signaling pathway. Front. Pharmacol. 2018, 9, 372. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Dai, Y.; Shen, W.; Liao, S.; Zou, Y. 1-Deoxynojirimycin modulates glucose homeostasis by regulating the combination of IR-GlUT4 and ADIPO-GLUT4 pathways in 3T3-L1 adipocytes. Mol. Biol. Rep. 2019, 46, 6277–6285. [Google Scholar] [CrossRef]
- Hu, T.G.; Wen, P.; Shen, W.Z.; Liu, F.; Li, Q.; Li, E.N.; Liao, S.T.; Wu, H.; Zou, Y.X. Effect of 1-Deoxynojirimycin isolated from mulberry leaves on glucose metabolism and gut microbiota in a streptozotocin-induced diabetic mouse model. J. Nat. Prod. 2019, 82, 2189–2200. [Google Scholar] [CrossRef]
- Parida, I.S.; Takasu, S.; Ito, J.; Ikeda, R.; Yamagishi, K.; Kimura, T.; Eitsuka, T.; Nakagawa, K. Supplementation of Bacillus amyloliquefaciens AS385 culture broth powder containing 1-deoxynojirimycin in high-fat diet altered the gene expressions related to lipid metabolism and insulin signaling in mice epididymal white adipose tissue. Food Funct. 2020, 11, 3926–3940. [Google Scholar] [CrossRef]
- Mathew, N.S.; Negi, P.S. Phenolic content and anti-oxidative attributes of various parts of wild banana (Ensete superbum Roxb. Cheesman) plant. J. Food Biochem. 2021, 45, e13657. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Nja, S. Anticancer properties of phenolic acids in colon cancer—A review. J. Nutr. Food Sci. 2016, 6, 1000468. [Google Scholar] [CrossRef]
- Qian, W.D.; Yang, M.; Wang, T.; Sun, Z.H.; Liu, M.; Zhang, J.N.; Zeng, Q.; Cai, C.L.; Li, Y.D. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.W.; Zhou, X.; Guo, K.X.; Zhou, F.; Yang, H.Q. Chlorogenic acid protects against indomethacin-induced inflammation and mucosa damage by decreasing Bacteroides-derived LPS. Front. Immunol. 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.H.; Wan, X.Y.; Liu, T.; Xiong, Y.; Xiang, G.; Peng, Y.L.; Zhu, R.H.; Zhou, Y.Q.; Liu, C.Q. Chlorogenic acid ameliorates Klebsiella pneumoniae-induced pneumonia in immunosuppressed mice via inhibiting the activation of NLRP3 inflammasomes. Food Funct. 2021, 12, 9466–9475. [Google Scholar] [CrossRef]
- Saraswat, N.; Sachan, N.; Chandra, P. Anti-diabetic, diabetic neuropathy protective action and mechanism of action involving oxidative pathway of chlorogenic acid isolated from Selinum vaginatum roots in rats. Heliyon 2020, 6, e05137. [Google Scholar] [CrossRef]
- Qin, L.H.; Zang, M.X.; Xu, Y.; Zhao, R.R.; Wang, Y.T.; Mi, Y.; Mei, Y.W. Chlorogenic acid alleviates hyperglycemia-induced cardiac fibrosis through activation of the NO/cGMP/PKG pathway in cardiac fibroblasts. Mol. Nutr. Food Res. 2021, 65, e2000810. [Google Scholar] [CrossRef]
- Peng, B.J.; Zhu, Q.; Zhong, Y.L.; Xu, S.H.; Wang, Z. Chlorogenic acid maintains glucose homeostasis through modulating the expression of SGLT-1, GLUT-2, and PLG in different intestinal segments of sprague-dawley rats fed a high-fat diet. Biomed. Environ. Sci. 2015, 28, 894–903. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere Groza, F.; Venter, A.C.; Vicas, S.I. Phytochemical composition of different botanical parts of Morus species, health benefits and application in food industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The impact of plant phytochemicals on the gut microbiota of humans for a balanced life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.; Ekpo, O. Inhibition of γH2AX, COX-2 and regulation of antioxidant enzymes in MPP(+)-exposed SH-SY5Y cells pre-treated with rutin. Metab. Brain Dis. 2021, 36, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Zheng, L.; Liu, M.; Mo, Y.Z. Protective effects of rutin on lipopolysaccharide-induced heart injury in mice. J. Toxicol. Sci. 2018, 43, 329–337. [Google Scholar] [CrossRef]
- Tsai, M.S.; Wang, Y.H.; Lai, Y.Y.; Tsou, H.K.; Liou, G.G.; Ko, J.L.; Wang, S.H. Kaempferol protects against propacetamol-induced acute liver injury through CYP2E1 inactivation, UGT1A1 activation, and attenuation of oxidative stress, inflammation and apoptosis in mice. Toxicol. Lett. 2018, 290, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Siddiqui, M.N.; Khatun, A.; Siddiky, M.N.A.; Bostami, A.B.M.R.; Selim, A.S.M. Dietary effect of mulberry leaf (Morus alba) meal on growth performance and serum cholesterol level of broiler chickens. SAARC J. Agric. 2015, 12, 79–89. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, J.; Li, H. Effect of green tea and mulberry leaf powders on the gut microbiota of chicken. BMC Veter Res. 2019, 15, 77. [Google Scholar] [CrossRef]
- Chen, X.L.; Sheng, Z.C.; Qiu, S.L.; Yang, H.F.; Jia, J.P.; Wang, J.; Jiang, C.M. Purification, characterization and in vitro and in vivo immune enhancement of polysaccharides from mulberry leaves. PLoS ONE 2019, 14, e0208611. [Google Scholar] [CrossRef]
- Wina, E.; Tangendjaja, B.; Pasaribu, T.; Purwadaria, T. Broiler performance fed Jatropha curcas seed meal detoxified by fermentation, physic and chemical treatments. Indones. J. Anim. Vet. Sci. 2010, 15, 174–181. [Google Scholar]
- Ding, Y.; Jiang, X.; Yao, X.; Zhang, H.; Song, Z.; He, X.; Cao, R. Effects of feeding fermented mulberry leaf powder on growth performance, slaughter performance, and meat quality in chicken broilers. Animals 2021, 11, 3294. [Google Scholar] [CrossRef]
- Che, C.T.; Wang, Z.J.; Chow, M.S.; Lam, C.W. Herb-herb combination for therapeutic enhancement and advancement: Theory, practice and future perspectives. Molecules 2013, 18, 5125–5141. [Google Scholar] [CrossRef]
- Jang, A.; Liu, X.D.; Shin, M.H.; Lee, B.D.; Lee, S.K.; Lee, J.H.; Jo, C. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult. Sci. 2008, 87, 2382–2389. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, M.T.; Chang, S.C.; Chang, Y.L.; Shih, C.H.; Yu, B.; Lee, T.T. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult. Sci. 2017, 96, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.W.; Lv, Z.P.; Dai, H.J.; Li, S.M.; Jiang, J.L.; Ye, N.W.; Zhu, S.L.; Wei, Q.W.; Shi, F.X. Dietary mulberry-leaf flavonoids supplementation improves liver lipid metabolism and ovarian function of aged breeder hens. J. Anim. Physiol. Anim. Nutr. 2021, 106, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.W.; Dai, H.J.; Jiang, J.L.; Ye, N.W.; Zhu, S.L.; Wei, Q.W.; Lv, Z.P.; Shi, F.X. Dietary mulberry-leaf flavonoids improve the eggshell quality of aged breeder hens. Theriogenology 2022, 179, 177–186. [Google Scholar] [CrossRef]
- Li, Y.G.; Zhang, L.; Zhong, H.S.; Zhang, X.D.; Li, Q.H.; Lu, Z.Q.; Ji, D.F. Effects of dietary mulberry leaf on growth performance, fat metabolism and meat quality of finishing pigs. Chin. J. Anim. Nutr. 2012, 24, 1805–1811. [Google Scholar] [CrossRef]
- Song, Q.L.; Wei, Q.P.; Zou, Z.H.; Zhou, Q.Y.; Liu, L.X.; Chen, X.L.; Dong, M.X.; Lai, Y.K.; Yan, J.S. Effects of mulberry leaf powder on growth performance, meat quality and serum biochemical indexes in finishing pigs. Chin. J. Anim. Nutr. 2016, 28, 541–547. [Google Scholar]
- Liu, Y.Y.; Li, Y.H.; Xiao, Y.; Peng, Y.L.; He, J.H.; Chen, C.; Xiao, D.F.; Yin, Y.L.; Li, F.N. Mulberry leaf powder regulates antioxidative capacity and lipid metabolism in finishing pigs. Anim. Nutr. 2021, 7, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.S.; Su, Y.Y.; Cai, Y.; He, L.H.; Yang, G. Comparative transcriptomic analysis reveals beneficial effect of dietary mulberry leaves on the muscle quality of finishing pigs. Veter Med. Sci. 2019, 5, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Xie, Y.Q.; Luo, J.Y.; Chen, T.; Xi, Q.Y.; Zhang, Y.L.; Sun, J.J. Dietary supplementation with Moringa oleifera and mulberry leaf affects pork quality from finishing pigs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 72–79. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, J.J.; Yu, J.; Mao, X.B.; Yu, B.; Chen, D.W. Effect of dietary supplementation with mulberry (Morus alba L.) leaves on the growth performance, meat quality and antioxidative capacity of finishing pigs. J. Integr. Agric. 2019, 18, 143–151. [Google Scholar] [CrossRef]
- Chen, G.S.; Shui, S.Z.; Chai, M.J.; Wang, D.; Su, Y.Y.; Wu, H.B.; Sui, X.D.; Yin, Y.L. Effects of paper mulberry (Broussonetia papyrifera) leaf extract on growth performance and fecal microflora of weaned piglets. Biomed. Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, J.; Zhu, M.; Zhu, C.; Yuan, L.; Li, Z.; Cai, D.; Chen, S.; Hu, P.; Liu, H.-Y. A meta-analysis of Lactobacillus-based probiotics for growth performance and intestinal morphology in piglets. Front. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Frontiers in Cellular and Infection Microbiology 2021, 11, 1750. [Google Scholar] [CrossRef]
- Fan, L.J.; Peng, Y.; Wu, D.; Hu, J.H.; Shi, X.; Yang, G.S.; Li, X. Morus nigra L. leaves improve the meat quality in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1904–1911. [Google Scholar] [CrossRef]
- Zhang, N.N.; Cao, H.Z.; Li, T.Z.; Yang, J.; Lu, C.L. Effect of feeding fermented feed mulberry powder on growth performance and meat quality indicators of fattening pigs. Chin. J. Vet. Sci. 2016, 36, 1005–4545. [Google Scholar]
- Ding, P.; Ding, Y.N.; Zeng, Q.H.; Li, X.; Song, Z.H.; Fan, Z.Y.; He, X. Effects of fermented forage mulberry powder on antioxidant performance and intestinal function of Ningxiang pigs. Chin. J. Anim. Nutr. 2019, 31, 4303–4313. [Google Scholar]
- Ding, P.; Li, X.; Ding, Y.N.; Song, Z.H.; Zhang, S.R.; Fan, Z.Y.; Li, Y.P.; He, X. Effects of fermented feed mulberry powder on growth performance, meat quality, and serum biochemical Iindexes of Ningxiang pigs. Chin. J. Anim. Nutr. 2018, 30, 1950–1957. [Google Scholar]
- Fan, L.J.; Peng, Y.; Wu, D.; Hu, J.H.; Shi, X.e.; Yang, G.S.; Li, X. Dietary supplementation of Morus nigra L. leaves decrease fat mass partially through elevating leptin-stimulated lipolysis in pig model. J. Ethnopharmacol. 2020, 249, 112416. [Google Scholar] [CrossRef]
- Maier, G.U.; Breitenbuecher, J.; Gomez, J.P.; Samah, F.; Fausak, E.; Van Noord, M. Vaccination for the prevention of neonatal calf diarrhea in cow-calf operations: A scoping review. Veter Anim. Sci. 2022, 15, 100238. [Google Scholar] [CrossRef]
- Roussel, A.J., Jr.; Brumbaugh, G.W. Treatment of diarrhea of neonatal calves. Vet. Clin. N. Am. Food Anim. Pract. 1991, 7, 713–728. [Google Scholar] [CrossRef]
- Bi, Y.L.; Yang, C.T.; Diao, Q.Y.; Tu, Y. Effects of dietary supplementation with two alternatives to antibiotics on intestinal microbiota of preweaned calves challenged with Escherichia coli K99. Sci. Rep. 2017, 7, 5439. [Google Scholar] [CrossRef]
- Kong, L.X.; Yang, C.T.; Dong, L.F.; Diao, Q.Y.; Si, B.W.; Ma, J.N.; Tu, Y. Rumen fermentation characteristics in pre- and post-weaning calves upon feeding with mulberry leaf flavonoids and Candida tropicalis individually or in combination as a supplement. Animals 2019, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, C.T.; Diao, Q.Y.; Tu, Y. The influence of mulberry leaf flavonoids and Candida tropicalis on antioxidant function and gastrointestinal development of preweaning calves challenged with Escherichia coli O141:K99. J. Dairy Sci. 2018, 101, 6098–6108. [Google Scholar] [CrossRef] [PubMed]
- Castillo-González, A.R.; Burrola-Barraza, M.E.; Viveros, J.; Chavez-Martinez, A. Rumen microorganisms and fermentation. Arch. de Med. Veter- 2013, 46, 349–361. [Google Scholar] [CrossRef]
- Diao, Q.; Zhang, R.; Fu, T. Review of strategies to promote rumen development in calves. Animals 2019, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Berends, H.; van Reenen, C.G.; Stockhofe-Zurwieden, N.; Gerrits, W.J. Effects of early rumen development and solid feed composition on growth performance and abomasal health in veal calves. J. Dairy Sci. 2012, 95, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Wang, X.; Pang, X.; Liu, G. Effects of supplementary feeding on the rumen morphology and bacterial diversity in lambs. PeerJ 2020, 8, e9353. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.D.; Wanapat, M.; Uriyapongson, S.; Cherdthong, A.; Pilajun, R. Enhancing mulberry leaf meal with urea by pelleting to improve rumen fermentation in cattle. Asian Australas. J. Anim. Sci. 2012, 25, 452–461. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Shen, Q.M.; Zhong, S.; Chen, Y.L.; Yang, Y.X. Comparison of rumen microbiota and serum biochemical indices in white cashmere goats fed ensiled or sun-dried mulberry leaves. Microorganisms 2020, 8, 981. [Google Scholar] [CrossRef]
- Wang, B.; Luo, H. Effects of mulberry leaf silage on antioxidant and immunomodulatory activity and rumen bacterial community of lambs. BMC Microbiol. 2021, 21, 250. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Mei, J.; Huang, L.; Liu, H. Effects of mulberry branch and leaves silage on microbial community, rumen fermentation characteristics, and milk yield in lactating dairy cows. Fermentation 2022, 8, 86. [Google Scholar] [CrossRef]
- Welte, C.U. Revival of archaeal methane microbiology. mSystems 2018, 3, e00181-17. [Google Scholar] [CrossRef] [PubMed]
- Borrel, G.; Adam, P.S.; McKay, L.J.; Chen, L.X.; Sierra-García, I.N.; Sieber, C.M.K.; Letourneur, Q.; Ghozlane, A.; Andersen, G.L.; Li, W.J.; et al. Wide diversity of methane and short-chain alkane metabolisms in uncultured archaea. Nat. Microbiol. 2019, 4, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Shekhawat, I.; Ujor, V.; Ezeji, T.; Lakritz, J.; Lal, R. Global climate change: Enteric methane reduction strategies in livestock. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S.M.K., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 469–499. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Tu, Y.; Wang, B.; Lou, C.; Ma, T.; Diao, Q. Effects of mulberry leaf flavonoid and resveratrol on methane emission and nutrient digestion in sheep. Anim. Nutr. 2015, 1, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, D.D.; Tu, Y.; Zhang, N.F.; Si, B.W.; Diao, Q.Y. Dietary supplementation with mulberry leaf flavonoids inhibits methanogenesis in sheep. Anim. Sci. J. 2017, 88, 72–78. [Google Scholar] [CrossRef]
- Liu, J.X.; Yao, J.; Yan, B.; Yu, J.Q.; Shi, Z.Q. Effects of mulberry leaves to replace rapeseed meal on performance of sheep feeding on ammoniated rice straw diet. Small Rumin. Res. 2001, 39, 131–136. [Google Scholar] [CrossRef]
- Salinas-Chavira, J.; Castillo-Martínez, O.; Ramirez-Bribiesca, J.E.; Mellado, M. Effect of increasing levels of white mulberry leaves (Morus alba) on ruminal dry matter degradability in lambs. Trop. Anim. Health Prod. 2011, 43, 995–999. [Google Scholar] [CrossRef]
- Doran, M.P.; Laca, E.A.; Sainz, R.D. Total tract and rumen digestibility of mulberry foliage (Morus alba), alfalfa hay and oat hay in sheep. Anim. Feed. Sci. Technol. 2007, 138, 239–253. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Zhou, B.; Ren, L.P.; Meng, Q.X. Effect of ensiled mulberry leaves and sun-dried mulberry fruit pomace on finishing steer growth performance, blood biochemical parameters, and carcass characteristics. PLoS ONE 2014, 9, e85406. [Google Scholar] [CrossRef]
- Niu, Y.H.; Meng, Q.X.; Li, S.L.; Ren, L.P.; Zhou, B.; Schonewille, T.; Zhou, Z.M. Effects of diets supplemented with ensiled mulberry leaves and sun-dried mulberry fruit pomace on the ruminal bacterial and archaeal community composition of finishing steers. PLoS ONE 2016, 11, e0156836. [Google Scholar] [CrossRef]
- Yulistiani, D.; Jelan, Z.A.; Liang, J.B.; Yaakub, H.; Abdullah, N. Effects of supplementation of mulberry (Morus alba) foliage and urea-rice bran as fermentable energy and protein sources in sheep fed urea-treated rice straw based diet. Asian Australas. J. Anim. Sci. 2015, 28, 494–501. [Google Scholar] [CrossRef][Green Version]
- David, C.M.G.; Costa, R.L.D.D.; Parren, G.A.E.; Rua, M.A.S.; Nordi, E.C.P.; Okamoto, F.; Paz, C.C.P. Sugarcane and mulberry silage supplementation of sheep during the peripartum period. Trop. Anim. Health Prod. 2015, 47, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Alpízar-Naranjo, A.; Arece-García, J.; Esperance, M.; López, Y.; Molina, M.; González-García, E. Partial or total replacement of commercial concentrate with on-farm-grown mulberry forage: Effects on lamb growth and feeding costs. Trop. Anim. Health Prod. 2017, 49, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, Y.; Zhao, F.; Fan, Y.; Ma, J.; Jin, Y.; Hou, Q.; Ahmed, G.; Wang, H. The effect of replacing wildrye hay with mulberry leaves on the growth performance, blood metabolites, and carcass characteristics of sheep. Animals 2020, 10, 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Qu, P.B.; Tu, Y.; Yang, C.T.; Diao, Q.Y. Effects of flavonoids from mulberry leaves and Candida tropicalis on performance and nutrient digestibility in calves. Kafkas Üniversitesi Vet. Fakültesi Derg. 2017, 23, 473–479. [Google Scholar] [CrossRef]
- Si, B.W.; Tao, H.; Zhang, X.L.; Guo, J.P.; Cui, K.; Tu, Y.; Diao, Q.Y. Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian Australas. J. Anim. Sci. 2018, 31, 1259–1266. [Google Scholar] [CrossRef]
- Hao, Y.Y.; Huang, S.; Si, J.F.; Zhang, J.; Gaowa, N.; Sun, X.G.; Lv, J.Y.; Liu, G.K.; He, Y.Q.; Wang, W.; et al. Effects of paper mulberry silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in holstein dairy cows. Animals 2020, 10, 1152. [Google Scholar] [CrossRef]
- Ouyang, J.; Hou, Q.; Wang, M.; Zhao, W.; Feng, D.; Pi, Y.; Sun, X. Effects of dietary mulberry leaf powder on growth performance, blood metabolites, meat quality and antioxidant enzyme related gene expression of fattening Hu lambs. Can. J. Anim. Sci. 2020, 100, 510–521. [Google Scholar] [CrossRef]
- Roothaert, R.L. Feed intake and selection of tree fodder by dairy heifers. Anim. Feed Sci. Technol. 1999, 79, 1–13. [Google Scholar] [CrossRef]
- Venkatesh, K.R.; Gautam, C.; Shobha, N.; Shankar, R.L. Use of mulberry leaves as supplementary food in cow and goat to improve milk production. Int. J. Appl. Res. 2015, 1, 81–84. [Google Scholar]
- Jeon, B.T.; Kim, K.H.; Kim, S.; Kim, D.H.; Kim, E.T.; Cho, W.-m.; Hwang, I.; Choi, N.J.; Moon, S.-H. Effects of mulberry (Morus alba L) silage supplementation on the haematological traits and meat compositions of Hanwoo (Bos taurus coreanae) steer. Afr. J. Biomed. Res. 2012, 7, 662–668. [Google Scholar] [CrossRef]
- Fu, Y.W.; Zhang, Q.Z.; Xu, D.H.; Xia, H.; Cai, X.X.; Wang, B.; Liang, J.H. Parasiticidal effects of Morus alba root bark extracts against Ichthyophthirius multifiliis infecting grass carp. Dis. Aquat. Org. 2014, 108, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Fu, Y.W.; Zhang, Q.Z.; Xu, D.H.; Wang, B.; Lin, D.J. Identification and effect of two flavonoids from root bark of Morus alba against Ichthyophthirius multifiliis in grass carp. J. Agric. Food Chem. 2015, 63, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Jeong, J.W.; Choi, E.O.; Lee, H.W.; Lee, K.W.; Kim, K.Y.; Kim, S.G.; Hong, S.H.; Kim, G.Y.; Park, C.; et al. Inhibitory effects on the production of inflammatory mediators and reactive oxygen species by Mori folium in lipopolysaccharide-stimulated macrophages and zebrafish. An. Acad. Bras. Cienc. 2017, 89, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Ergün, S.; Yigit, M.; Yilmaz, E.; Ahmadifar, E. Dietary supplementation of black mulberry (Morus nigra) syrup improves the growth performance, innate immune response, antioxidant status, gene expression responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 107, 211–217. [Google Scholar] [CrossRef]
- Neamat-Allah, A.N.F.; Mahmoud, E.A.; Mahsoub, Y. Effects of dietary white mulberry leaves on hemato-biochemical alterations, immunosuppression and oxidative stress induced by Aeromonas hydrophila in Oreochromis niloticus. Fish Shellfish Immunol. 2021, 108, 147–156. [Google Scholar] [CrossRef]
- Tang, T.; Bai, J.; Ao, Z.; Wei, Z.; Hu, Y.; Liu, S. Effects of dietary paper mulberry (Broussonetia papyrifera) on growth performance and muscle quality of grass carp (Ctenopharyngodon idella). Animals 2021, 11, 1655. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Li, H.Y.; Qiu, N.; Su, L.X.; Huang, Z.L.; Song, L.R.; Wang, J.W. Bioconcentration and depuration of cadmium in the selected tissues of rare minnow (Gobiocypris rarus) and the effect of dietary mulberry leaf supplementation on depuration. Environ. Toxicol. Pharmacol. 2020, 73, 103278. [Google Scholar] [CrossRef]
| Authors | Species | Source | Country | Season | Fe | Zn | Ca | Mg, | K | Na | Mn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ercisli et al., 2007 | Morus alba L. | Fresh | Turkey | - | 4.2 | - | 152 | 106 | 1668 | - | - | - |
| Morus rubra | 4.2 | - | 132 | 106 | 922 | - | - | - | ||||
| Morus nigra | 4.2 | - | 132 | 115 | 834 | - | - | - | ||||
| Koca et al., 2008 | Morus latifolia | Fresh | Turkey | - | 2.85 | 0.52 | 88.9 | 19.4 | 210.75 | 11.89 | 0.35 | 0.31 |
| Akbulut et al., 2009 | Morus nigra | Freeze-dried | Turkey | - | 3.72 | 0.96 | 304.4 | 95.9 | 1086.0 | 11.5 | - | 0.41 |
| Morus rubra | 1.22 | 1.16 | 443.7 | 103.3 | 1526.9 | 21.8 | - | 0.65 | ||||
| Morus alba L. | 5.29 | 0.90 | 292.7 | 90.4 | 1310.2 | 21.5 | - | 0.68 | ||||
| Sheng et al., 2009 | - | Fresh | China | - | 1.17 | 0.14 | 38.89 | 12.21 | 234.65 | 16.1 | 0.03 | 0.04 |
| Imran et al., 2010 | Morus alba L. | Fresh | Pakistan | Spring | 73.0 | 50.20 | 576 | 240 | 1731 | 280 | - | - |
| Morus nigra | 77.6 | 59.20 | 470 | 240 | 1270 | 272 | - | - | ||||
| Morus laevigata (Large white fruit) | 48.6 | 53.40 | 440 | 360 | 1650 | 260 | - | - | ||||
| Morus laevigata (Large black fruit) | 63.6 | 50.80 | 576 | 240 | 1644 | 264 | - | - | ||||
| Altundag et al. 2011 | Morus alba L. | Dry | Turkey | - | 37.45 | 4.47 | - | - | - | - | 4.36 | 1.28 |
| Wet | 40.80 | 4.38 | - | - | - | - | 4.25 | 1.31 | ||||
| Microwave | 36.17 | 3.78 | - | - | - | - | 4.16 | 1.23 | ||||
| Sánchez-Salcedo et al., 2015 | Morus alba | Fresh | Italy | - | 2.82–4.67 | 1.49–1.96 | 190–370 | 120–190 | 1620–2130 | 10 | 1.23–1.94 | 0.45–0.64 |
| Morus nigra | 2.39–3.71 | 1.57–2.25 | 210–430 | 130–190 | 1480–2170 | 10 | 1.23–1.81 | 0.28–0.52 | ||||
| Jiang et al., 2015 | Morus alba L. | Fresh | China | Summer | 6.96 | 0.21 | 71 | 32.5 | 239 | 6.2 | 0.31 | 0.10 |
| M. alba var. tatarica L. | 11.40 | 0.32 | 124 | 55.8 | 350 | 6.5 | 0.70 | 0.13 | ||||
| Morus nigra | 11.90 | 0.10 | 113 | 36.9 | 297 | 5.9 | 0.40 | 0.10 | ||||
| Sun et al., 2018 | - | Fresh | China | Spring | 7.72–30.13 | 4.06–10.58 | 180.61–423.30 | 13.96–33.38 | 87.70–208.44 | - | - | 0.04–0.50 |
| Authors | Species | Parts | Sources | Animal | Age of Animals | Dose or Concentration | Main Effects |
|---|---|---|---|---|---|---|---|
| Jang et al., 2008 | - | Leaves | Mixed herbs containing mulberry leaf | Broiler | 1–35 d | 0.3 and 1.0% | Increased the antioxidative potential and overall preference of breast meat during cold storage |
| Islam et al., 2015 | Morus alba L. | Leaves | Mulberry leaf powders | Broiler | 1–42 d | 2.5, 3.5, and 4.5% | Improved the performances and had a positive effect on the serum lipids |
| Mulberry leaf powder extract | 3.50% | ||||||
| Lin et al., 2017 | Morus latifolia | Leaves | Mulberry leaves | Laying hens | 22–34 weeks | 0.5, 1, and 2% | Modulated the antioxidative status of laying hens and improved their production performance and egg quality |
| Chen et al., 2019c | - | Leaves | Mulberry leaf powders | Broiler | 120–157 d | 4% | Changed the composition of the gut microbiota |
| Chen et al., 2019b | Morus alba L. | Leaves | Mulberry leaf extract | Egg Chickens | 14–21 d | Orally administered 0.5, 1, 2, 4, 8, and 12 mg | Increased the serum Newcastle disease antibody titers, enhanced the immune effect of the vaccine, caused significant weight gain, and enhanced the intestinal and tracheal mucosal immune function |
| Ding et al., 2021 | - | Leaves | Fermented mulberry leaf powder | Broilers | 1–56 d | 3, 6, and 9% | Improved the digestion and absorption of nutrients, growth performance, slaughter performance, and meat quality |
| Huang et al., 2021 | - | Leaves | Mulberry leaf extract | Hens | 60–68 weeks | 0, 30, and 60 mg/kg | Improved the reproduction performance of aged breeder hens through improving the ovary function and hepatic lipid metabolism |
| Huang et al., 2022 | - | Leaves | Mulberry leaf extract | Hens | 60–68 weeks | 0, 30, and 60 mg/kg | Ameliorated the eggshell quality of aged hens by improving the antioxidative capability and Ca deposition in the shell gland of the uterus |
| Authors | Species | Leaves | Sources | Animal | Test Duration | Dose or Concentration | Main Effects |
|---|---|---|---|---|---|---|---|
| Li et al., 2012 | - | Leaves | Mulberry leaf | Finishing pigs | 65 d | 10 and 20% | Does not affect the growth rate of finishing pigs, it can decrease the leaf lard percentage and back fat thickness, increase the contents of inosinic acid and fat in muscle |
| Song et al., 2016 | - | Leaves | Mulberry leaf powder | Finishing pigs | 50 d | 5, 10, 15, and 20% | Has less effect on the growth rate of finishing pigs, while it can improve the meat quality |
| Zhang et al., 2016 | - | - | Fermented feed mulberry powder | Fattening pigs | 66 d | 15% | Increased the average daily gain of pigs, increased the content of unsaturated fatty acids in pork, reduced the content of saturated fatty acids to a certain extent, improved the quality of pork, and realized the health care function of pork |
| Liu et al., 2019 | Morus latifolia | Leaves | Mulberry leaves | Finishing pigs | 50 d | 3, 6, 9, and 12% | Mulberry in the diet at <12% is an effective feed crop to improve the meat quality and the chemical composition of the muscle without negatively affecting the growth performance |
| Chen et al., 2019a | - | Leaves | Mulberry leaves | Finishing pigs | 67 d | 3, 6, and 9% | May improve the muscle quality of pigs by modulating the expression of several key genes |
| Zeng et al., 2019 | Morus alba L. | Leaves | Mulberry leaves | Finishing pigs | 85 d | 15% | Reduced the growth performance and carcass traits, but improved the meat quality of finishing pigs possibly through the change of the myofiber characteristics, the enhancement of the antioxidative capacity and the increase of intramuscular fat |
| Ding et al., 2019 | Forage Mulberry | Forage mulberry | Fermented forage mulberry powder | Ningxiang Pigs | 75 d | 9, 12, and 15% | Improved the antioxidant performance, ameliorated the intestinal micro environment, and reduced the negative effects of mulberry powder on the intestine |
| Chen et al., 2020 | Broussonetia papyrifera | Leaves | Paper mulberry leaf extract | Weaned Piglets | 15 d | 150 and 300 g/t | Increased the growth performance and the antioxidant capacity, reduced the occurrence of diarrhea, enhanced the immune functions and disease resistance, and affected the composition of fecal microflora |
| Fan et al., 2020a | Morus nigra L. | Leaves | Non- or fermented mulberry leaf powder | Finishing pigs | 45 d | 5% | Promoted the feed conversion ratio, reduced the backfat thickness, increased the fat deposition in the muscle, and reduced the rancidity of pork |
| Fan et al., 2020b | Morus nigra L. | Leaves | Mulberry leaves | Finishing pigs | 45 d | 5% | Have obvious anti-obesity effects |
| Liu et al., 2021 | - | Leaves | Mulberry leaf powder | Finishing pigs | 50 d | 3, 6, 9, and 12% | Enhanced the serum antioxidant property, increased the polyunsaturated fatty acid content, and inhibited lipid oxidation by regulating gene expression levels of lipid metabolism and mitochondrial uncoupling protein in muscle tissue |
| Chen et al., 2021 | Morus alba L. | Leaves | Mulberry leaves | Finishing pigs | 31 d | 4% | Promoted the growth performance, improved carcass traits, better quality of pork, and improved meat flavor |
| Authors | Species | Parts | Sources | Animal | Test Duration | Dose or Concentration | Main Effects |
|---|---|---|---|---|---|---|---|
| Roothaert et al., 1999 | Morus alba | Leaves and succulent twigs | Mulberry leaves and succulent twigs | Dairy heifers | 14 d | Supplements at 25% of the estimated daily dry matter intake | Had a higher voluntary intake and a higher potential of milk production than the cassava tree (Manihot glaziovii), and fresh leucaena (Leucaena diversifolia) |
| Liu et al., 2001 | Morus alba | Leaves | Mulberry leaves | Sheep | 75 d | Replace 25, 50, 75, and 100% rapeseed meal | Mulberry leaves may be used as a proteinsupplement to ammoniated straw diets to fully substitute for rapeseed meal |
| Doran et al., 2007 | Morus alba L. | Leaves | Mulberry leaves | Sheep | 14 d | 20 g DM/kg BW per day | Mulberry appears to be an excellent forage with many qualities, comparable and in some cases, superior to alfalfa. In addition, the combination of mulberry foliage with such a low-quality roughage as oat hay could produce a positive interaction on the protein and neutral detergent fiber digestibility |
| Kandylis et al., 2009 | Morus latifolia | Leaves | Mulberry leaves | Sheep | 19 d | Fed the prescribed ration | Mulberry leaves have an appreciable potential as a protein source in sheep feeding |
| Vu et al., 2011 | Morus alba L. | Leaves | Mulberry leaves | Cattle | 15 d | 5, 10, and 15% | Improved digestibility of the crude protein and organic matter |
| Salinas-Chavira, et al., 2011 | Morus alba L. | Leaves | Mulberry leaves | Lambs | 10 d | 2.5% or 5% | Partly replace the expensive protein-rich ingredients, such as oilseed cakes and help increase the profitability of lamb fattening |
| Tan et al., 2012 | - | Leaves and stems | Mulberry leaf meal | Beef cattle | 84 d | 0, 200, 400 and 600 g/hd/d | Improved the dry matter intake, ruminal NH3-N, and cellulolytic bacteria thus improved the rumen ecology in beef cattle fed with rice straw |
| Jeon et al., 2012 | Morus alba L. | Leaves and stems | Mulberry silage | Hanwoo (Bos taurus coreanae) steer | 36 d | 10% | Improved the hematological traits and composition of fatty acids and amino acids |
| Zhou et al., 2014 | - | Leaves | Ensiled mulberry leaves | Finishing steer | 112 d | 8% | Had similar effects to corn grain and cottonseed meals on the steer performance, blood biochemical parameters, and carcass characteristics, with the exception of ruminal VFA concentrations and lower intramuscular fat content |
| Fruit | Sun-dried mulberry fruit | 6.30% | |||||
| Yulistiani et al., 2015 | Morus alba L. | Leaves | Mulberry leaves | Sheep | 22 d | 50% or 100% mulberry replaced with rice bran and urea | Had a similar effect to urea rice bran supplementation on the dry matter intake, nutrient digestibility and N utilization that create an efficient rumen ecosystem and microbial protein supply |
| David et al., 2015 | - | Mulberry branches | Mulberry silage | Sheep | 150 d | 100% sugarcane silage, 75% sugarcane silage plus 25% mulberry branches silage, and 50% sugarcane with 50% mulberry branches silage | The protein in mulberry silage replaced the protein offered in the concentrate, making this mulberry silage a cost-effective alternative |
| Venkatesh et al., 2015 | - | Leaves | Mulberry leaves | Goat and cow | 60 d | Fed a basal diet supplemented daily with mulberry leaf flavonoids (5.0%, w/w; 3 g/calf) and then orally challenged with E. coli K99 | Enhanced the quality and quantity of goat and cow’s milk |
| Niu et al., 2016 | Leaves | Ensiled mulberry leaves | Finishing steers | - | 8% | The partial replacement of corn grain and cottonseed meal with ensiled mulberry leaves or sundried mulberry fruit pomace had no substantial effect on the ruminal microflora composition | |
| Fruit | Sundried mulberry fruit pomace | 6.30% | |||||
| Bi et al., 2017 | - | Leaves | Mulberry leaf extract | Pre-weaned calves | 36 d | Fed a basal diet supplemented daily with mulberry leaf flavonoids (5.0%, w/w; 3 g/calf) and then orally challenged with E. coli K99 (30 mL; 1.0 × 109 CFU/mL) | Had a higher average daily weight gain and feed efficiency, reduced days of diarrhea, improved intestinal health, and beneficially manipulated the intestinal microbiota in pre-weaned calves |
| Ma et al., 2017 | - | Leaves | Mulberry leaf extract | Sheep | 14 d | 2 g/head/day | Improved the digestibility of the organic matter and reduced the enteric methane (CH4) output by inhibiting the populations ofmicrobes involved in methanogenesis |
| 42 d | |||||||
| Alpízar-Naranjo et al., 2017 | Morus alba L. | - | Mulberry foliage | Lambs | 84 d | Inclusion of MLP at 0.75 and 1%in a concentrate diet | Replaced the imported concentrate while increasing the level of substitution of mulberry in the ration of fattening lambs |
| Zhang et al., 2017 | - | Leaves | Mulberry leaf extract | Calves | 60 d | Starter at 2 g/d per calf before weaning, or 4 g/d per calf after weaning | Increased growth performance and nutrient digestibility |
| Wang et al., 2018 | Morus alba L. | Leaves | Mixed extract of mulberry leaf | Preweaning calves | 35 d | 3 g/calf per day | Improve the antioxidant function and reduce the incidence of oxidative stress after challenge with E. coli in 28-d-old preweaning calves |
| Ouyang et al., 2019 | - | Leaves | Mulberry leaf powder | Fattening Hu sheep | 84 d | 0, 15, 30, 45, and 60% | Promoted the nutrient digestibility in the rumen and promoted the development and the metabolic properties of the rumen epithelium |
| Kong et al. 2019 | Morus alba L. | Leaves and stems | Mulberry leaf extract | Calves | 60 d | 3 g/d | Increased the proportion of propionate among rumen fermentation products, increased the growth performance and decreased the fecal scores |
| Mengistu et al., 2020 | Morus indica | Leaves | Mixed meal containing mulberry leaf | Sheep | 90 d | The dietary treatments were a replacement of the protein in noug seed cake of concentrate mix at 25, 50, 75, and 100% proportions with dried mulberry and vernonia mixed leaves’ meal | Resulted in the optimum performances in terms of feed intakes, digestibility of the feeds, and the growth performances |
| Wang et al., 2020 | - | Leaves | Ensiled mulberry leaves | Goats | 70 d | 10, 15, and 20% | Keeps rumen healthy (changes in the ruminal microbiota and fermentation parameters) and results in a potential positive impact on the host health |
| Sun-dried mulberry leaves | 10 and 15% | ||||||
| Sun et al., 2020 | - | Leaves | Mulberry leaves | Sheep | 65 d | 8, 24 and 32% replace Chinese wildrye | Had a beneficial influence on the growth performance, blood metabolites, and carcass characteristics |
| Hao et al., 2020 | Broussonetia papyrifera | Leaves and stems | Paper mulberry silage | Holstein dairy cows | 28 d | 4.5 and 9.0% | Enhanced the antioxidant capacity and immunity of dairy cows, but did not influence the milk yield, dry matter digestibility, and fecal bacteria composition |
| Wang et al., 2021 | Morus alba L. | Leaves | Mulberry leaf silage | Lambs | 84 d | 20% mulberry leaf silage | Improved the antioxidant capacity and immune function, and modified the rumen microbial community |
| Li et al., 2022 | - | Branch and leaves | Mulberry branch and leaves silage | Cows | 56 d | 5 and 10% | Modulated the rumen microbiota and fermentation, increased the abundance of fiber-digesting, propionic acid synthesis, and milk fat-related microorganisms, thus improving the milk yield in dairy cows |
| Authors | Species | Parts | Sources | Animal | Test Duration | Dose or Concentration | Main Effects |
|---|---|---|---|---|---|---|---|
| Fu et al., 2014 | Morus alba L. | Root bark | Mulberry root bark extract | Grass carp | 96 h | 2,4, and 8 mg/L | Protected fish from I. multifiliis infection |
| Liang et al., 2015 | Morus alba L. | Root bark | Mulberry root bark extract | Grass carp | 96 h | 0.125, 0.25, 0.5, 1, and 2 mg/L | Controls Ichthyophthirius multifiliis |
| Kwon et al., 2017 | Morus alba L. | Leaves | Mulberry leaves | Zebrafish larvae | - | 800 μg/mL | Exerted potent anti-inflammatory and antioxidant effects in zebrafish |
| Yilmaz et al., 2020 | Morus latifolia | - | Mulberry extract | Nile tilapia (Oreochromis niloticus) | 60 d | 0.75, 1.5, 2.0, and 3.0% | Improved the growth performance, innate immune parameters, antioxidant related gene expression responses, and disease resistance against Aeromonas veronii |
| Xiong et al., 2020 | - | Leaves | Mulberry leaves | Rare minnow (Gobiocypris rarus) | 28 d | 10 and 30% | Decreased the Cd residues in the liver |
| Neamat-Allah et al., 2021 | Morus alba L. | Leaves | Mulberry leaves | Oreochromis niloticus | 30 d | 1, 3 and 5 g/kg | Protected tilapias from hemato-biochemical alterations and enhanced its immune feedback, antioxidant defense, and resistance against A. hydrophila |
| Tang et al., 2021 | Broussonetia papyrifera | - | Mulberry stems and leaves | Grass Carp (Ctenopharyngodon idella) | 56 d | 5, 10, 15, and 20% | Improved the muscle quality through improving the muscle hardness, reducing the fat accumulation, and the muscle fiber diameter, at the cost of reducing growth performance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Gao, H.; Sun, C.; Huang, L. Protective Application of Morus and Its Extracts in Animal Production. Animals 2022, 12, 3541. https://doi.org/10.3390/ani12243541
Wang L, Gao H, Sun C, Huang L. Protective Application of Morus and Its Extracts in Animal Production. Animals. 2022; 12(24):3541. https://doi.org/10.3390/ani12243541
Chicago/Turabian StyleWang, Lixue, Huaqi Gao, Cui Sun, and Lingxia Huang. 2022. "Protective Application of Morus and Its Extracts in Animal Production" Animals 12, no. 24: 3541. https://doi.org/10.3390/ani12243541
APA StyleWang, L., Gao, H., Sun, C., & Huang, L. (2022). Protective Application of Morus and Its Extracts in Animal Production. Animals, 12(24), 3541. https://doi.org/10.3390/ani12243541






