Effects of Strategic Supplementation with Lupinus angustifolius and Avena sativa Grains on Colostrum Quality and Passive Immunological Transfer to Newborn Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
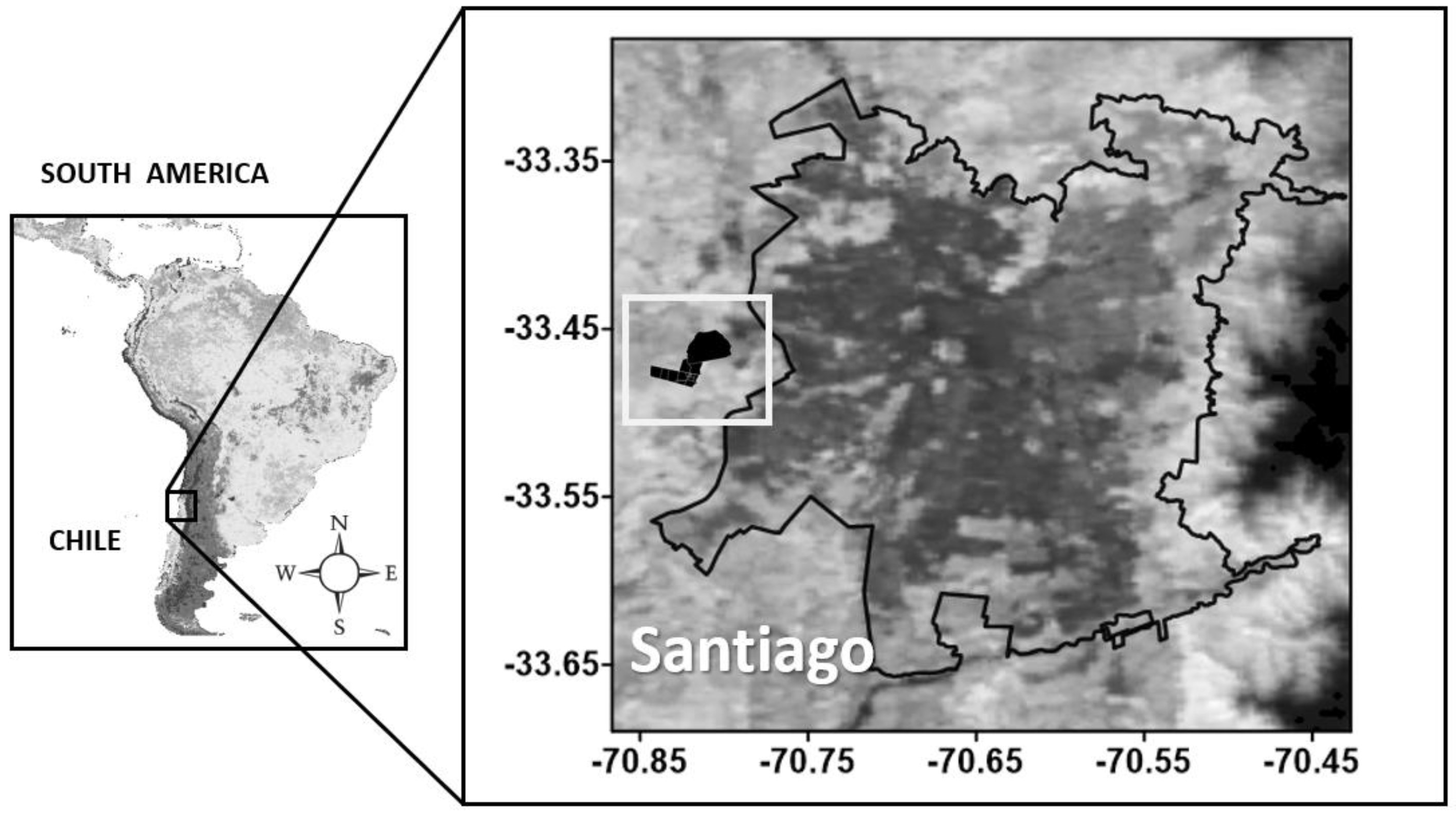
2.1. Study Area
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Chemical Composition and Energy Value of Colostrum
3.2. Colostrum IgG Concentration
3.3. Concentration of IgG in the Blood Serum of Newborn Lambs
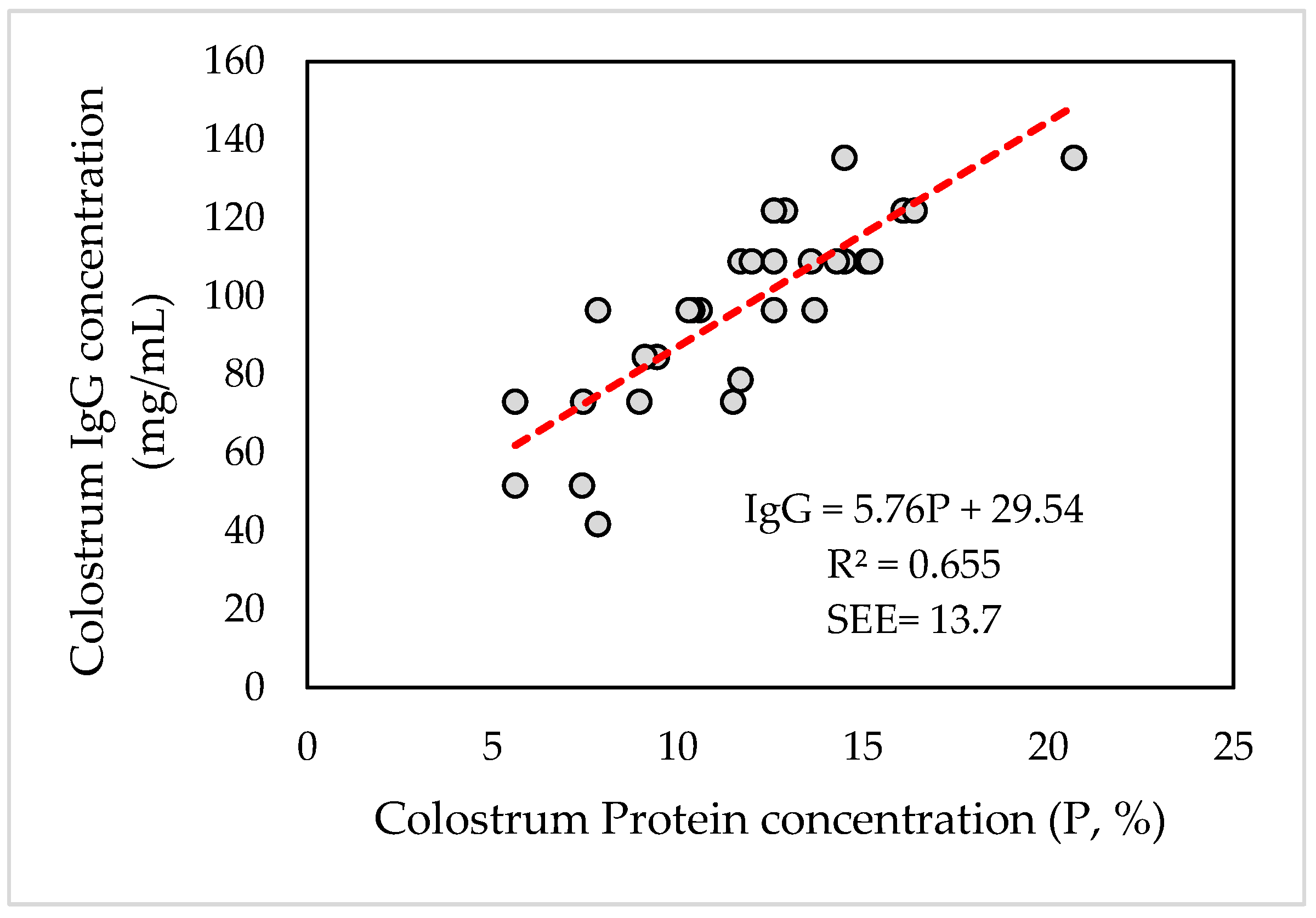
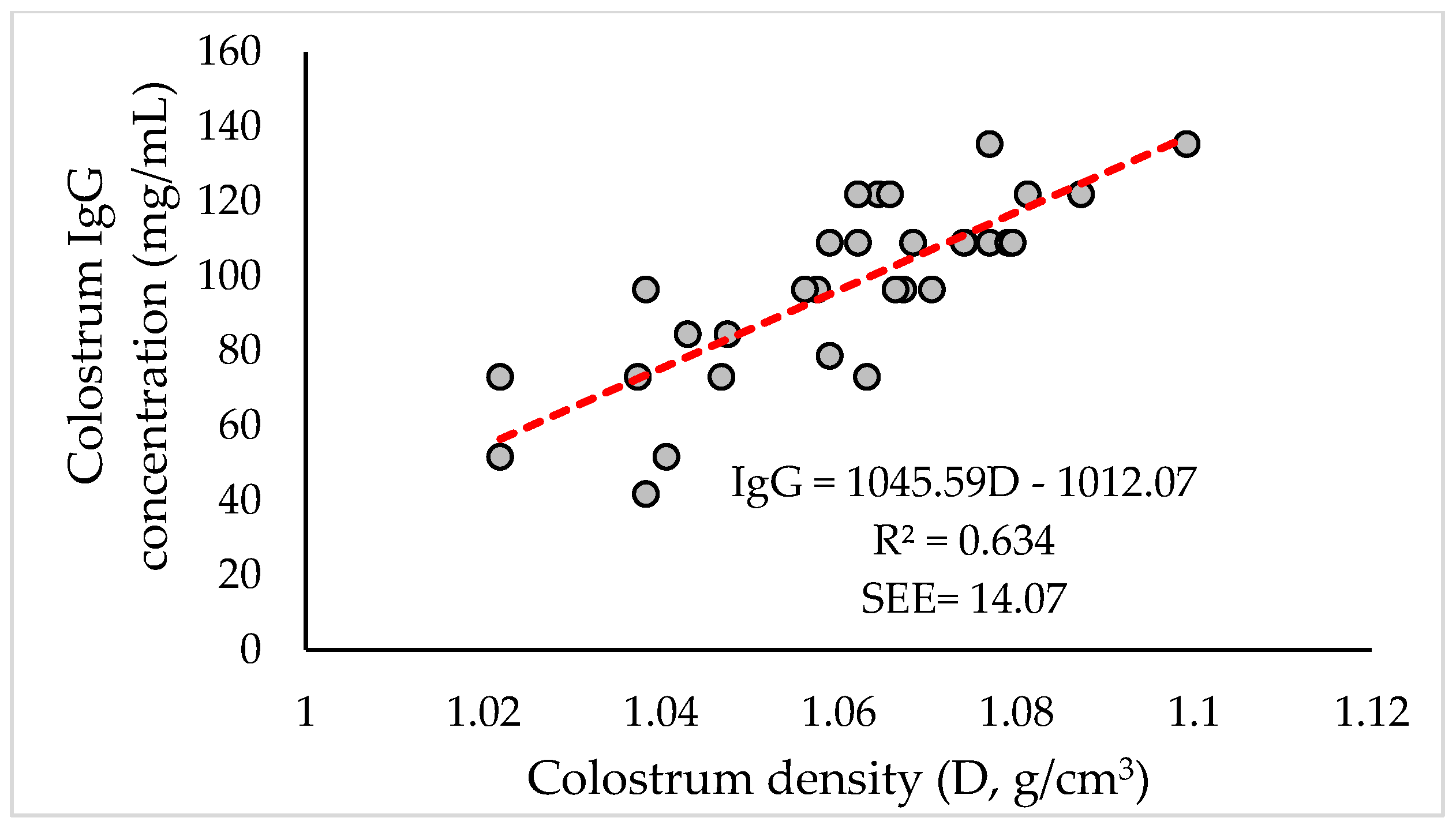
3.4. Correlations and Linear Regressions between Some Variables
4. Discussion
4.1. Nutritional Composition and Energy Value of Colostrum
4.2. Concentration of IgG in Ewe’s Colostrum
4.3. Concentration of IgG in Lamb’s Blood Serum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loste, A.; Ramos, J.J.; Fernández, A.; Ferrer, L.M.; Lacasta, D.; Verde, M.T.; Marca, M.C.; Ortín, A. Effect of colostrum treated by heat on immunological parameters in newborn lambs. Livest. Sci. 2008, 117, 176–183. [Google Scholar] [CrossRef]
- Hart, K.W.; Contou, C.; Blackberry, M.; Blache, D. Merino ewes divergently selected for calm temperament have a greater concentration of immunoglobulin G in their colostrum than nervous ewes. Proc. Assoc. Advmt. Anim. Breed. Genet. 2009, 18, 576–579. [Google Scholar]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barringtonm, G.M. Passive Transfer of Colostral Immunoglobulins in Calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef]
- Tabatabaei, S.; Nikbakht Brujeni, G.; Vatankhah, M.; Sharifi, H.; Alidadi, N. Variation in colostral immunoglobulin G concentration in fat tailed sheep and evaluation of methods for estimation of colostral immunoglobulin content. Acta Vet. Brno 2013, 82, 271275. [Google Scholar] [CrossRef]
- Ocak, N.; Cam, M.A.; Kuran, M. The effect of high dietary protein levels during late gestation on colostrum yield and lamb survival rate in singleton-bearing ewes. Small Rum. Res. 2005, 56, 89–94. [Google Scholar] [CrossRef]
- Swanson, T.J.; Hammer, C.J.; Luther, J.S.; Carlson, D.B.; Taylor, J.B.; Redmer, D.A.; Neville, T.L.; Reed, J.J.; Reynolds, L.P.; Caton, J.S.; et al. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J. Anim. Sci. 2008, 86, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Leal Yepes, F.A.; Overton, T.R.; Lock, A.L.; Lamb, S.V.; Wakshlag, J.J.; Nydam, D.V. Effect of dry period dietary energy level in dairy cattle on volume, concentrations of immunoglobulin G, insulin, and fatty acid composition of colostrum. J. Dairy Sci. 2016, 99, 1515–1526. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Salem, A.Z.; Olafadehan, O.A.; Kfolif, A.E.; Rivero, N.; Mariezcurrena, M.; Camacho, L.M.; Elghandour, M.M.; Alonso, M.U.; Almaz, A.H. Effect of pre-and pots partum dietary crude protein level on the performance of ewes and their lambs. Small Rum. Res. 2016, 136, 221–226. [Google Scholar] [CrossRef][Green Version]
- Wallace, M.M.; Jarvie, B.D.; Perkins, N.R.; Leslie, K.E. A comparison of serum harvesting methods and type of refractometer for determining total solids to estimate failure of passive transfer in calves. Can. Vet. J. 2006, 47, 573–575. [Google Scholar]
- Blair, H.T.; Jenkinson, C.M.; Peterson, S.W.; Kenyon, P.R.; Van der Linden, D.S.; Davenport, L.C.; Mackenzie, D.D.; Morris, S.T.; Firth, E.C. Dam and granddam feeding during pregnancy in sheep affects milk supply in offspring and reproductive performance in grand-offspring. J. Anim. Sci. 2010, 88 (Suppl. 13), E40–E50. [Google Scholar] [CrossRef]
- Banchero, G.E.; Quintans, G.; Vázquez, A.; Gigena, F.; La Manna, A.; Lindsay, D.R. Effect of supplementation of ewes with barley or maize during the last week of pregnancy on colostrum production. Animal 2007, 1, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Pulina, G.; Nudda, A.; Battacone, G.; Cannas, A. Effects of nutrition on the contents of fat, protein, somatic cells, aromatic compounds, and undesirable substances in sheep milk. Anim. Feed Sci. Technol. 2006, 131, 255–291. [Google Scholar] [CrossRef]
- Santibáñez, F.P.; Santibáñez, C.; Caroca, P.; Morales, P.; González, N.; Gajardo, P.; Perry, C. Atlas del Cambio Climático en las Zonas de Régimen Arido y Semiárido de Chile; Universidad de Chile, Ministerio del Medio Ambiente: Santiago de Chile, Chile, 2014; p. 136. [Google Scholar]
- CIREN-CORFO. Estudio Agrológico. In Región Metropolitana, Descripciones de Suelos, Materiales y Símbolos; Publicación CIREN 115; Centro de Información de Recursos Naturales (CIREN): Santiago de Chile, Chile, 1996; p. 464. [Google Scholar]
- Castellaro, G.; Silva, M.; Santibáñez, F. Efecto de la radiación solar y la temperatura sobre las fenofases de las principales especies del pastizal mediterráneo anual. Av. Prod. Anim. 1994, 19, 65–75. [Google Scholar]
- Olivares, A. El Espinal: Manejo Silvopastoril de un Recurso Olvidado; Editorial Universitaria: Santiago de Chile, Chile, 2017; p. 184. [Google Scholar]
- Haydock, K.; Shaw, N. The comparative yield method for estimating dry matter yield of pasture. Aus. J. Exp. Agric. Anim. Husb. 1975, 15, 662–670. [Google Scholar] [CrossRef]
- Russel, A.J.F.; Doney, J.M.; Gunn, G. Subjective assessment of body fat in live sheep. J. Agric. Sci. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist. Official Methods of Analysis of the AOAC International, 17th ed.; Association of Official Analytical Chemist: Arlington, VA, USA, 2000. [Google Scholar]
- Standing Committee on Agriculture, Ruminants Subcommittee (SCA). Nutrient Requirement of Domesticated Ruminants; CSIRO Publications: Melbourne, Australia, 2007; p. 296. [Google Scholar]
- Nicol, A.M.; Brookes, I.M. The metabolizable energy requirements of grazing livestock. In Pasture, and Supplements for Grazing Animals; Rattray, P.V., Brookes, I.M., Nicol, A.M., Eds.; New Zealand Society of Animal Production: Hamilton, New Zealand, 2007; Volume 10, pp. 151–172, 309. [Google Scholar]
- Ahmad, R.; Khan, A.; Javed, M.T.; Hussain, I. The level of immunoglobulins in relation to neonatal lamb mortality in Pak-Karkul sheep. Vet. Archiv. 2000, 70, 129–139. [Google Scholar]
- Johnson, J.L.; Godden, S.M.; Molitor, T.; Ames, T.; Hagman, D. Effects of feeding heat-treated colostrum on passive transfer of immune and nutritional parameters in neonatal dairy calves. J. Dairy Sci. 2007, 90, 5189–5198. [Google Scholar] [CrossRef]
- Angulo, J.; Gómez, L.M.; Mahecha, L.; Mejía, E.; Henao, J.; Mesa, C. Calf’s sex, parity, and the hour of harvest after calving affect colostrum quality of dairy cows grazing under high tropical conditions. Trop. Anim. Health Prod. 2015, 47, 699–705. [Google Scholar] [CrossRef]
- Morsy, A.S.; Eissa, M.M.; Answer, M.M.; Ghobashy, H.; Sallam, S.M.A.; Soltan, Y.A.; Saber, A.M.; El-Wakeel, E.A.; Sadik, W.M. Colostral immunoglobulin concentration and milk production of ewes fed salt tolerant forages as alternatives to berseem hay. Livestock Sci. 2018, 210, 125–128. [Google Scholar] [CrossRef]
- Fahey, J.; McKelvey, J. Quantitative determination of serum immunoglobulins in antibody agar plates. J. Immunol. 1965, 94, 84–90. [Google Scholar]
- Mancini, G.; Carbonara, A.O.; Heremans, J.F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry 1965, 2, 235–254. [Google Scholar] [CrossRef]
- Waldner, C.L.; Rosengren, L.B. Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes. Can. Vet. J. 2009, 50, 275–281. [Google Scholar] [PubMed]
- Di Rienzo, J.; Cadanoves, A.; Balzarini, M.; Gonzalez, L.; Tablada, M.; Roberdo, C. InfoStat Versión 2011. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: https://www.infostat.com.ar/ (accessed on 8 March 2021).
- Ciuryk, S.; Molik, E.; Kaczor, U.; Bonczar, G. Chemical composition of colostrum and milk of Polish Merino sheep lambing at different times. Arch. Tierz. 2004, 47, 129–134. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.616.4848&rep=rep1&type=pdf (accessed on 13 April 2021).
- Althaus, R.L.; Sosa, J.; Gapel, C.; Scaglione, L.; Moreyra, E.; Coraza, M. Leche y calostro de ovejas Corriedale: Composición química y mineral. Revista FAVE 2001, 15, 7–13. [Google Scholar] [CrossRef]
- Wohlt, J.E.; Kleyn, D.H.; Vandernoot, G.W.; Selfrigge, D.J.; Novotney, C.A. Effect of stage of lactation, age of ewe, sibling status, and sex of lamb on gross and minor constituents of Dorset ewe milk. J. Dairy Sci. 1981, 64, 2175–2184. [Google Scholar] [CrossRef]
- O’Doherty, J.V.; Crosby, T.F. The effect of diet in late pregnancy on colostrum production and immunoglobulin absorption in sheep. Anim. Sci. 1997, 64, 87–96. [Google Scholar] [CrossRef]
- Abd-Allah, M. Effects of parity and nutrition plane during late pregnancy on metabolic responses, colostrum production and lamb output of Rahmani ewes. Egypt. J. Anim. Prod. 2013, 50, 132–142. [Google Scholar]
- Banchero, G.E.; Milton, J.T.; Lindsay, D.R.; Martin, G.B.; Quintans, G. Colostrum production in ewes: A review of regulation mechanisms and of energy supply. Animal 2015, 9, 831–837. [Google Scholar] [CrossRef]
- Getahun, D.; Alemneh, T.; Akeberegn, D.; Getabalew, M.; Zewdie, D. Urea metabolism and recycling in ruminants. Biomed. J. Sci. Tech. Res. 2019, 20, 14790–14796. [Google Scholar] [CrossRef]
- Bocquier, F.; Caja, G. Production et composition du lait de brebis: Effets de l’alimentation. INRAE Prod. Anim. 2001, 14, 129–140. [Google Scholar] [CrossRef]
- Cannas, A.; Pes, A.; Mancuso, R.; Vodret, B.; Nudda, A. Effect of dietary energy and protein concentration on the concentration of milk urea nitrogen in dairy ewes. J. Dairy Sci. 1998, 81, 499–508. [Google Scholar] [CrossRef]
- Rowe, J.B.; Pethick, D.W. Starch digestion in ruminants. Problems, solutions, and opportunities. In Proceedings of the Nutrition Society of Australia, V18, Twentieth Annual Scientific Meeting, Newcastle, NSW, Australia, 26–28 September 1994; pp. 40–52. Available online: https://researchrepository.murdoch.edu.au/id/eprint/22547/ (accessed on 26 October 2022).
- Nudda, A.; Fancellu, S.; Porcu, F.; Boe, F.; Cannas, A. Responses of milk fat composition to dietary non-fiber carbohydrates in Sarda dairy sheep. J. Dairy Sci. 2004, 87, 310–318. [Google Scholar]
- Hunter, A.G.; Reneau, J.K.; Williams, J.B. Factors affecting IgG concentration in day-old lambs. J. Anim. Sci. 1977, 45, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Boucher, Z. Breed and Diet Effects on Ewe Colostrum Quality, Lamb Birthweight and the Transfer of Passive Immunity. Master’s Thesis, Charles Sturt University, Wagga Wagga, Australia, 2014; p. 47. [Google Scholar]
- Vatankhah, M. Relationship between immunoglobulin concentrations in the ewe’s serum and colostrum, and lamb’s serum in Lori-Bakhtiari sheep. Iranian J. App. Anim. Sci. 2013, 3, 539–544. Available online: https://ijas.rasht.iau.ir/article_513845_ece477e895e4c62ac246e33dc4602031.pdf (accessed on 26 October 2022).
- De, K.; Swarnkar, C.P.; Prince, L.L.L.; Ali, S.F. Interrelationship between late gestational ewe factor and early life lamb factors in semi-arid tropical region. Trop. Anim. Health Prod. 2018, 51, 249–255. [Google Scholar] [CrossRef]
- Banchero, G.E.; Perez, R.; Bencini, R.; Lindsay, D.R.; Milton, J.T.; Martin, G.B. Endocrine and metabolic factors involved in the effect of nutrition on the production of colostrum in female sheep. Reprod. Nutr. Dev. 2006, 46, 447–460. [Google Scholar] [CrossRef]
- Banchero, G.E.; Quintans, G.; Martin, G.B.; Milton, J.T.; Lindsay, D.R. Nutrition, and colostrum production in sheep. 2. Metabolic and hormonal responses to different energy sources in the final stages of pregnancy. Reprod. Fertil. Dev. 2004, 16, 645–653. [Google Scholar] [CrossRef]
- Wallace, J.; Milne, J.; Aitken, R. The effect of overnourishing singleton-bearing adult ewes on nutrient partitioning to the gravid uterus. Br. J. Nutr. 2005, 94, 533–539. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Zhao, Y.; Kacskovics, I.; Eijk, M.; Groot, N.; Li, N.; Hammarstrom, L. Association of FcRn heavy chain encoding gene (FCGRT) polymorphisms with IgG content in bovine colostrum. Anim. Biotechnol. 2009, 20, 242–246. [Google Scholar] [CrossRef]
- Mayer, B.; Doleschall, M.; Bender, B.; Bartyik, J.; Bosze, Z.; Frenyó, L.V.; Kacskovics, I. Expression of the neonatal Fc receptor (FcRn) in the bovine mammary gland. J. Dairy Res. 2005, 72, 107–112. [Google Scholar] [CrossRef]
- Mayer, B.; Zolnai, A.; Frenyo, L.V.; Jancsik, V.; Szentirmay, Z.; Hammar-Strom, L.; Kacskovics, I. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology 2002, 107, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Fallah, R.; Kiani, A.; Khaldari, M. Supplementing lycopene combined with corn improves circulating IgG concentration in pregnant ewes and their lambs. Trop. Anim. Health Prod. 2021, 53, 360. [Google Scholar] [CrossRef] [PubMed]
- Campion, F.P.; McGovern, F.M.; Lott, S.; Fahey, A.G.; Creighton, P.; Boland, T.M. Comparison of energy rationing systems for late gestation ewes: Impacts on ewe and lamb performance. J. Anim. Sci. 2016, 94, 3441–3456. [Google Scholar] [CrossRef] [PubMed]
- Jaster, E.H. Evaluation of quality, quantity, and timing of colostrum feeding on immunoglobulin G1 absorption in Jersey calves. J. Dairy Sci. 2005, 88, 296–302. [Google Scholar] [CrossRef]
- Altiner, A.; Ozplnar, A.; Erhard, M.H. Serum immunoglobulin G levels in lambs fed colostrum and dam milk or cow milk and milk replacer after birth. Med. Wet. 2005, 61, 1135–1137. [Google Scholar]
- Monteiro, A.P.A.; Tao, S.; Thompson, M.; Dahl, G.E. Effect of heat stress during late gestation on immune function and growth performance of calves: Isolation of altered colostral and calf factors. J. Dairy Sci. 2014, 97, 6426–6439. [Google Scholar] [CrossRef]
- Nikbakht, G.; Jani, S.; Alidadi, N.; Tabatabaei, S.; Sharifi, H.; Mohri, M. Passive immune transfer in fat-tailed sheep: Evaluation with different methods. Small Rum. Res. 2010, 90, 146–149. [Google Scholar] [CrossRef]

| Treatment | MS 1 (%) | ME 2 (MJ/kg) | CP 1 (%) | EfD 2 (%) | NDF 1 (%) | ADF 1 (%) |
|---|---|---|---|---|---|---|
| Lupine grain | 86.80 | 13.72 | 29.45 | 80.10 | 25.10 | 18.70 |
| Oat grain | 89.81 | 11.55 | 11.52 | 71.70 | 33.38 | 26.40 |
| Pasture | 30.00 | 10.40 | 19.90 | 75.60 | 40.40 | 29.52 |
| Components of Colostrum | Supplementation Type | ||
|---|---|---|---|
| Lupine Grain | Oat Grain | Control | |
| P (%) | 9.05 ± 0.68 c | 14.93 ± 0.68 a | 11.28 ± 0.68 b |
| F (%) | 12.57 ± 0.97 a | 8.23 ± 0.97 b | 8.41 ± 0.97 b |
| NFS (%) | 15.16 ± 0.82 c | 21.68 ± 0.82 a | 17.57 ± 0.82 b |
| TS (%) | 27.73 ± 1.20 | 29.91 ± 1.20 | 25.98 ± 1.71 |
| D (g/cm3) | 1.046 ± 0.004 c | 1.078 ± 0.004 a | 1.060 ± 0.004 b |
| EV (MJ/kg, DM basis) | 30.23 ± 0.92 | 28.69 ±0.92 | 28.15 ± 0.92 |
| EV (MJ/kg, offered basis) | 8.53 ± 0.67 | 8.30 ± 0.67 | 8.77 ± 0.67 |
| Ewe’s Supplementation Type | IgG (mg/mL) | Range (mg/mL) |
|---|---|---|
| Lupine grain | 86.0 ± 5.1 b | 41.7–121.9 |
| Oat grain | 118.8 ± 5.2 a | 96.4–135.4 |
| Control | 97.4 ± 5.1 b | 73.0–121.9 |
| Newborn Lamb’s Sex | IgG (mg/mL) | Range (mg/mL) |
|---|---|---|
| Male | 110.1 ± 4.3 a | 73.0–135.4 |
| Female | 91.4 ± 4.1 b | 41.7–135.4 |
| Ewe’s Supplementation Type | IgG (mg/mL) in Blood Serum | Range (mg/mL) |
|---|---|---|
| Lupine grain | 51.2 ± 3.8 b | 21.5–64.2 |
| Oat grain | 72.0 ± 3.8 a | 64.3–81.3 |
| Control | 57.7 ± 3.8 b | 27.8–72.6 |
| P (%) | F (%) | NFS (%) | TS (%) | D (g/cm3) | IgG (mg/mL) | |
|---|---|---|---|---|---|---|
| P (%) | 1 | −0.365 * | 0.990 ** | 0.857 ** | 0.974 ** | 0.816 ** |
| F (%) | 1 | −0.380 * | 0.162 ns | −0.530 ** | −0.308 ns | |
| NFS (%) | 1 | 0.849 ** | 0.986 ** | 0.816 ** | ||
| TS (%) | 1 | 0.749 ** | 0.698 ** | |||
| D (g/cm3) | 1 | 0.803 ** | ||||
| IgG (mg/mL) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellaro, G.; Ochoa, I.; Borie, C.; Parraguez, V.H. Effects of Strategic Supplementation with Lupinus angustifolius and Avena sativa Grains on Colostrum Quality and Passive Immunological Transfer to Newborn Lambs. Animals 2022, 12, 3159. https://doi.org/10.3390/ani12223159
Castellaro G, Ochoa I, Borie C, Parraguez VH. Effects of Strategic Supplementation with Lupinus angustifolius and Avena sativa Grains on Colostrum Quality and Passive Immunological Transfer to Newborn Lambs. Animals. 2022; 12(22):3159. https://doi.org/10.3390/ani12223159
Chicago/Turabian StyleCastellaro, Giorgio, Isaí Ochoa, Consuelo Borie, and Víctor H. Parraguez. 2022. "Effects of Strategic Supplementation with Lupinus angustifolius and Avena sativa Grains on Colostrum Quality and Passive Immunological Transfer to Newborn Lambs" Animals 12, no. 22: 3159. https://doi.org/10.3390/ani12223159
APA StyleCastellaro, G., Ochoa, I., Borie, C., & Parraguez, V. H. (2022). Effects of Strategic Supplementation with Lupinus angustifolius and Avena sativa Grains on Colostrum Quality and Passive Immunological Transfer to Newborn Lambs. Animals, 12(22), 3159. https://doi.org/10.3390/ani12223159






