Can Sound Alone Act as a Virtual Barrier for Horses? A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Procedures of the Sound Tunnel Test
2.2.1. General Conditions and Test Preparation
2.2.2. Carrying Out the Test
- First group—30 m
- Second group—15 m
- Third group—5 m
- Variant F—the motivator (additionally to a near pasture) for movement was a food reward
- Variant S—the motivator (additionally to a near pasture) for movement was a familiar and friendly horse that was not included in the study (social reward)
- A—recalling the direction of movement and showing the reward; no measurements
- B—a control trial; behavioural measurements
- C—an experimental trial; behavioural and cardiac activity measurements
2.2.3. Sound Stimulus
2.3. Behavioural Data Collection and Analyses
2.4. Cardiac Activity Data Collection and Analysis
- -
- RMSSD (ms)—standard deviation of differences between successive IBIs (time domain),
- -
- HF (ms2)—high-frequency component (0.07–0.5 Hz) of HRV determined by spectral analysis (frequency domain),
- -
- LF (ms2)—low-frequency component (0.005–0.07 Hz) of HRV determined by spectral analysis (frequency domain),
- -
- LF/HF (%)—low frequencies/high-frequencies ratio (frequency domain).
2.5. Statistical Analyses
3. Results
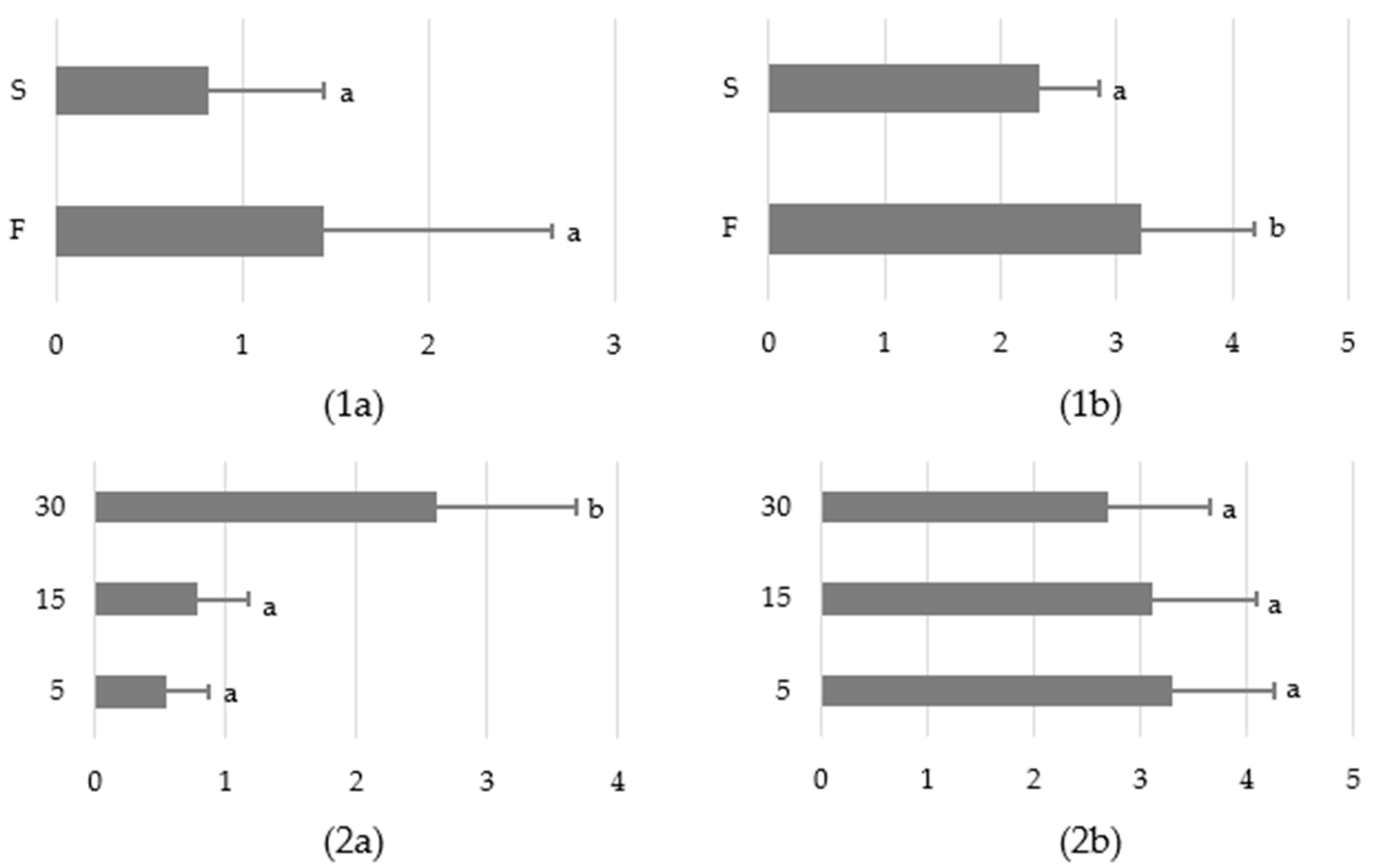
3.1. Behavioural Responses
3.1.1. The Variant of the Study
3.1.2. Sound Exposure Distance
3.1.3. The Sound Barrier Effect
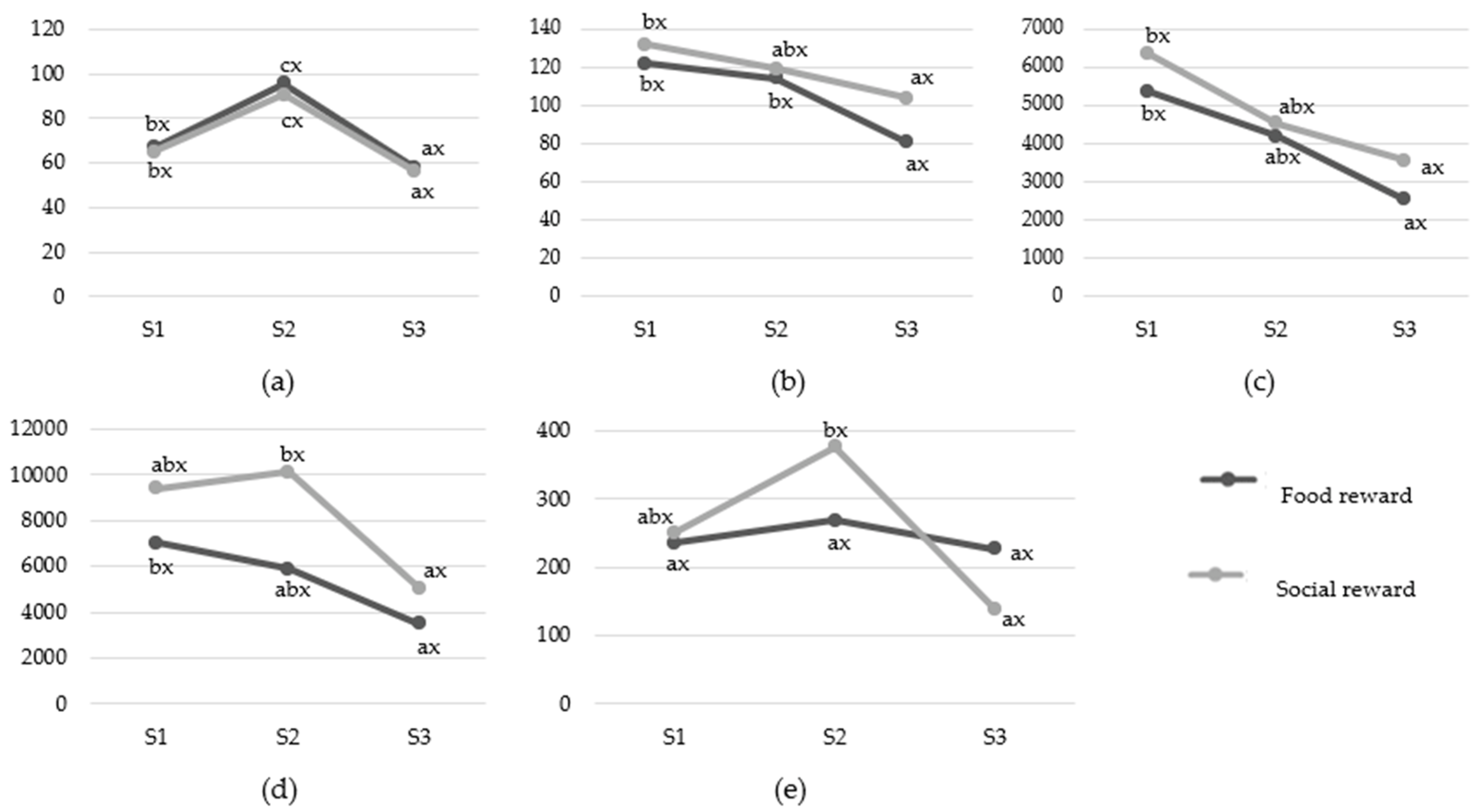
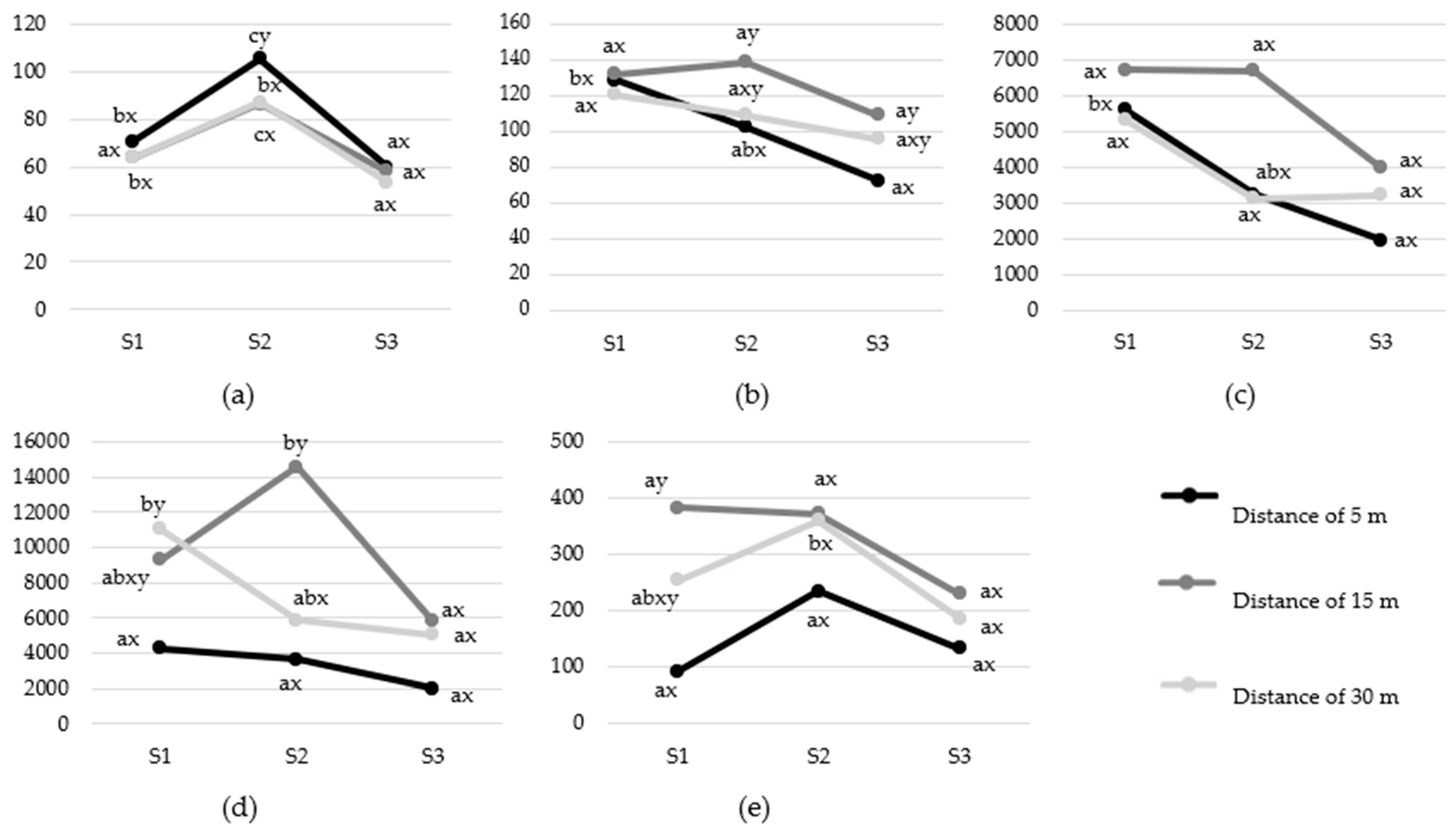
3.2. Cardiac Activity Responses
3.2.1. The Variant of the Study
3.2.2. Sound Exposure Distance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marini, D.; Meuleman, M.D.; Belson, S.; Rodenburg, T.B.; Llewellyn, R.; Lee, C. Developing an Ethically Acceptable Virtual Fencing System for Sheep. Animals 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Markus, S.B.; Bailey, D.W.; Jensen, D. Comparison of electric fence and a simulated fenceless control system on cattle movements. Livest. Sci. 2014, 170, 203–209. [Google Scholar] [CrossRef]
- Lee, C.; Campbell, D.L.M. A Multi-Disciplinary Approach to Assess the Welfare Impacts of a New Virtual Fencing Technology. Front. Vet. Sci. 2021, 8, 637709. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; Lea, J.M.; Farrer, W.J.; Haynes, S.J.; Lee, C. Tech-Savvy Beef Cattle? How Heifers Respond to Moving Virtual Fence Lines. Animals 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Hurley, G.J.; Swain, D.L.; Anderson, D.M.; Sikka, P.; Crossman, C.; Corke, P. Virtual fencing applications: Implementing and testing an automated cattle control system. Comput. Electron. Agric. 2007, 56, 14–22. [Google Scholar] [CrossRef]
- Umstatter, C.; Brocklehurst, S.; Ross, D.V.; Haskell, M.J. Can the location of cattle be managed using broadcast audio cues? Appl. Anim. Behav. Sci. 2013, 147, 34–42. [Google Scholar] [CrossRef]
- Lomax, S.; Colusso, P.; Clark, C.E.F. Does Virtual Fencing Work for Grazing Dairy Cattle? Animals 2019, 9, 429. [Google Scholar] [CrossRef]
- Marini, D.; Llewellyn, R.; Belson, S.; Lee, C. Controlling Within-Field Sheep Movement Using Virtual Fencing. Animals 2018, 8, 31. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; Haynes, S.J.; Lea, J.M.; Farrer, W.J.; Lee, C. Temporary Exclusion of Cattle from a Riparian Zone Using Virtual Fencing Technology. Animals 2018, 9, 5. [Google Scholar] [CrossRef]
- Jouven, M.; Leroy, H.; Ickowicz, A.; Lapeyronie, P. Can virtual fences be used to control grazing sheep? Rangel. J. 2012, 34, 111–123. [Google Scholar] [CrossRef]
- Herlin, A.; Brunberg, E.; Hultgren, J.; Högberg, N.; Rydberg, A.; Skarin, A. Animal Welfare Implications of Digital Tools for Monitoring and Management of Cattle and Sheep on Pasture. Animals 2021, 11, 829. [Google Scholar] [CrossRef]
- Umstatter, C.; Ross, D.; Haskell, M.J. Audio approaches in Virtual Fencing. In Precision Livestock Farming ’11; Lokhorst, C., Berckmans, D., Eds.; Czech Centre for Science and Society: Prague, Czech Republic, 2011; pp. 177–182. [Google Scholar]
- Butler, Z.; Corke, P.; Peterson, R.; Rus, D. Virtual Fences for Controlling Cows. In Proceedings of the 2004 IEEE International Conference on Robotics 8 Automation, New Orleans, LA, USA, 26 April–1 May 2004. [Google Scholar]
- Umstatter, C.; Tailleur, C.; Ross, D.; Haskell, M.J. Could virtual fences work without giving cows electric shocks? In Precision Livestock Farming ’09; Lokhorst, C., Groot Koerkamp, P.W.G., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2009; pp. 161–168. [Google Scholar]
- Butler, Z.; Corke, P.; Peterson, R.; Rus, D. From Robots to Animals: Virtual Fences for Controlling Cattle. Int. J. Robot. Res. 2006, 25, 485–508. [Google Scholar] [CrossRef]
- Umstatter, C. The evolution of virtual fences: A review. Comput. Electron. Agric. 2011, 75, 10–22. [Google Scholar] [CrossRef]
- Janicka, W.; Wilk, I. Praktyczne znaczenie badań nad behawiorem antydrapieżniczym w utrzymywaniu zwierząt gospodarskich (Practical importance of anti-predatory behavior research in keeping farm animals). Med. Weter. 2022, 78, 126–132. [Google Scholar] [CrossRef]
- Marliani, G.; Sprocatti, I.; Schiavoni, G.; Bellodi, A.; Accorsi, P.A. Evaluation of Horses’ Daytime Activity Budget in a Model of Ethological Stable: A Case Study in Italy. J. Appl. Anim. Welf. Sci. 2021, 24, 200–213. [Google Scholar] [CrossRef]
- Cooper, J.J.; Albentosa, M.J. Behavioural adaptation in the domestic horse: Potential role of apparently abnormal responses including stereotypic behaviour. Livest. Prod. Sci. 2005, 92, 177–182. [Google Scholar] [CrossRef]
- Squibb, K.; Griffin, K.; Favier, R.; Ijichi, C. Poker Face: Discrepancies in behaviour and affective states in horses during stressful handling procedures. Appl. Anim. Behav. Sci. 2018, 202, 34–38. [Google Scholar] [CrossRef]
- Rørvang, M.V.; Nielsen, B.L.; McLean, A.N. Sensory Abilities of Horses and Their Importance for Equitation Science. Front. Vet. Sci. 2020, 7, 663. [Google Scholar] [CrossRef]
- Janczarek, I.; Stachurska, A.; Kędzierski, W.; Wiśniewska, A.; Ryżak, M.; Kozioł, A. The intensity of physiological and behavioral responses of horses to predator vocalizations. BMC Vet. Res. 2020, 16, 431. [Google Scholar] [CrossRef]
- Heffner, H.E.; Heffner, R.S. The Evolution of Mammalian Sound Localization. Acoust. Today 2016, 12, 20–27. [Google Scholar]
- Barrera, J.P.; Chong, L.; Judy, K.N.; Blumstein, D.T. Reliability of public information: Predators provide more information about risk than conspecifics. Anim. Behav. 2011, 81, 779–787. [Google Scholar] [CrossRef]
- Adcock, S.J.J.; Tuckerm, C.B. Naïve domestic Bos taurus calves recognize the scent of a canine predator. Anim. Behav. 2020, 164, 173–180. [Google Scholar] [CrossRef]
- Arnould, C.; Malosse, C.; Signoret, J.-P.; Descoins, C. Which chemical constituents from dog feces are involved in its food repellent effect in sheep? J. Chem. Ecol. 1998, 24, 559–576. [Google Scholar] [CrossRef]
- Janczarek, I.; Wiśniewska, A.; Chruszczewski, M.H.; Tkaczyk, E.; Górecka– Bruzda, A. Social Behaviour of Horses in Response to Vocalisations of Predators. Animals 2020, 10, 2331. [Google Scholar] [CrossRef] [PubMed]
- Apfelbach, R.; Blanchard, C.D.; Blanchard, R.J.; Hayes, R.A.; McGregor, I.S. The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neurosci. Biobehav. Rev. 2005, 29, 1123–1144. [Google Scholar] [CrossRef]
- Aflitto, N.C.; Hofstetter, R.W. Use of acoustics to deter bark beetles from entering tree material. Pest Manag. Sci. 2014, 70, 1808–1814. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L. Human–caused Disturbance Stimuli as a Form of Predation Risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Janicka, W.; Wilk, I.; Ryżak, M. Horses’ perception of a threat posed by sounds of different origin. Med. Weter. 2022, 78, 401–4213. [Google Scholar] [CrossRef]
- Rochais, C.; Henry, S.; Hausberger, M. Spontaneous attention–capture by auditory distractors as predictor of distractibility: A study of domestic horses (Equus caballus). Sci. Rep. 2017, 7, 15283. [Google Scholar] [CrossRef]
- Christensen, J.W.; Keeling, L.J.; Nielsen, B.L. Responses of horses to novel visual, olfactory and auditory stimuli. Appl. Anim. Behav. Sci. 2005, 93, 53–65. [Google Scholar] [CrossRef]
- Seamana, S.C.; Davidson, H.P.B.; Waran, N.K. How reliable is temperament assessment in the domestic horse (Equus caballus)? Appl. Anim. Behav. Sci. 2002, 78, 175–191. [Google Scholar] [CrossRef]
- Stomp, N.; Leroux, M.; Cellier, M.; Henry, S.; Hausberger, M.; Lemasson, A. Snort acoustic structure codes for positive emotions in horses. Sci. Nat. 2018, 105, 57. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, T.; Bienboire-Frosini, C.; Kowalczyk, I.; Leclercq, J.; Arroub, S.; Pageat, P. Equine Activities Influence Horses’ Responses to Different Stimuli: Could This Have an Impact on Equine Welfare? Animals 2019, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, K.; Hall, C.; Royle, C.; Walker, S.L. Domesticated horses differ in their behavioural and physiological responses to isolated and group housing. Physiol. Behav. 2015, 143, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, E.; Jensen, M.B.; Nicol, H.J. Motivation for social contact in horses measured by operant conditioning. Appl. Anim. Behav. Sci. 2011, 132, 131–137. [Google Scholar] [CrossRef]
- Christensen, J.W.; Rundgren, M. Predator odour per se does not frighten domestic horses. Appl. Anim. Behav. Sci. 2008, 112, 136–145. [Google Scholar] [CrossRef]
- Scopa, C.; Palagi, E.; Sighieri, C.; Baragli, P. Physiological outcomes of calming behaviors support the resilience hypothesis in horses. Sci. Rep. 2018, 8, 17501. [Google Scholar] [CrossRef]
- Ricci–Bonot, C.; Romero, T.; Nicol, C.; Mills, D. Social buffering in horses is influenced by context but not by the familiarity and habituation of a companion. Sci. Rep. 2021, 11, 8862. [Google Scholar] [CrossRef]
- Christensen, J.W.; Malmkvist, J.; Nielsen, B.L.; Keeling, L.J. Effects of a calm companion on fear reactions in naïve test horses. Equine Vet. J. 2008, 40, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Safryghin, A.; Hebesberger, D.V.; Wascher, C.A.F. Testing for Behavioral and Physiological Responses of Domestic Horses (Equus caballus) Across Different Contexts–Consistency Over Time and Effects of Context. Front. Psychol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Floyd, T.; Erb, H.; Houpt, K. Preference and demand for exercise in stabled horses. Appl. Anim. Behav. Sci. 2011, 130, 91–100. [Google Scholar] [CrossRef]
- Lenoir, A.; Trachsel, D.S.; Younes, M.; Barrey, E.; Robert, C. Agreement between Electrocardiogram and Heart Rate Meter Is Low for the Measurement of Heart Rate Variability during Exercise in Young Endurance Horses. Front. Vet. Sci. 2017, 4, 170. [Google Scholar] [CrossRef] [PubMed]

| Day | Variant | Group of the Horses | Day | Variant | Group of the Horses |
|---|---|---|---|---|---|
| Week 1 | Week 2 | ||||
| 1 | Variant F | a | 7 | Variant S | a |
| 2 | Variant S | b | 8 | Variant F | b |
| 3 | Variant F | c | 9 | Variant S | c |
| 4 | Variant S | d | 10 | Variant F | d |
| 5 | Variant F | e | 11 | Variant S | e |
| 6 | Variant S | f | 12 | Variant F | f |
| Behaviour | Description |
|---|---|
| Tunnel completion [s] | Time taken by the horse to cross the tunnel from the start line to the finish line |
| Walk/trot/canter [%] | Time spent walking/trotting/cantering |
| Locomotion [%] | Total time of locomotion (walk, trot and canter) |
| Vigilance [%] | Time spent standing alert; standing still with elevated neck, intently orientated head and pinnae [34] |
| Snoring [freq.] | Total number of a very short raspy inhalation sound [35] |
| * Defecation [freq.] | Total number of repetitions of the elimination of faeces |
| ** Barrier effect [scale 1–4] | The first reaction to the sound; 1—continuing forward movement, 2—stopping, 3—going away, 4—running away |
| ** Latency time [s] | Latency time to respond to the sound; assessed only for horses that stopped, went away or ran away in response to the sound stimulus |
| Behavioural Variable | Study Factors | Stage of the Study | |
|---|---|---|---|
| B | C | ||
| Test variant (food—F/social—S reward) | |||
| Tunnel completion time [s] | F | 31.97 ± 20.27 ax | 99.67 ± 97.95 by |
| S | 25.27 ± 15.90 ax | 27.07 ± 25.45 ax | |
| Total locomotion [%] | F | 94.57 ± 6.28 bx | 68.57 ± 25.07 ax |
| S | 98.50 ± 5.01 ax | 93.50 ± 13.21 ay | |
| Vigilance [%] | F | 2.29 ± 3.98 ay | 18.60 ± 14.29 by |
| S | 0.00 ± 0.00 ax | 5.02 ± 12.32 bx | |
| Snoring [freq.] | F | 0.60 ± 1.99 ax | 4.03 ± 4.42 by |
| S | 0.33 ± 1.30 ax | 0.60 ± 2.30 ax | |
| Sound exposure distance (5/15/30 m from the speaker) | |||
| Vigilance [%] | 5 | 0.48 ± 2.13 ax | 8.29 ± 10.38 bx |
| 15 | 1.28 ± 3.58 ax | 13.69 ± 18.75 bx | |
| 30 | 1.67 ± 3.20 ax | 13.45 ± 18.75 bx | |
| Study Factors | Ignoring | Stopping | Going Away | Running Away |
|---|---|---|---|---|
| Variant of the study | ||||
| Food reward | 6 | 9 | 1 | 14 |
| Social reward | 24 | 4 | 2 | 0 |
| Sound exposure distance | ||||
| 5 m | 10 | 3 | 1 | 6 |
| 15 m | 10 | 4 | 1 | 5 |
| 30 m | 10 | 6 | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicka, W.; Wilk, I.; Próchniak, T.; Janczarek, I. Can Sound Alone Act as a Virtual Barrier for Horses? A Preliminary Study. Animals 2022, 12, 3151. https://doi.org/10.3390/ani12223151
Janicka W, Wilk I, Próchniak T, Janczarek I. Can Sound Alone Act as a Virtual Barrier for Horses? A Preliminary Study. Animals. 2022; 12(22):3151. https://doi.org/10.3390/ani12223151
Chicago/Turabian StyleJanicka, Wiktoria, Izabela Wilk, Tomasz Próchniak, and Iwona Janczarek. 2022. "Can Sound Alone Act as a Virtual Barrier for Horses? A Preliminary Study" Animals 12, no. 22: 3151. https://doi.org/10.3390/ani12223151
APA StyleJanicka, W., Wilk, I., Próchniak, T., & Janczarek, I. (2022). Can Sound Alone Act as a Virtual Barrier for Horses? A Preliminary Study. Animals, 12(22), 3151. https://doi.org/10.3390/ani12223151





