Do Spiders Ride on the Fear of Scorpions? A Cross-Cultural Eye Tracking Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
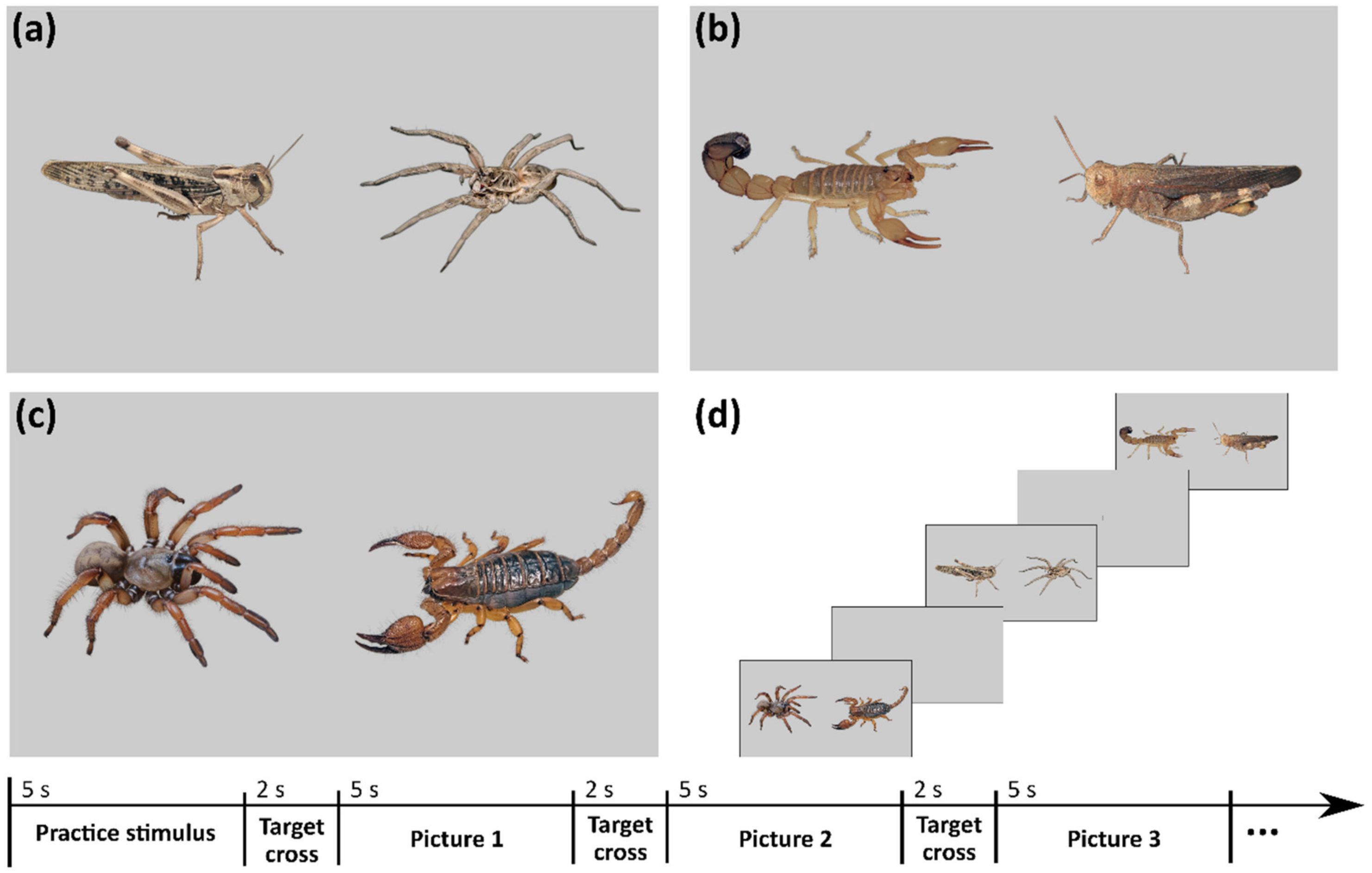
2.1. Selection and Preparation of the Stimuli
2.2. The Experimental Procedure
2.3. Pilot Experiment
2.4. Participants
2.5. Data Extraction and Curation
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. Biophilia; Harvard Univesity Press: Cambridge, UK, 1984. [Google Scholar]
- Ulrich, R.S. Biophilia, biophobia, and natural landscapes. In The Biophilia Hypothesis, 1st ed.; Kellert, S.R., Wilson, E.O., Eds.; Island Press: Washington, DC, USA, 1993; pp. 73–137. [Google Scholar]
- Patuano, A. Biophobia and urban restorativeness. Sustainability 2020, 12, 4312. [Google Scholar] [CrossRef]
- Seligman, M.E. Phobias and preparedness. Behav. Ther. 1971, 2, 307–320. [Google Scholar] [CrossRef]
- New, J.J.; German, T.C. Spiders at the cocktail party: An ancestral threat that surmounts inattentional blindness. Evol. Hum. Behav. 2015, 36, 165–173. [Google Scholar] [CrossRef]
- Gao, H.; Jia, Z. Detection of threats under inattentional blindness and perceptual load. Curr. Psychol. 2017, 36, 733–739. [Google Scholar] [CrossRef]
- Bannerman, R.L.; Milders, M.; De Gelder, B.; Sahraie, A. Orienting to threat: Faster localization of fearful facial expressions and body postures revealed by saccadic eye movements. Proc. R. Soc. B Biol. Sci. 2009, 276, 1635–1641. [Google Scholar] [CrossRef]
- Fox, E.; Russo, R.; Bowles, R.; Dutton, K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J. Exp. Psychol. Gen. 2001, 130, 681–700. [Google Scholar] [CrossRef]
- Bjärtå, A.; Flykt, A.; Sundin, Ö. The effect of using different distractor sets in visual search with spiders and snakes on spider-sensitive and nonfearful participants. Swiss J. Psychol. 2013, 72, 171–179. [Google Scholar] [CrossRef]
- Calvillo, D.P.; Hawkins, W.C. Animate objects are detected more frequently than inanimate objects in inattentional blindness tasks independently of threat. J. Gen. Psychol. 2016, 143, 101–115. [Google Scholar] [CrossRef]
- Calvillo, D.P.; Jackson, R.E. Animacy, perceptual load, and inattentional blindness. Psychon. B. Rev. 2014, 21, 670–675. [Google Scholar] [CrossRef]
- Gerdes, A.B.; Alpers, G.W.; Pauli, P. When spiders appear suddenly: Spider-phobic patients are distracted by task-irrelevant spiders. Behav. Res. Ther. 2008, 46, 174–187. [Google Scholar] [CrossRef]
- Yorzinski, J.L.; Penkunas, M.J.; Platt, M.L.; Coss, R.G. Dangerous animals capture and maintain attention in humans. Evol. Psychol. 2014, 12, 534–548. [Google Scholar] [CrossRef]
- Isbell, L.A. Snakes as agents of evolutionary change in primate brains. J. Hum. Evol. 2006, 51, 1–35. [Google Scholar] [CrossRef]
- Jensen, C.H.; Caine, N.G. Preferential snake detection in a simulated ecological experiment. Am. J. Phys. Anthropol. 2021, 175, 895–904. [Google Scholar] [CrossRef]
- Shibasaki, M.; Kawai, N. Rapid detection of snakes by Japanese monkeys (Macaca fuscata): An evolutionarily predisposed visual system. J. Comp. Psychol. 2009, 123, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.C.; Lindström, B.; Esteves, F.; Öhman, A. The hidden snake in the grass: Superior detection of snakes in challenging attentional conditions. PLoS ONE 2014, 9, e114724. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, J.W.; Isbell, L.A. Snake scales, partial exposure, and the Snake Detection Theory: A human event-related potentials study. Sci. Rep. 2017, 7, 46331. [Google Scholar] [CrossRef] [PubMed]
- Wombolt, J.R.; Caine, N.G. Patterns on serpentine shapes elicit visual attention in marmosets (Callithrix jacchus). Am. J. Primatol. 2016, 78, 928–936. [Google Scholar] [CrossRef]
- Bjerke, T.; Ødegårdstuen, T.S.; Kaltenborn, B.P. Attitudes toward animals among Norwegian children and adolescents: Species preferences. Anthrozoös 1998, 11, 227–235. [Google Scholar] [CrossRef]
- Oosterink, F.M.; De Jongh, A.; Hoogstraten, J. Prevalence of dental fear and phobia relative to other fear and phobia subtypes. Eur. J. Oral Sci. 2009, 117, 135–143. [Google Scholar] [CrossRef]
- Staňková, H.; Janovcová, M.; Peléšková, Š.; Sedláčková, K.; Landová, E.; Frynta, D. The Ultimate List of the Most Frightening and Disgusting Animals: Negative Emotions Elicited by Animals in Central European Respondents. Animals 2021, 11, 747. [Google Scholar] [CrossRef]
- Shibasaki, M.; Kawai, N. Visual searching for fear-relevant stimuli: Snakes draw our attention more strongly than spiders do. Cogn. Stud. B Jpn. Cogn. Sci. Soc. 2011, 18, 158–172. [Google Scholar]
- Soares, S.C.; Esteves, F. A glimpse of fear: Fast detection of threatening targets in visual search with brief stimulus durations. PsyCh J. 2013, 2, 11–16. [Google Scholar] [CrossRef]
- He, H.; Kubo, K.; Kawai, N. Spider is not special comparing with other animals in human early visual attention: Evidence from event-related potentials. JCSS Jpn. Cong. Sci. Soc. 2014, 31, 187–190. [Google Scholar]
- Van Strien, J.W.; Christiaans, G.; Franken, I.H.; Huijding, J. Curvilinear shapes and the snake detection hypothesis: An ERP study. Psychophysiology 2016, 53, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Kawai, N.; Koda, H. Japanese monkeys (Macaca fuscata) quickly detect snakes but not spiders: Evolutionary origins of fear-relevant animals. J. Comp. Psychol. 2016, 130, 299–303. [Google Scholar] [CrossRef]
- Kawai, N. Do Snakes Draw Attention More Strongly than Spiders or Other Animals? In The Fear of Snakes; Springer: Singapore, 2019; pp. 73–94. [Google Scholar] [CrossRef]
- Polák, J.; Rádlová, S.; Janovcová, M.; Flegr, J.; Landová, E.; Frynta, D. Scary and nasty beasts: Self-reported fear and disgust of common phobic animals. Br. J. Psychol. 2020, 111, 297–321. [Google Scholar] [CrossRef]
- Hauke, T.J.; Herzig, V. Dangerous arachnids—Fake news or reality? Toxicon 2017, 138, 173–183. [Google Scholar] [CrossRef]
- Herman, B.E.; Skokan, E.G. Bites that poison: A tale of spiders, snakes, and scorpions. Contemp. Pediatr. 1999, 16, 41. [Google Scholar]
- Nentwig, W. Human health impact by alien spiders and scorpions. In Invasive Species and Human Health; Mazza, G., Tricarico, E., Eds.; CABI: Oxfordshire, UK, 2018; pp. 34–49. ISBN 1786390981. [Google Scholar]
- World Health Organization. Snakebite Envenoming. Available online: http://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 15 February 2022).
- Askew, C.; Field, A.P. Vicarious learning and the development of fears in childhood. Behav. Res. Ther. 2007, 45, 2616–2627. [Google Scholar] [CrossRef]
- Davey, G.C. The “disgusting” spider: The role of disease and illness in the perpetuation of fear of spiders. Soc. Anim. 1994, 2, 17–25. [Google Scholar] [CrossRef]
- Frynta, D.; Janovcová, M.; Štolhoferová, I.; Peléšková, Š.; Vobrubová, B.; Frýdlová, P.; Skalíková, H.; Šípek, P.; Landová, E. Emotions triggered by live arthropods shed light on spider phobia. Sci. Rep. 2021, 11, 22268. [Google Scholar] [CrossRef]
- Vetter, R.S.; Draney, M.L.; Brown, C.A.; Trumble, J.T.; Gouge, D.H.; Hinkle, N.C.; Pace-Schott, E.F. Spider Fear Versus Scorpion Fear in Undergraduate Students at Five American Universities. Am. Entomol. 2018, 64, 79–82. [Google Scholar] [CrossRef]
- Landová, E.; Janovcová, M.; Štolhoferová, I.; Rádlová, S.; Frýdlová, P.; Sedláčková, K.; Frynta, D. Specificity of spiders among fear-and disgust-eliciting arthropods: Spiders are special, but phobics not so much. PLoS ONE 2021, 16, e0257726. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Emerging options for the management of scorpion stings. Drug Des. Dev. Ther. 2012, 6, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Muris, P.; du Plessis, M.; Loxton, H. Origins of common fears in South African children. J. Anxiety Disord. 2008, 22, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Prokop, P.; Tolarovičová, A.; Camerik, A.M.; Peterková, V. High school students’ attitudes towards spiders: A cross-cultural comparison. Int. J. Sci. Educ. 2010, 32, 1665–1688. [Google Scholar] [CrossRef]
- Lemelin, R.H.; Yen, A. Human-spider entanglements: Understanding and managing the good, the bad, and the venomous. Anthrozoös 2015, 28, 215–228. [Google Scholar] [CrossRef]
- Gerdes, A.B.; Pauli, P.; Alpers, G.W. Toward and away from spiders: Eye-movements in spider-fearful participants. J. Neural Transm. 2009, 116, 725–733. [Google Scholar] [CrossRef]
- Öhman, A.; Flykt, A.; Esteves, F. Emotion drives attention: Detecting the snake in the grass. J. Exp. Psychol. Gen. 2001, 130, 466–478. [Google Scholar] [CrossRef]
- Wiemer, J.; Gerdes, A.B.; Pauli, P. The effects of an unexpected spider stimulus on skin conductance responses and eye movements: An inattentional blindness study. Psychol. Res. 2013, 77, 155–166. [Google Scholar] [CrossRef]
- Berger, L.R.; Hawks, J.; Dirks, P.H.; Elliott, M.; Roberts, E.M. Homo naledi and Pleistocene hominin evolution in subequatorial Africa. eLife 2017, 6, e24234. [Google Scholar] [CrossRef] [PubMed]
- Hublin, J.J.; Ben-Ncer, A.; Bailey, S.E.; Freidline, S.E.; Neubauer, S.; Skinner, M.M.; Bergmann, I.; Le Cabec, A.; Benazzi, S.; Harvati, K.; et al. New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature 2017, 546, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Haile-Selassie, Y.; Gibert, L.; Melillo, S.M.; Ryan, T.M.; Alene, M.; Deino, A.; Levin, N.E.; Scott, G.; Saylor, B.Z. New species from Ethiopia further expands Middle Pliocene hominin diversity. Nature 2015, 521, 483–488. [Google Scholar] [CrossRef]
- Leakey, M.G.; Spoor, F.; Dean, M.C.; Feibel, C.S.; Antón, S.C.; Kiarie, C.; Leakey, L.N. New fossils from Koobi Fora in northern Kenya confirm taxonomic diversity in early Homo. Nature 2012, 488, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Papac, L.; Ernée, M.; Dobeš, M.; Langová, M.; Rohrlach, A.B.; Aron, F.; Neumann, G.U.; Spyrou, M.A.; Rohland, N.; Velemínský, P.; et al. Dynamic changes in genomic and social structures in third millennium BCE central Europe. Sci. Adv. 2021, 7, eabi6941. [Google Scholar] [CrossRef]
- Orquin, J.L.; Holmqvist, K. Threats to the validity of eye-movement research in psychology. Behav. Res. Methods 2018, 50, 1645–1656. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 April 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-155. Available online: https://CRAN.R-project.org/package=nlme (accessed on 23 January 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. _emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.8.2. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 17 November 2022).
- Armstrong, T.; Olatunji, B.O. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clin. Psychol. Rev. 2012, 32, 704–723. [Google Scholar] [CrossRef]
- Hermans, D.; Vansteenwegen, D.; Eelen, P. Eye movement registration as a continuous index of attention deployment: Data from a group of spider anxious students. Cogn. Emot. 1999, 13, 419–434. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hyönä, J.; Calvo, M.G. Eye movement assessment of selective attentional capture by emotional pictures. Emotion 2006, 6, 257–268. [Google Scholar] [CrossRef]
- Andersen, N.E.; Dahmani, L.; Konishi, K.; Bohbot, V.D. Eye tracking, strategies, and sex differences in virtual navigation. Neurobiol. Learn. Mem. 2012, 97, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Coutrot, A.; Binetti, N.; Harrison, C.; Mareschal, I.; Johnston, A. Face exploration dynamics differentiate men and women. J. Vis. 2016, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Sargezeh, B.A.; Tavakoli, N.; Daliri, M.R. Gender-based eye movement differences in passive indoor picture viewing: An eye-tracking study. Physiol. Behav. 2019, 206, 43–50. [Google Scholar] [CrossRef]
- McNally, R.J. The legacy of Seligman’s “phobias and preparedness” (1971). Behav. Ther. 2016, 47, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Åhs, F.; Rosén, J.; Kastrati, G.; Fredrikson, M.; Agren, T.; Lundström, J.N. Biological preparedness and resistance to extinction of skin conductance responses conditioned to fear relevant animal pictures: A systematic review. Neurosci. Biobehav. Rev. 2018, 95, 430–437. [Google Scholar] [CrossRef]
- Coelho, C.M.; Suttiwan, P.; Faiz, A.M.; Ferreira-Santos, F.; Zsido, A.N. Are humans prepared to detect, fear, and avoid snakes? The mismatch between laboratory and ecological evidence. Front. Psychol. 2019, 10, 2094. [Google Scholar] [CrossRef]
- Flykt, A.; Caldara, R. Tracking fear in snake and spider fearful participants during visual search: A multi-response domain study. Cogn. Emot. 2006, 20, 1075–1091. [Google Scholar] [CrossRef]
- Matchett, G.; Davey, G.C. A test of a disease-avoidance model of animal phobias. Behav. Res. Ther. 1991, 29, 91–94. [Google Scholar] [CrossRef]
- Sulikowski, D. Are natural threats superior threats? Evol. Hum. Behav. 2022, 43, 34–43. [Google Scholar] [CrossRef]
- Wardenaar, K.J.; Lim, C.C.; Al-Hamzawi, A.O.; Alonso, J.; Andrade, L.H.; Benjet, C.D.; Bunting, B.; de Girolamo, G.; Demyttenaere, K.; Florescu, S.E.; et al. The cross-national epidemiology of specific phobia in the World Mental Health Surveys. Psychol. Med. 2017, 47, 1744–1760. [Google Scholar] [CrossRef] [PubMed]
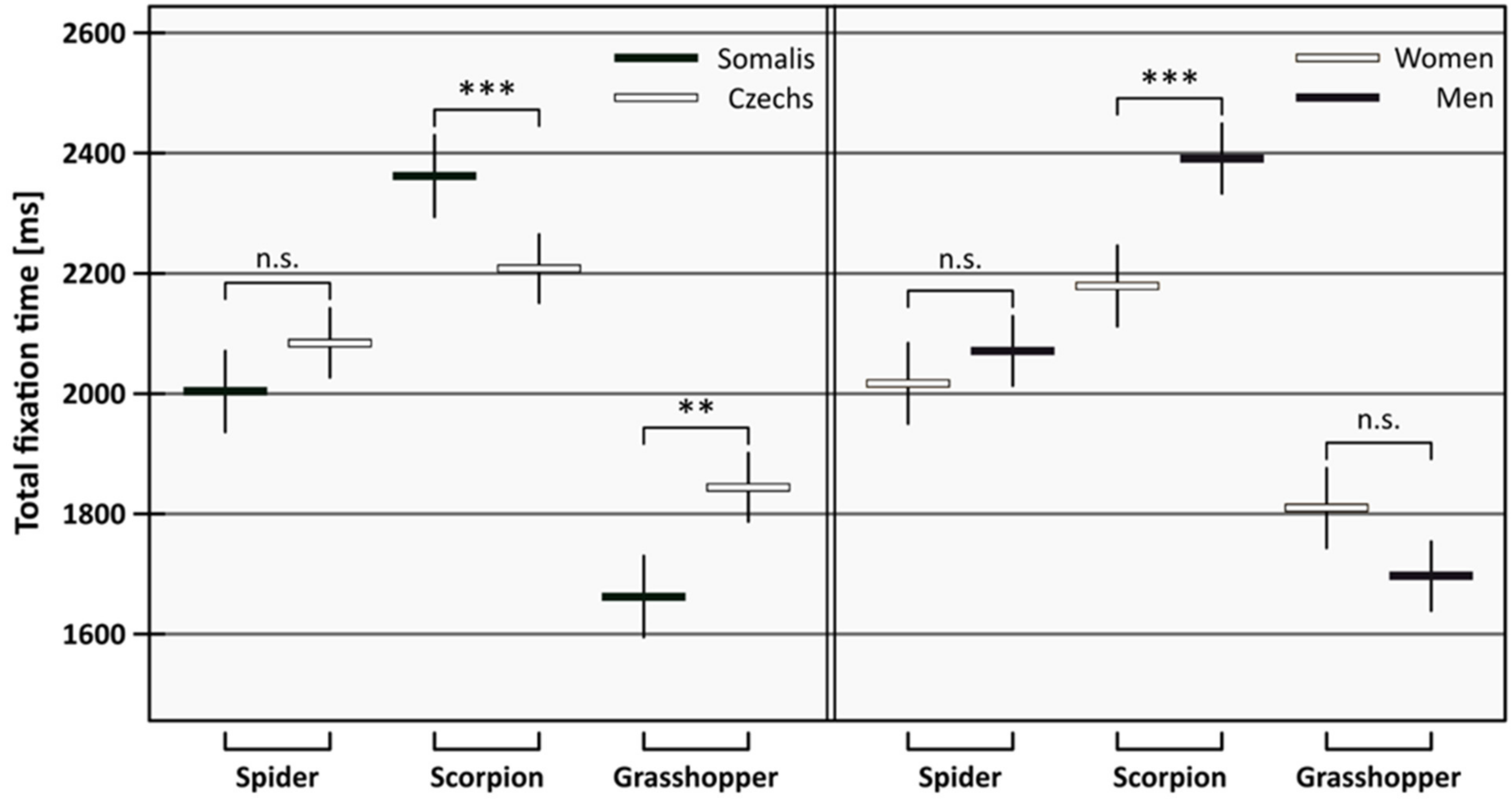


| Animal | Somalis | Czechs | Women | Men | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||||
| Spider | 2004 | 1936, 2071 | 2084 | 2027, 2142 | 2017 | 1950, 2084 | 2071 | 2013, 2129 | ||||
| Scorpion | 2362 | 2294, 2430 | 2208 | 2151, 2265 | 2179 | 2112, 2246 | 2391 | 2333, 2449 | ||||
| Grasshopper | 1662 | 1595, 1730 | 1844 | 1787, 1901 | 1810 | 1743, 1876 | 1697 | 1639, 1754 | ||||
| Contrast | Est. | t-rat. | p | Est. | t-rat. | p | Est. | t-rat. | p | Est. | t-rat. | p |
| Spider–Grasshopper | 341 | 7.25 | <0.001 | 240 | 6.07 | <0.001 | 207 | 4.47 | <0.001 | 375 | 9.32 | <0.001 |
| Scorpion–Grasshopper | 700 | 14.82 | <0.001 | 364 | 9.22 | <0.001 | 370 | 7.96 | <0.001 | 694 | 17.27 | <0.001 |
| Scorpion–Spider | 358 | 7.57 | <0.001 | 124 | 3.12 | 0.022 | 163 | 3.49 | 0.006 | 319 | 7.93 | <0.001 |
| Pair of Stimuli | Somalis | Czechs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | t | p | Mean | 95% CI | t | p | ||
| Women | Spider–Grasshopper | 414.54 | 152.92, 676.16 | 3.12 | 0.002 | 92.39 | −148.74, 333.51 | 0.75 | 0.453 |
| Scorpion–Grasshopper | 741.86 | 479.76, 1003.96 | 5.55 | <0.001 | 419.71 | 178.75, 660.67 | 3.42 | <0.001 | |
| Scorpion–Spider | 322.81 | 60.24, 585.38 | 2.41 | 0.016 | 0.66 | −240.72, 242.04 | 0.01 | 0.996 | |
| Men | Spider–Grasshopper | 709.93 | 485.04, 934.81 | 6.19 | <0.001 | 387.77 | 152.63, 622.91 | 3.23 | 0.001 |
| Scorpion–Grasshopper | 1037.25 | 812.02, 1262.48 | 9.03 | <0.001 | 715.09 | 480.33, 949.86 | 5.97 | <0.001 | |
| Scorpion–Spider | 618.20 | 392.60, 843.79 | 5.37 | <0.001 | 296.04 | 61.02, 531.07 | 2.47 | 0.014 | |
| Pair of Stimuli | Somalis | Czechs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | t | p | Mean | 95% CI | t | p | ||
| Women | Spider–Grasshopper | 1.23 | 0.22, 2.24 | 2.39 | 0.017 | 0.23 | −0.72, 1.17 | 0.47 | 0.639 |
| Scorpion–Grasshopper | 3.40 | 2.38, 4.41 | 6.58 | <0.001 | 2.39 | 1.45, 3.34 | 4.96 | <0.001 | |
| Scorpion–Spider | 1.28 | 0.27, 2.30 | 2.48 | 0.013 | 0.28 | −0.67, 1.23 | 0.58 | 0.563 | |
| Men | Spider–Grasshopper | 2.49 | 1.62, 3.35 | 5.62 | <0.001 | 1.48 | 0.56, 2.41 | 3.15 | 0.002 |
| Scorpion–Grasshopper | 4.65 | 3.78, 5.52 | 10.49 | <0.001 | 3.65 | 2.72, 4.57 | 7.75 | <0.001 | |
| Scorpion–Spider | 2.54 | 1.67, 3.41 | 5.72 | <0.001 | 1.53 | 0.62, 2.46 | 3.26 | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolfová, V.; Štolhoferová, I.; Elmi, H.S.A.; Rádlová, S.; Rexová, K.; Berti, D.A.; Král, D.; Sommer, D.; Landová, E.; Frýdlová, P.; et al. Do Spiders Ride on the Fear of Scorpions? A Cross-Cultural Eye Tracking Study. Animals 2022, 12, 3466. https://doi.org/10.3390/ani12243466
Rudolfová V, Štolhoferová I, Elmi HSA, Rádlová S, Rexová K, Berti DA, Král D, Sommer D, Landová E, Frýdlová P, et al. Do Spiders Ride on the Fear of Scorpions? A Cross-Cultural Eye Tracking Study. Animals. 2022; 12(24):3466. https://doi.org/10.3390/ani12243466
Chicago/Turabian StyleRudolfová, Veronika, Iveta Štolhoferová, Hassan S. A. Elmi, Silvie Rádlová, Kateřina Rexová, Daniel A. Berti, David Král, David Sommer, Eva Landová, Petra Frýdlová, and et al. 2022. "Do Spiders Ride on the Fear of Scorpions? A Cross-Cultural Eye Tracking Study" Animals 12, no. 24: 3466. https://doi.org/10.3390/ani12243466
APA StyleRudolfová, V., Štolhoferová, I., Elmi, H. S. A., Rádlová, S., Rexová, K., Berti, D. A., Král, D., Sommer, D., Landová, E., Frýdlová, P., & Frynta, D. (2022). Do Spiders Ride on the Fear of Scorpions? A Cross-Cultural Eye Tracking Study. Animals, 12(24), 3466. https://doi.org/10.3390/ani12243466







