Effects of Genetic Variation of the Sorting Nexin 29 (SNX29) Gene on Growth Traits of Xiangdong Black Goat
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Bioinformatics Analysis
2.2. Samples and Phenotypic Data Collection
2.3. Total RNA Isolation, DNA Extraction and Primer Design
2.4. Copy Number Analysis and mRNA Expression of SNX29 Gene
2.5. Polymerase Chain Reaction Amplification and Indel Genotyping of the SNX29 Gene
2.6. Statistical Analyses
3. Results
3.1. Biological Evolution and Conservation Analysis of SNX29
3.2. Distribution of Copy Numbers Variation and Indels of SNX29 in XBD Goats
3.3. Population Genetics Analysis for Two SNX29 Indels in XBD Goats
3.4. Association Analysis for the Genetic Variations of SNX29 and XBD Goat Growth Traits
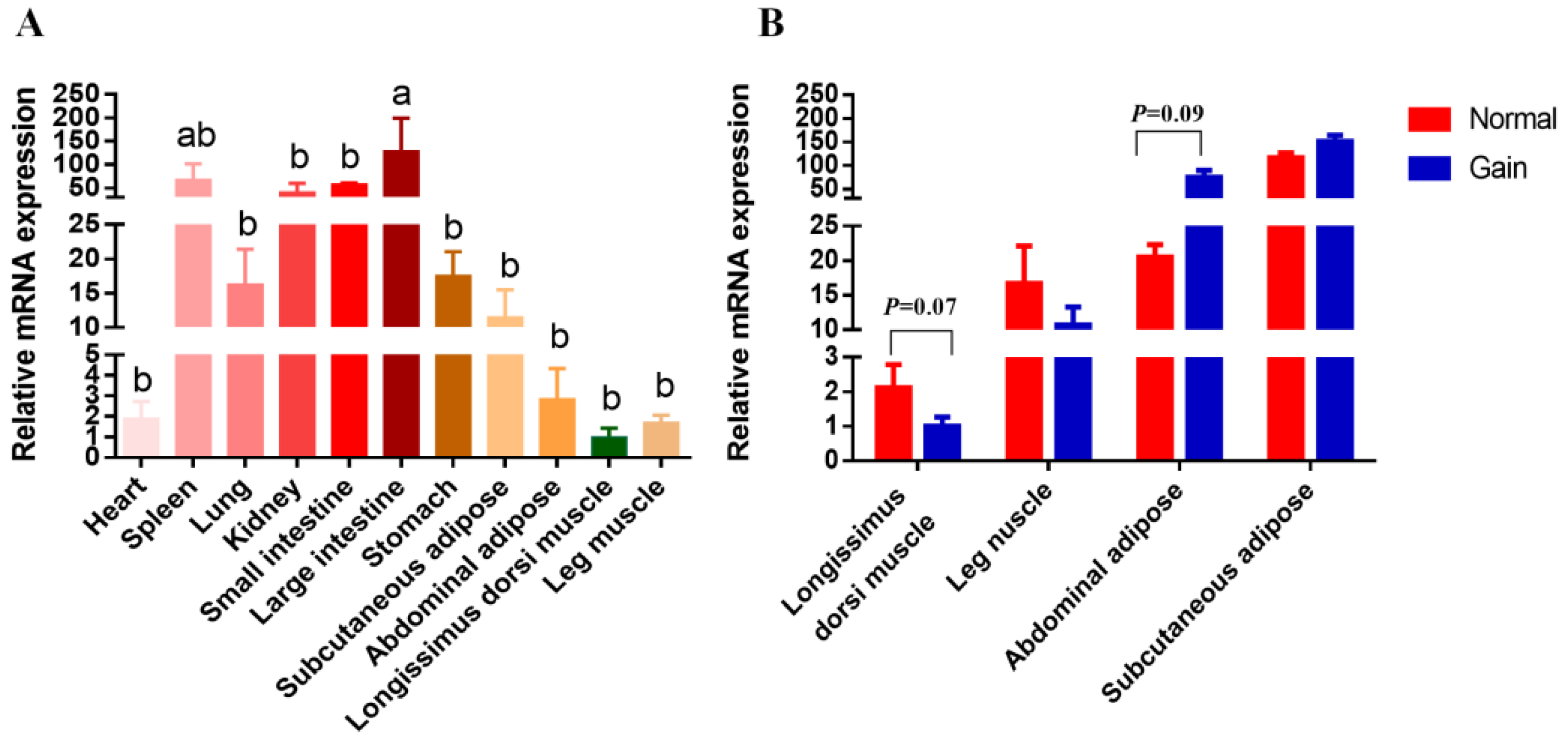
3.5. Analysis for the SNX29 Gene Expression and Its Associations with the SNX29 CNV in XBD Goats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mielczarek, M.; Frąszczak, M.; Giannico, R.; Minozzi, G.; Williams, J.L.; Wojdak-Maksymiec, K.; Szyda, J. Analysis of copy number variations in Holstein-Friesian cow genomes based on whole-genome sequence data. J. Dairy Sci. 2017, 100, 5515–5525. [Google Scholar] [CrossRef] [PubMed]
- Wolfner, M.F.; Miller, D.E. Alfred Sturtevant Walks into a Bar: Gene Dosage, Gene Position, and Unequal Crossing Over in Drosophila. Genetics 2016, 204, 833–835. [Google Scholar] [CrossRef]
- Sohrabi, S.S.; Mohammadabadi, M.; Wu, D.D.; Esmailizadeh, A. Detection of breed-specific copy number variations in domestic chicken genome. Genome 2018, 61, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, G.E.; Bickhart, D.M.; Cardone, M.F.; Wang, K.; Kim, E.S.; Matukumalli, L.K.; Ventura, M.; Song, J.; VanRaden, P.M.; et al. Genomic characteristics of cattle copy number variations. BMC Genom. 2011, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, G.E.; Bickhart, D.M.; Matukumalli, L.K.; Li, C.; Song, J.; Gasbarre, L.C.; Van Tassell, C.P.; Sonstegard, T.S. Genomic regions showing copy number variations associate with resistance or susceptibility to gastrointestinal nematodes in Angus cattle. Funct. Integr. Genom. 2012, 12, 81–92. [Google Scholar] [CrossRef]
- Guo, X.; Pei, J.; Wu, X.; Bao, P.; Ding, X.; Xiong, L.; Chu, M.; Lan, X.; Yan, P. Detection of InDel and CNV of SPAG17 gene and their associations with bovine growth traits. Anim. Biotechnol. 2020, 33, 440–447. [Google Scholar] [CrossRef]
- Ma, T.; Liu, Y.; Wei, X.; Xue, Q.; Zheng, Z.; Xu, X. Polymorphism of coupled indels in porcine TNNC2 alters its transcript splicing and is associated with meat quality traits. Anim. Genet. 2022, 53, 175–182. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Wei, Z.; Gao, J.; Yu, T.; Chen, R.; Lv, X.; Pan, C. Pig KDM5B: mRNA expression profiles of different tissues and testicular cells and association analyses with testicular morphology traits. Gene 2018, 650, 27–33. [Google Scholar] [CrossRef]
- Shi, T.; Xu, Y.; Yang, M.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Yang, X.; Chen, H. Copy number variations at LEPR gene locus associated with gene expression and phenotypic traits in Chinese cattle. Anim. Sci. J. 2016, 87, 336–343. [Google Scholar] [CrossRef]
- Park, C.; Makova, K.D. Coding region structural heterogeneity and turnover of transcription start sites contribute to divergence in expression between duplicate genes. Genome Biol. 2009, 10, R10. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, H.; Wang, K.; Xu, H.; Zhang, X.; Zhu, H.; Liu, J.; Qu, L.; Lan, X.; Pan, C. Insertion/Deletion Within the KDM6A Gene Is Significantly Associated with Litter Size in Goat. Front. Genet. 2018, 9, 91. [Google Scholar] [CrossRef]
- Upadhyay, M.; da Silva, V.H.; Megens, H.J.; Visker, M.H.P.W.; Ajmone-Marsan, P.; Bâlteanu, V.A.; Dunner, S.; Garcia, J.F.; Ginja, C.; Kantanen, J.; et al. Distribution and Functionality of Copy Number Variation across European Cattle Populations. Front. Genet. 2017, 8, 108. [Google Scholar] [CrossRef]
- Peng, S.; Song, C.; Li, H.; Cao, X.; Ma, Y.; Wang, X.; Huang, Y.; Lan, X.; Lei, C.; Chaogetu, B.; et al. Circular RNA SNX29 Sponges miR-744 to Regulate Proliferation and Differentiation of Myoblasts by Activating the Wnt5a/Ca2+ Signaling Pathway. Molecular therapy. Nucleic Acids 2019, 16, 481–493. [Google Scholar] [CrossRef]
- Cheever, M.L.; Sato, T.K.; de Beer, T.; Kutateladze, T.G.; Emr, S.D.; Overduin, M. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol. 2001, 3, 613–618. [Google Scholar] [CrossRef]
- Sparks, A.M.; Watt, K.; Sinclair, R.; Pilkington, J.G.; Pemberton, J.M.; McNeilly, T.N.; Nussey, D.H.; Johnston, S.E. The genetic architecture of helminth-specific immune responses in a wild population of Soay sheep (Ovis aries). PLoS Genet. 2019, 15, e1008461. [Google Scholar] [CrossRef]
- Wei, X.; Li, H.; Yang, J.; Hao, D.; Dong, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Lin, F.; et al. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell Death Dis. 2017, 8, e3153. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, H.; Li, X.; Wei, W.; Zhao, S.; Cao, J. A genome-wide association study of five meat quality traits in Yorkshire pigs. Front. Agric. Sci. Eng. 2014, 1, 137–143. [Google Scholar] [CrossRef]
- Liu, M.; Woodward-Greene, J.; Kang, X.; Pan, M.G.; Rosen, B.; Van Tassell, C.P.; Chen, H.; Liu, G.E. Genome-wide CNV analysis revealed variants associated with growth traits in African indigenous goats. Genomics 2020, 112, 1477–1480. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, Y.; Xin, D.; Liu, T.; He, L.; Kang, Y.; Pan, C.; Shen, W.; Lan, X.; Liu, M. Effect of indel variants within the sorting nexin 29 (SNX29) gene on growth traits of goats. Anim. Biotechnol. 2020, 33, 914–919. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Lu, F.; Marchler, G.H.; Song, J.S.; Thanki, N.; Wang, Z.; Yamashita, R.A.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar]
- Müllenbach, R.; Lagoda, P.J.; Welter, C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989, 5, 391. [Google Scholar] [PubMed]
- Liu, M.; Cheng, J.; Chen, Y.; Yang, L.; Raza, S.H.A.; Huang, Y.; Lei, C.; Liu, G.E.; Lan, X.; Chen, H. Distribution of DGAT1 copy number variation in Chinese goats and its associations with milk production traits. Anim. Biotechnol. 2021, 1–6. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, E.; Wang, K.; Zhang, Y.; Yan, H.; Qu, L.; Chen, H.; Lan, X.; Pan, C. Two Insertion/Deletion Variants within SPAG17 Gene Are Associated with Goat Body Measurement Traits. Animals 2019, 9, 379. [Google Scholar] [CrossRef]
- Liu, N.; Cui, W.; Chen, M.; Zhang, X.; Song, X.; Pan, C. A 21-bp indel within the LLGL1 gene is significantly associated with litter size in goat. Anim. Biotechnol. 2021, 32, 213–218. [Google Scholar] [CrossRef]
- Li, X.; Gao, W.; Guo, H.; Zhang, X.; Fang, D.D.; Lin, Z. Development of EST-based SNP and InDel markers and their utilization in tetraploid cotton genetic mapping. BMC Genom. 2014, 15, 1046. [Google Scholar] [CrossRef]
- Canterini, S.; Carletti, V.; Nusca, S.; Mangia, F.; Fiorenza, M.T. Multiple TSC22D4 iso-/phospho-glycoforms display idiosyncratic subcellular localizations and interacting protein partners. FEBS J. 2013, 280, 1320–1329. [Google Scholar] [CrossRef]
- Choi, S.J.; Moon, J.H.; Ahn, Y.W.; Ahn, J.H.; Kim, D.U.; Han, T.H. Tsc-22 enhances TGF-beta signaling by associating with Smad4 and induces erythroid cell differentiation. Mol. Cell. Biochem. 2005, 271, 23–28. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.; Wang, S.; Xu, Y.; Lan, X.; Li, Z.; Lei, C.; Yang, D.; Jia, Y.; Chen, H. Association analysis of bovine Foxa2 gene single sequence variant and haplotype combinations with growth traits in Chinese cattle. Gene 2014, 536, 385–392. [Google Scholar] [CrossRef]
- Xia, L.; Ou, J.; Li, K.; Guo, H.; Hu, Z.; Bai, T.; Zhao, J.; Xia, K.; Zhang, F. Genome-wide association analysis of autism identified multiple loci that have been reported as strong signals for neuropsychiatric disorders. Autism Res. 2020, 13, 382–396. [Google Scholar] [CrossRef]
- Hamilton, C.K.; Verduzco-Gómez, A.R.; Favetta, L.A.; Blondin, P.; King, W.A. Testis-specific protein Y-encoded copy number is correlated to its expression and the field fertility of Canadian Holstein bulls. Sex. Dev. 2012, 6, 231–239. [Google Scholar] [CrossRef]
- Wang, X.; Nahashon, S.; Feaster, T.K.; Bohannon-Stewart, A.; Adefope, N. An initial map of chromosomal segmental copy number variations in the chicken. BMC Genom. 2010, 11, 351. [Google Scholar] [CrossRef]
- Yi, G.; Qu, L.; Liu, J.; Yan, Y.; Xu, G.; Yang, N. Genome-wide patterns of copy number variation in the diversified chicken genomes using next-generation sequencing. BMC Genom. 2014, 15, 962. [Google Scholar] [CrossRef]
- Cheng, J.; Jiang, R.; Cao, X.K.; Liu, M.; Huang, Y.Z.; Lan, X.Y.; Cao, H.; Lei, C.Z.; Chen, H. Association analysis of SSTR2 copy number variation with cattle stature and its expression analysis in Chinese beef cattle. J. Agric. Sci. 2019, 157, 365–374. [Google Scholar] [CrossRef]
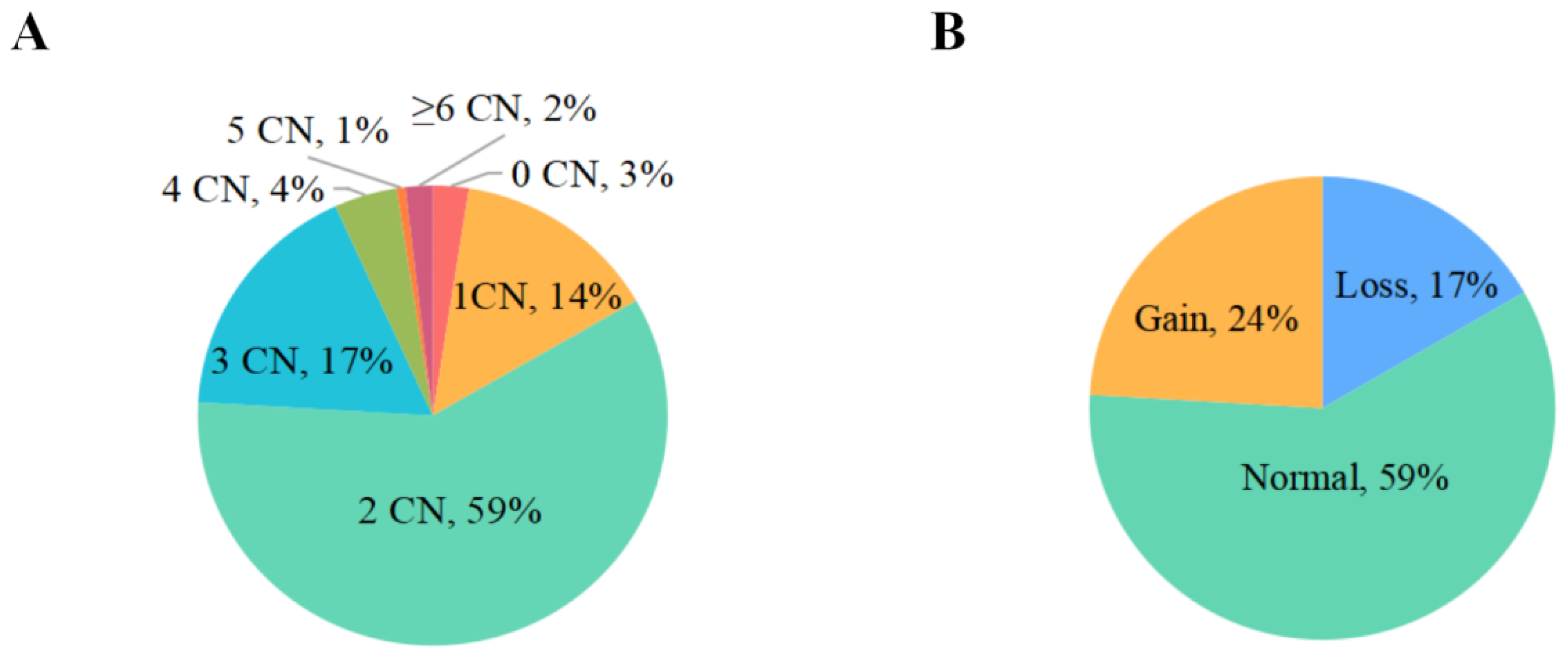
| CNV Types (Mean ± SE) | ||||
|---|---|---|---|---|
| Growth Traits | Loss (n = 88) | Normal (n = 304) | Gain (n = 124) | p Value |
| Age (day) | 738.48 ± 17.48 | 732.96 ± 16.73 | 721.36 ± 16.29 | 0.281 |
| Body height (BH) | 55.30 ± 0.98 | 56.30 ± 0.44 | 56.76 ± 0.65 | 0.407 |
| Body length (BL) | 57.96 ± 0.96 | 59.47 ± 0.51 | 58.09 ± 1.11 | 0.262 |
| Body oblique length (OL) | 58.48 ± 1.41 | 59.89 ± 0.47 | 58.52 ± 1.11 | 0.278 |
| Chest circumference (CC) | 72.04 ± 1.50 a,b | 74.89 ± 0.72 a | 70.09 ± 1.42 b | 0.004 ** |
| Abdominal circumference (AC) | 81.56 ± 1.91 b | 86.50 ± 1.01 a | 81.24 ± 1.62 b | 0.007 ** |
| Cross height (CH) | 55.96 ± 0.96 | 57.80 ± 0.39 | 56.91 ± 0.70 | 0.103 |
| Cannon Circumference (CAC) | 11.05 ± 0.30 | 10.80 ± 0.11 | 10.61 ± 0.21 | 0.216 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, L.; Lin, X.; Peng, P.; Shen, W.; Tang, S.; Lan, X.; Wan, F.; Yin, Y.; Liu, M. Effects of Genetic Variation of the Sorting Nexin 29 (SNX29) Gene on Growth Traits of Xiangdong Black Goat. Animals 2022, 12, 3461. https://doi.org/10.3390/ani12243461
Chen Y, Yang L, Lin X, Peng P, Shen W, Tang S, Lan X, Wan F, Yin Y, Liu M. Effects of Genetic Variation of the Sorting Nexin 29 (SNX29) Gene on Growth Traits of Xiangdong Black Goat. Animals. 2022; 12(24):3461. https://doi.org/10.3390/ani12243461
Chicago/Turabian StyleChen, Yuhan, Long Yang, Xiaoding Lin, Peiya Peng, Weijun Shen, Sipei Tang, Xianyong Lan, Fachun Wan, Yulong Yin, and Mei Liu. 2022. "Effects of Genetic Variation of the Sorting Nexin 29 (SNX29) Gene on Growth Traits of Xiangdong Black Goat" Animals 12, no. 24: 3461. https://doi.org/10.3390/ani12243461
APA StyleChen, Y., Yang, L., Lin, X., Peng, P., Shen, W., Tang, S., Lan, X., Wan, F., Yin, Y., & Liu, M. (2022). Effects of Genetic Variation of the Sorting Nexin 29 (SNX29) Gene on Growth Traits of Xiangdong Black Goat. Animals, 12(24), 3461. https://doi.org/10.3390/ani12243461





