Higher Concentration of Dietary Selenium, Zinc, and Copper Complex Reduces Heat Stress-Associated Oxidative Stress and Metabolic Alteration in the Blood of Holstein and Jersey Steers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Diet
2.2. Recording of Temperature-Humidity Index (THI)
2.3. Sample Collection, and Processing
2.4. Analysis of Serum Biochemistry, Trace Minerals, SOD, and HSPs
2.5. Analysis of Serum Metabolites
2.5.1. Gas Chromatography–Tandem Mass Spectrometry
2.5.2. Sample Preparation for Profiling Analysis of OAs and FAs in Sera
2.5.3. Liquid Chromatography–Tandem Mass Spectrometry
2.5.4. Star Pattern Recognition and Multivariate Statistical Analysis in Sera
2.6. Statistical Analysis
3. Results
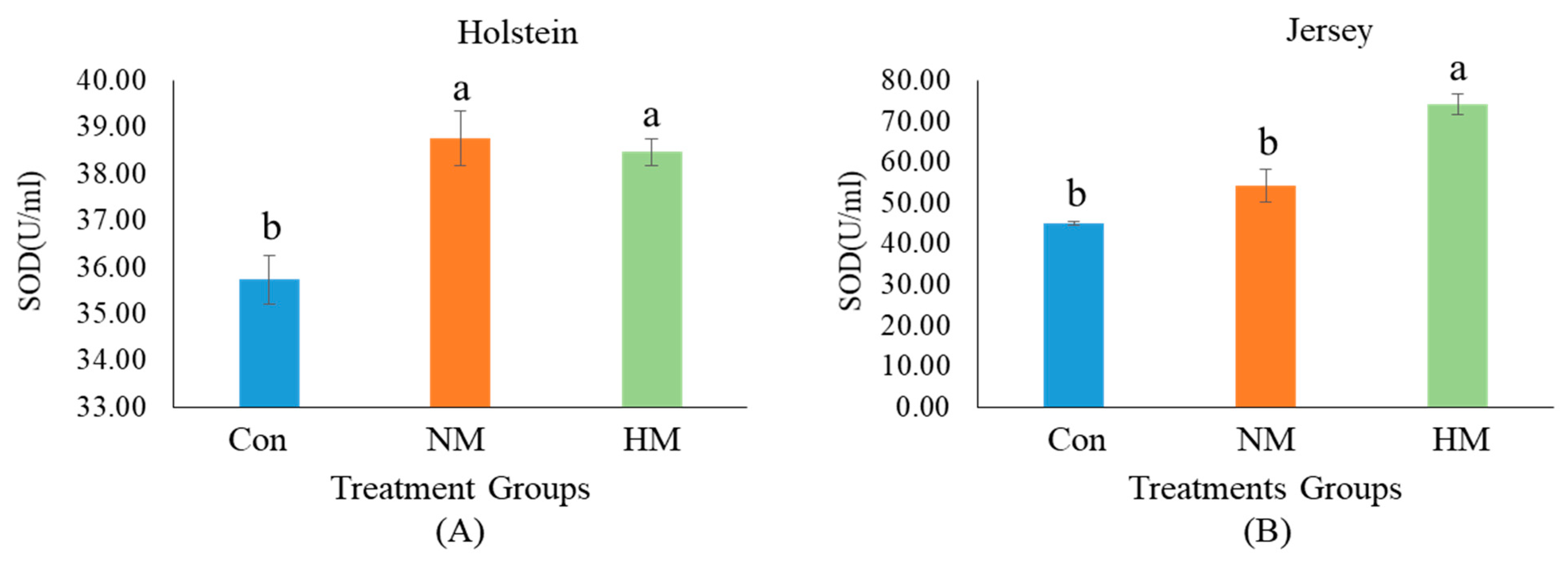
3.1. Growth Performance, Serum Biochemistry, Trace Minerals, SOD, and HSPs
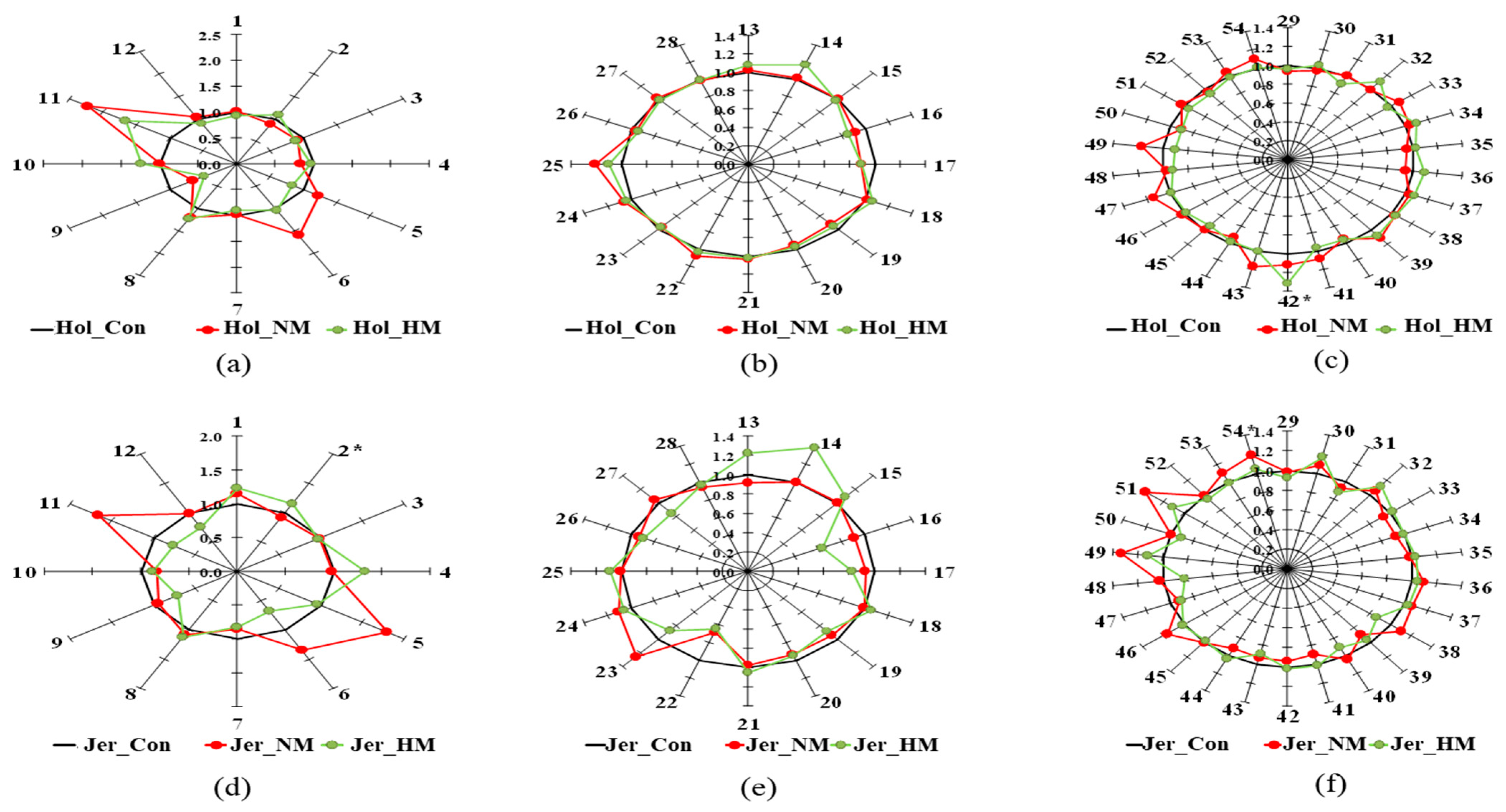
3.2. Serum Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: Consequences and opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ramos, S.C.; Valencia, R.A.; Cho, Y.I.; Lee, S.S. Heat Stress: Effects on Rumen Microbes and Host Physiology, and Strategies to Alleviate the Negative Impacts on Lactating Dairy Cows. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Trifković, J.; Jovanović, L.; Bošnjaković, D.; Savić, Đ.; Stefanović, S.; Krajišnik, T.; Sladojević, Ž.; Kirovski, D. Summer Season-Related Heat Stress Affects the Mineral Composition of Holstein Dams’ Colostrum, and Neonatal Calves’ Mineral Status and Hematological Profile. Biol. Trace Elem. Res. 2022, 200, 2122–2134. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Global Warming of 1.5 Degree Celsius: IPCC Special Report on the Impacts of Global Warming of 1.5 Degree Celsius. Intergovernmental Panel on Climate Change: Geneva, Switzerland. Available online: https://www.ipcc.ch/sr15 (accessed on 3 March 2021).
- Kim, E.S. Statistical interpretation of climate change in Seoul, Korea, over the last 98 years. J. Ecol. F. Biol. 2010, 33, 37–45. [Google Scholar] [CrossRef][Green Version]
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Min, L.; Li, D.; Tong, X.; Nan, X.; Ding, D.; Xu, B.; Wang, G. Nutritional strategies for alleviating the detrimental effects of heat stress in dairy cows: A review. Int. J. Biometeorol. 2019, 63, 1283–1302. [Google Scholar] [CrossRef]
- Islam, M.; Kim, S.-H.; Son, A.; Ramos, S.C.; Jeong, C.-D.; Yu, Z.; Kang, S.H.; Cho, Y.-I.; Lee, S.-S.; Cho, K.-K. Seasonal Influence on Rumen Microbiota, Rumen Fermentation, and Enteric Methane Emissions of Holstein and Jersey Steers under the Same Total Mixed Ration. Animals 2021, 11, 1184. [Google Scholar] [CrossRef]
- Eom, J.S.; Kim, E.T.; Kim, H.S.; Choi, Y.Y.; Lee, S.J.; Lee, S.S.; Kim, S.H.; Lee, S.S. Metabolomics comparison of serum and urine in dairy cattle using proton nuclear magnetic resonance spectroscopy. Anim. Biosci. 2021, 34, 1930–1939. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Zheng, N.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteomics 2015, 125, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, X.; Ping, J.; Lei, Z.; Gao, Y.; Ma, Z.; Jia, C.; Zhang, Z.; Li, X.; Jin, M. Metabonomics approach to assessing the modulatory effects of kisspeptin-10 on liver injury induced by heat stress in rats. Sci. Rep. 2017, 7, 7020. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, S.; Ouyang, J.; Ma, L.; Bu, D. Impacts of Heat Stress-Induced Oxidative Stress on the Milk Protein Biosynthesis of Dairy Cows. Animals 2021, 11, 726. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Rashamol, V.P.; Bagath, M.; Sejian, V.; Dunshea, F.R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 2021, 65, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Doctrow, S.R.; Oberley, L.W.; Kregel, K.C. Chronic antioxidant enzyme mimetic treatment differentially modulates hyperthermia-induced liver HSP70 expression with aging. J. Appl. Physiol. 2006, 100, 1385–1391. [Google Scholar] [CrossRef][Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Parashuramulu, S.; Nagalakshmi, D.; Rao, D.S.; Kumar, M.K.; Swain, P.S. Effect of Zinc supplementation on antioxidant status and immune response in buffalo calves. Anim. Nutr. Feed Technol. 2015, 15, 179–188. [Google Scholar] [CrossRef]
- Cortinhas, C.S.; Botaro, B.G.; Sucupira, M.C.A.; Renno, F.P.; Santos, M. V Antioxidant enzymes and somatic cell count in dairy cows fed with organic source of zinc, copper and selenium. Livest. Sci. 2010, 127, 84–87. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting oxidative stress and the use of organic selenium in dairy cow nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and Activators of SOD, GSH-Px, and CAT. Enzym. Inhib. Act. 2017, 29, 207–224. [Google Scholar]
- Zimmerman, M.T.; Bayse, C.A.; Ramoutar, R.R.; Brumaghim, J.L. Sulfur and selenium antioxidants: Challenging radical scavenging mechanisms and developing structure–activity relationships based on metal binding. J. Inorg. Biochem. 2015, 145, 30–40. [Google Scholar] [CrossRef]
- Conte, G.; Ciampolini, R.; Cassandro, M.; Lasagna, E.; Calamari, L.; Bernabucci, U.; Abeni, F. Feeding and nutrition management of heat-stressed dairy ruminants. Ital. J. Anim. Sci. 2018, 17, 604–620. [Google Scholar] [CrossRef]
- Weng, X.; Monteiro, A.P.A.; Guo, J.; Li, C.; Orellana, R.M.; Marins, T.N.; Bernard, J.K.; Tomlinson, D.J.; DeFrain, J.M.; Wohlgemuth, S.E. Effects of heat stress and dietary zinc source on performance and mammary epithelial integrity of lactating dairy cows. J. Dairy Sci. 2018, 101, 2617–2630. [Google Scholar] [CrossRef]
- López-Alonso, M.; Miranda, M. Copper supplementation, a challenge in cattle. Animals 2020, 10, 1890. [Google Scholar] [CrossRef]
- Sun, L.L.; Gao, S.T.; Wang, K.; Xu, J.C.; Sanz-Fernandez, M.V.; Baumgard, L.H.; Bu, D.P. Effects of source on bioavailability of selenium, antioxidant status, and performance in lactating dairy cows during oxidative stress-inducing conditions. J. Dairy Sci. 2019, 102, 311–319. [Google Scholar] [CrossRef]
- National Research Council Nutrient Requirements of Beef Cattle: Seventh Revised Edition: Update 2000; The National Academies Press: Washington, DC, USA, 2000.
- Kim, D.-H.; Kim, M.-H.; Kim, S.-B.; Son, J.-K.; Lee, J.-H.; Joo, S.-S.; Gu, B.-H.; Park, T.; Park, B.-Y.; Kim, E.-T. Differential dynamics of the ruminal microbiome of Jersey Cows in a heat stress environment. Animals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Matamoros, C.; Salfer, I.J.; Bartell, P.A.; Harvatine, K.J. Effect of the timing of sodium acetate infusion on the daily rhythms of milk synthesis and plasma metabolites and hormones in Holstein cows. J. Dairy Sci. 2022, 105, 7432–7445. [Google Scholar] [CrossRef]
- Zeyner, A.; Romanowski, K.; Vernunft, A.; Harris, P.; Müller, A.-M.; Wolf, C.; Kienzle, E. Effects of different oral doses of sodium chloride on the basal acid-base and mineral status of exercising horses fed low amounts of hay. PLoS ONE 2017, 12, e0168325. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.L.; Brümmer-Holder, M.; Dawson, K.A. Dietary trace mineral level and source affect fecal bacterial mineral incorporation and mineral leaching potential of equine feces. Sustainability 2019, 11, 7107. [Google Scholar] [CrossRef]
- Bharanidharan, R.; Arokiyaraj, S.; Bae Kim, E.; Hyun Lee, C.; Won Woo, Y.; Na, Y.; Kim, D.; Hoon Kim, K. Ruminal Methane Emissions, Metabolic, and Microbial Profile of Holstein Steers Fed Forage and Concentrate, Separately or as a Total Mixed Ration. PLoS ONE 2018, 13, e0202446. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 2005.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Van Soest, P. Collaborative study of acid-detergent fiber and lignin. J. Assoc. Off. Anal. Chem. 1973, 56, 781–784. [Google Scholar] [CrossRef]
- Davis, M.S.; Mader, T.L.; Holt, S.M.; Parkhurst, A.M. Strategies to reduce feedlot cattle heat stress: Effects on tympanic temperature. J. Anim. Sci. 2003, 81, 649–661. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.-H.; Oh, S.-J.; Lee, H.-S.; Ji, M.; Choi, S.; Lee, S.-S.; Paik, M.-J. Metabolomic analysis of organic acids, amino acids, and fatty acids in plasma of Hanwoo beef on a high-protein diet. Metabolomics 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Paik, M.-J.; Kim, K.-R. Sequential ethoxycarbonylation, methoximation and tert-butyldimethylsilylation for simultaneous determination of amino acids and carboxylic acids by dual-column gas chromatography. J. Chromatogr. A 2004, 1034, 13–23. [Google Scholar] [CrossRef]
- Paik, M.-J.; Lee, H.-J.; Kim, K.-R. Simultaneous retention index analysis of urinary amino acids and carboxylic acids for graphic recognition of abnormal state. J. Chromatogr. B 2005, 821, 94–104. [Google Scholar] [CrossRef]
- Seo, C.; Hwang, Y.-H.; Kim, Y.; Joo, B.S.; Yee, S.-T.; Kim, C.M.; Paik, M.-J. Metabolomic study of aging in mouse plasma by gas chromatography–mass spectrometry. J. Chromatogr. B 2016, 1025, 1–6. [Google Scholar] [CrossRef]
- Seo, C.; Kim, S.-H.; Lee, H.-S.; Ji, M.; Min, J.; Son, Y.-J.; Kim, I.-H.; Lee, K.; Paik, M.-J. Metabolomic study on bleomycin and polyhexamethylene guanidine phosphate-induced pulmonary fibrosis mice models. Metabolomics 2019, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, B.A.; Powers, J.B.; Campagna, S.R.; Seay, T.B.; Embree, M.M.; Myer, P.R. Rumen fluid metabolomics of beef steers differing in feed efficiency. Metabolomics 2020, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Seo, C.; Kim, Y.-A.; Park, M.; Choi, B.; Ji, M.; Lee, S.; Paik, M.-J. Metabolomic study of polyamines in rat urine following intraperitoneal injection of γ-hydroxybutyric acid. Metabolomics 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Ametaj, B.N.; Bouatra, S.; Mandal, R.; Zebeli, Q.; Dunn, S.M.; Wishart, D.S. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J. Dairy Sci. 2012, 95, 6606–6623. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Xia, C.; Zhang, H.; Qian, W.; Cao, Y. Pathway analysis of plasma different metabolites for dairy cow ketosis. Ital. J. Anim. Sci. 2016, 15, 545–551. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis Systems for Windows; Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Armstrong, D. Heat stress interaction with shade and cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Hahn, G.L.; Mader, T.L.; Spiers, D.E.; Gaughan, J.B.; Nienaber, J.A.; Eigenberg, R.; Brown-Brandl, T.; Hu, Q.; Griffin, D.; Hungerford, L.; et al. Heat wave impacts on feedlot cattle: Considerations for improved environmental management. In Proceedings of the Sixth International Livestock Environment Symposium, Louisville, Kentucky, 21–23 May 2001; ASAE: St. Joseph, MI, USA, 2001. [Google Scholar]
- Vitali, A.; Segnalini, M.; Bertocchi, L.; Bernabucci, U.; Nardone, A.; Lacetera, N. Seasonal pattern of mortality and relationships between mortality and temperature-humidity index in dairy cows. J. Dairy Sci. 2009, 92, 3781–3790. [Google Scholar] [CrossRef]
- Pambu-Gollah, R.; Cronje, P.B.; Casey, N.H. An evaluation of the use of blood metabolite concentrations as indicators of nutritional status in free-ranging indigenous goats. S. Afr. J. Anim. Sci. 2000, 30, 115–120. [Google Scholar] [CrossRef][Green Version]
- Joo, S.S.; Lee, S.J.; Park, D.S.; Kim, D.H.; Gu, B.-H.; Park, Y.J.; Rim, C.Y.; Kim, M.; Kim, E.T. Changes in blood metabolites and immune cells in holstein and jersey dairy cows by heat stress. Animals 2021, 11, 974. [Google Scholar] [CrossRef]
- Srikandakumar, A.; Johnson, E.H. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian Milking Zebu cows. Trop. Anim. Health Prod. 2004, 36, 685–692. [Google Scholar] [CrossRef]
- Ganong, W.F. Energy balance, metabolism, and nutrition. In Review of Medical Physiology, 14th ed.; Appleton and Lange Publishers: New York, NY, USA, 1989. [Google Scholar]
- Bannister, J.; Bannister, W.H.; Rotilio, G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit. Rev. Biochem. 1987, 22, 111–180. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.-S.; Ko, Y.-H.; Moon, Y.-S.; Sohn, S.-H. Effects of vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian-Australas. J. Anim. Sci. 2014, 27, 749. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Werner, T.M.; Butler, J.M. Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poult. Sci. 2007, 86, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, S.; Khan, A.Z.; Parveen, F.; Nizamani, Z.A.; Siyal, F.A.; Abd El-Hack, M.E.; Gan, F.; Liu, Y.; Hamid, M.; Nido, S.A. Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature. Amb Express 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Auffret, M.D.; Dewhurst, R.J.; Duthie, C.A.; Rooke, J.A.; John Wallace, R.; Freeman, T.C.; Stewart, R.; Watson, M.; Roehe, R. The rumen microbiome as a reservoir of antimicrobial resistance and pathogenicity genes is directly affected by diet in beef cattle. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Banu, G.S.; Kumar, G.; Murugesan, A.G. Ethanolic leaves extract of Trianthema portulacastrum L. ameliorates aflatoxin B 1 induced hepatic damage in rats. Indian J. Clin. Biochem. 2009, 24, 250–256. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, J.-S.; Jeon, S.W.; Peng, D.Q.; Kim, Y.S.; Bae, M.H.; Jo, Y.H.; Lee, H.G. Correlation between blood, physiological and behavioral parameters in beef calves under heat stress. Asian-Australasian J. Anim. Sci. 2018, 31, 919. [Google Scholar] [CrossRef]
- Fan, C.; Di Su, H.T.; Li, X.; Li, Y.; Ran, L.; Hu, R.; Cheng, J. Liver metabolic perturbations of heat-stressed lactating dairy cows. Asian-Australasian J. Anim. Sci. 2018, 31, 1244. [Google Scholar] [CrossRef]
- Abbas, Z.; Sammad, A.; Hu, L.; Fang, H.; Xu, Q.; Wang, Y. Glucose metabolism and dynamics of facilitative glucose transporters (Gluts) under the influence of heat stress in dairy cattle. Metabolites 2020, 10, 312. [Google Scholar] [CrossRef]
- Hocquette, J.-F.; Abe, H. Facilitative glucose transporters in livestock species. Reprod. Nutr. Dev. 2000, 40, 517–533. [Google Scholar] [CrossRef]
- Liao, Y.; Hu, R.; Wang, Z.; Peng, Q.; Dong, X.; Zhang, X.; Zou, H.; Pu, Q.; Xue, B.; Wang, L. Metabolomics profiling of serum and urine in three beef cattle breeds revealed different levels of tolerance to heat stress. J. Agric. Food Chem. 2018, 66, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Birungi, G.; Chen, S.M.; Loy, B.P.; Ng, M.L.; Li, S.F.Y. Metabolomics approach for investigation of effects of dengue virus infection using the EA. hy926 cell line. J. Proteome Res. 2010, 9, 6523–6534. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Pan, G.; Qiu, Y.; Yang, L.; Su, M.; Liu, Y.; Chen, J.; Feng, G.; Fang, Y.; Jia, W. Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J. Proteome Res. 2011, 10, 5433–5443. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, D. Implication of metabolomic profiles to wide thermoneutral zone in Mongolian gerbils (Meriones unguiculatus). Integr. Zool. 2016, 11, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Jodar, A.; Nayan, N.H.; Hamzaoui, S.; Caja, G.; Salama, A.A.K. Heat stress modifies the lactational performances and the urinary metabolomic profile related to gastrointestinal microbiota of dairy goats. PLoS ONE 2019, 14, e0202457. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, X.; Lei, Z.; Ping, J.; Liu, J.; Ma, Z.; Zhang, Z.; Jia, C.; Jin, M.; Li, X. Heat-stress-induced metabolic changes and altered male reproductive function. J. Proteome Res. 2015, 14, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.T.; Guo, J.; Quan, S.Y.; Nan, X.M.; Fernandez, M.V.S.; Baumgard, L.H.; Bu, D.P. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017, 100, 5040–5049. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. The effects of hyperthermia on nutrient partitioning. In Proceedings of the Cornell Nutrition Conference for Feed Manufacturers; Cornell University Department of Animal Science: New York, NY, USA, 2007. [Google Scholar]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]



| Ingredients | Compositions (% of DM) |
|---|---|
| Corn gluten feed | 8.40 |
| Soybean | 6.24 |
| Beet pulp | 4.20 |
| Wheat bran | 3.15 |
| Corn flakes | 2.21 |
| Molasses | 1.04 |
| Rice wine residue | 5.25 |
| Brewer’s grain residue | 21.01 |
| Annual ryegrass straw | 27.29 |
| Orchard grass straw | 21.01 |
| Limestone | 0.10 |
| Sodium bicarbonate | 0.01 |
| Salt | 0.09 |
| Total | 100.00 |
| Chemical composition (DM basis) | % or ppm |
| DM (fresh basis) | 58.98% |
| CP | 13.55% |
| Crude Fiber | 21.92% |
| Crude fat | 3.02% |
| Ash | 9.21% |
| Calcium | 1.22% |
| Phosphorus | 0.47% |
| NDF | 48.00% |
| ADF | 25.36% |
| Zinc | 77.35 ppm |
| Copper | 17.31 ppm |
| Selenium | 0.05 ppm |
| Period | Mean Ambient Temp (°C) | rH (%) | THI |
|---|---|---|---|
| Period 1 | 30.50 ± 0.60 | 80.67 ± 5.63 | 83.77 ± 0.55 |
| Period 2 | 28.49 ± 1.24 | 88.13 ± 3.98 | 81.60 ± 2.01 |
| Period 3 | 29.74 ± 1.35 | 83.86 ± 6.15 | 83.00 ± 1.69 |
| Average | 29.58 ± 1.02 | 84.22 ± 3.74 | 82.79 ± 1.10 |
| Parameters | Breed | Treatment | SEM (4) | GLM p-Value (5) | Mixed p-Value (6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Con (1) | NM (2) | HM (3) | IN | All | B | T | B × T | |||
| IBW | Holstein | 783.67 | 782.33 | 782.00 | 25.355 | 19.174 | 0.9988 | <0.0001 | 0.9934 | 0.9995 |
| Jersey | 579.67 | 577.00 | 577.00 | 12.994 | 0.9895 | |||||
| DMI (kg/d) | Holstein | 12.74 | 13.32 | 12.90 | 1.541 | 1.172 | 0.9665 | 0.1250 | 0.9474 | 0.9816 |
| Jersey | 11.02 | 11.29 | 11.37 | 0.803 | 0.9571 | |||||
| FBW | Holstein | 793.00 | 792.33 | 792.33 | 25.265 | 19.246 | 0.9998 | <0.0001 | 0.9992 | 0.9974 |
| Jersey | 592.67 | 595.00 | 594.00 | 13.227 | 0.9941 | |||||
| ADG (kg) | Holstein | 0.78 | 0.83 | 0.86 | 0.090 | 0.096 | 0.877 | 0.0002 | 0.1476 | 0.3498 |
| Jersey | 1.08 | 1.50 | 1.42 | 0.102 | 0.1250 | |||||
| FE | Holstein | 0.06 | 0.07 | 0.07 | 0.009 | 0.010 | 0.8830 | <0.001 | 0.2305 | 0.4543 |
| Jersey | 0.10 | 0.13 | 0.13 | 0.012 | 0.2427 | |||||
| Ca (mg/dL) | Holstein | 8.97 | 8.80 | 8.73 | 0.179 | 0.118 | 0.6643 | 0.0482 | 0.0931 | 0.8349 |
| Jersey | 9.17 | 9.00 | 8.90 | 0.075 | 0.2519 | |||||
| Mg (mg/dL) | Holstein | 2.28 | 2.44 | 2.41 | 0.079 | 0.070 | 0.3743 | 0.1064 | 0.5210 | 0.0625 |
| Jersey | 2.62 | 2.39 | 2.57 | 0.071 | 0.1394 | |||||
| P (mg/dL) | Holstein | 6.95 | 7.15 | 7.30 | 0.367 | 0.392 | 0.8465 | 0.7570 | 0.7987 | 0.9852 |
| Jersey | 7.15 | 7.20 | 7.40 | 0.417 | 0.9235 | |||||
| BUN (mg/dL) | Holstein | 10.50 | 9.00 | 9.00 | 1.167 | 0.778 | 0.7542 | 0.5529 | 0.2266 | 0.9033 |
| Jersey | 11.50 | 9.67 | 9.00 | 0.389 | 0.1003 | |||||
| Glu (mg/dL) | Holstein | 81.33 | 78.50 | 78.50 | 0.889 | 2.170 | 0.1280 | 0.2234 | 0.5971 | 0.9513 |
| Jersey | 78.50 | 76.33 | 76.00 | 3.229 | 0.8587 | |||||
| TP (g/dL) | Holstein | 8.05 | 8.30 | 8.75 | 0.300 | 4.103 | 0.5801 | 0.2822 | 0.3284 | 0.3151 |
| Jersey | 8.45 | 8.20 | 8.05 | 0.238 | 0.5827 | |||||
| CHOL (mg/dL) | Holstein | 138.00 | 139.50 | 128.50 | 7.333 | 11.540 | 0.6413 | 0.6214 | 0.9427 | 0.7271 |
| Jersey | 148.50 | 141.33 | 141.00 | 12.734 | 0.9018 | |||||
| Cu (μg/L) | Holstein | 740 | 830 | 940 | 50.00 | 100.34 | 0.1542 | 0.3943 | 0.7605 | 0.8748 |
| Jersey | 880 | 970 | 1080 | 50.00 | 0.2162 | |||||
| Zn (μg/L) | Holstein | 750 | 855 | 910 | 71.67 | 50.00 | 0.4562 | 0.0235 | 0.0346 | 1.000 |
| Jersey | 815 | 900 | 930 | 71.44 | 0.6261 | |||||
| Se (μg/L) | Holstein | 106.50 c | 142.00 b | 218.50 a | 4.000 | 8.825 | 0.0017 | 0.0626 | 0.0004 | 0.2701 |
| Jersey | 77.00 b | 143.33 a | 174.50 a | 13.651 | 0.0383 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, A.-R.; Kim, S.-H.; Islam, M.; Oh, S.-J.; Paik, M.-J.; Lee, S.-S.; Lee, S.-S. Higher Concentration of Dietary Selenium, Zinc, and Copper Complex Reduces Heat Stress-Associated Oxidative Stress and Metabolic Alteration in the Blood of Holstein and Jersey Steers. Animals 2022, 12, 3104. https://doi.org/10.3390/ani12223104
Son A-R, Kim S-H, Islam M, Oh S-J, Paik M-J, Lee S-S, Lee S-S. Higher Concentration of Dietary Selenium, Zinc, and Copper Complex Reduces Heat Stress-Associated Oxidative Stress and Metabolic Alteration in the Blood of Holstein and Jersey Steers. Animals. 2022; 12(22):3104. https://doi.org/10.3390/ani12223104
Chicago/Turabian StyleSon, A-Rang, Seon-Ho Kim, Mahfuzul Islam, Song-Jin Oh, Man-Jeong Paik, Sung-Sill Lee, and Sang-Suk Lee. 2022. "Higher Concentration of Dietary Selenium, Zinc, and Copper Complex Reduces Heat Stress-Associated Oxidative Stress and Metabolic Alteration in the Blood of Holstein and Jersey Steers" Animals 12, no. 22: 3104. https://doi.org/10.3390/ani12223104
APA StyleSon, A.-R., Kim, S.-H., Islam, M., Oh, S.-J., Paik, M.-J., Lee, S.-S., & Lee, S.-S. (2022). Higher Concentration of Dietary Selenium, Zinc, and Copper Complex Reduces Heat Stress-Associated Oxidative Stress and Metabolic Alteration in the Blood of Holstein and Jersey Steers. Animals, 12(22), 3104. https://doi.org/10.3390/ani12223104







