Compensability of Enhanced Cytoplasmic Droplet Rates in Boar Semen: Insights of a Retrospective Field Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Boar and Semen Samples
2.2. Semen Quality Records
2.3. Heat and Cold Resistance of Semen Samples
2.4. Fertility Records
2.5. Statistical Analysis
3. Results
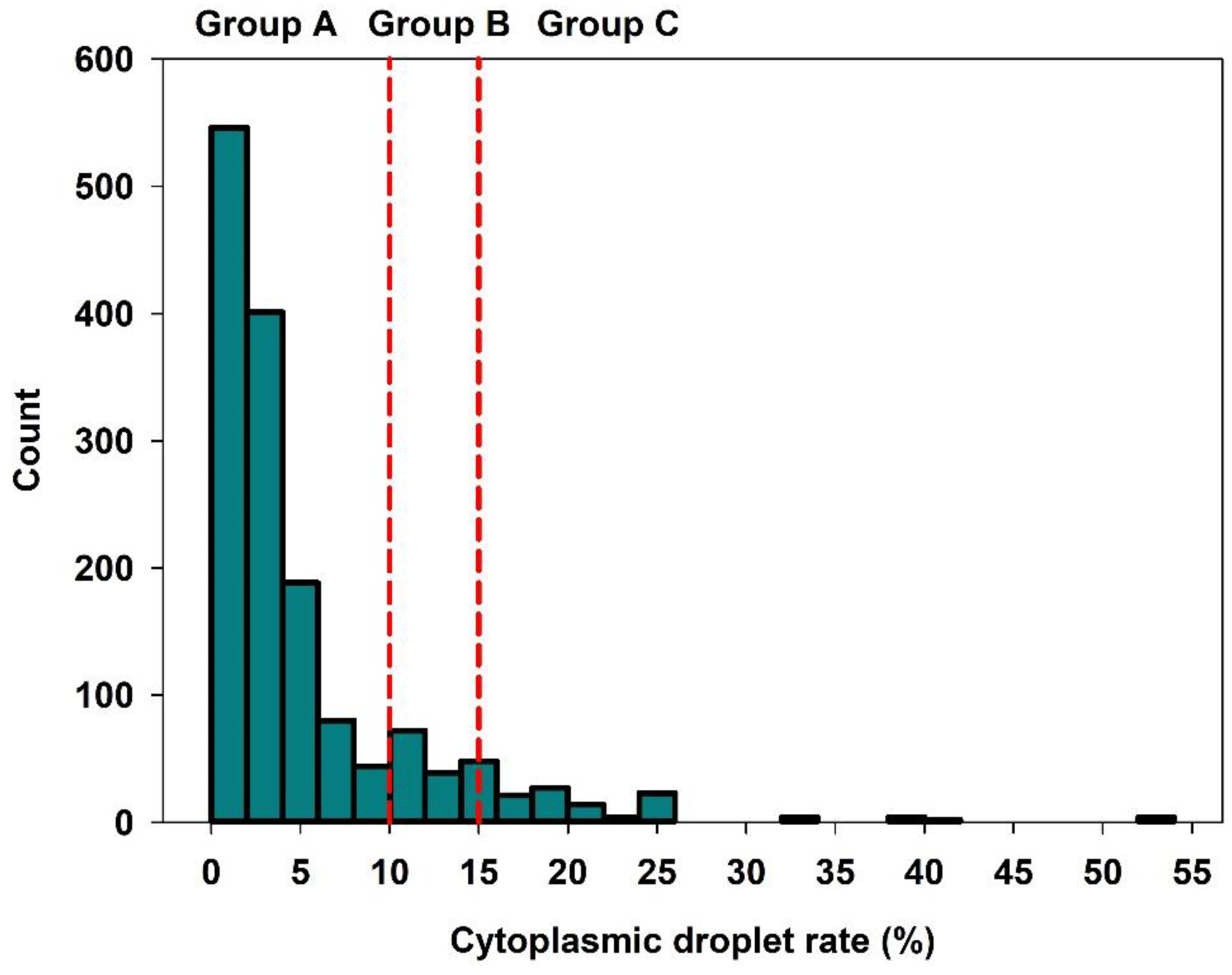
3.1. Descriptive Statistics of Semen Related Factors
3.2. Descriptive Statistics of Sow Related Factors
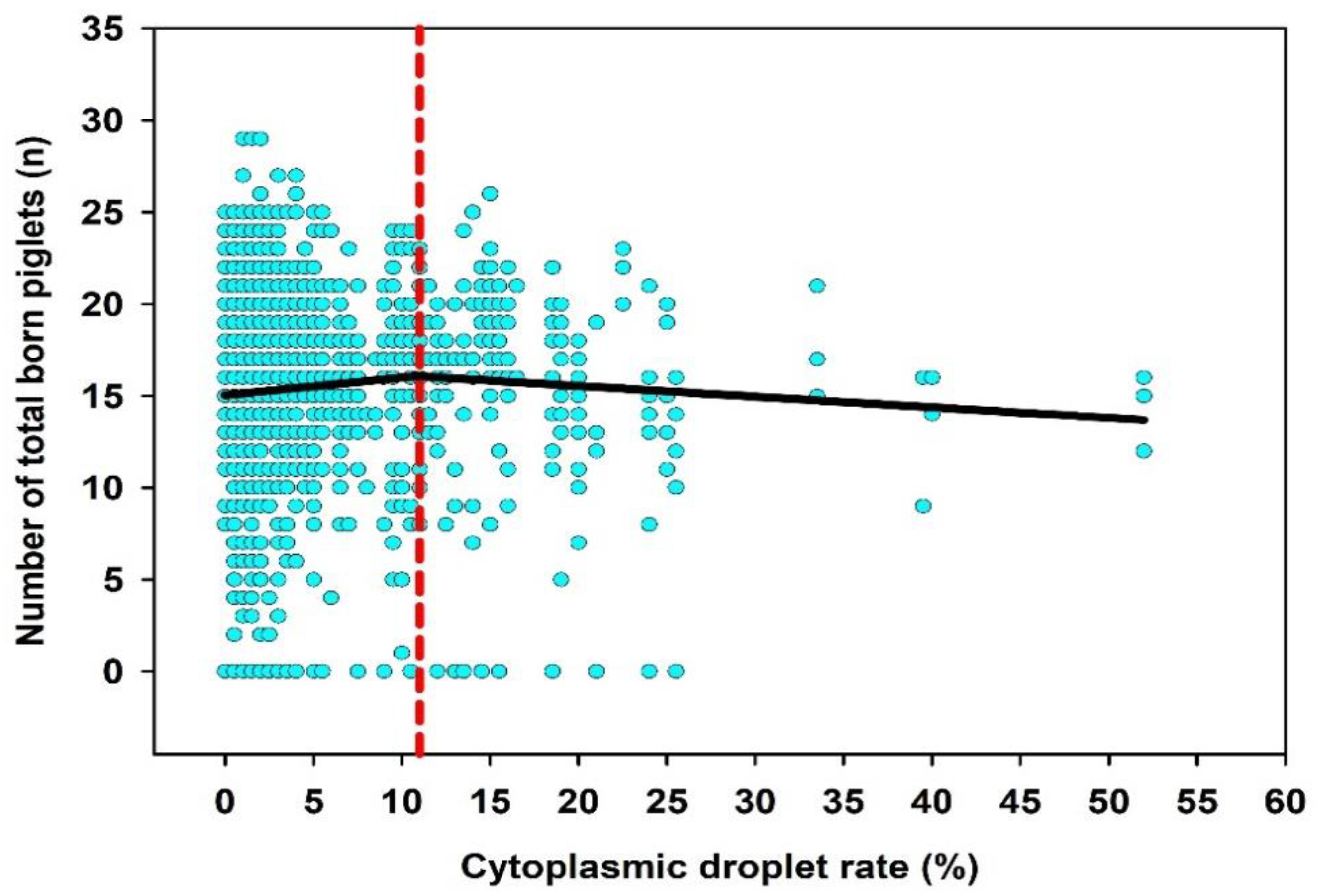
3.3. Relation between the CD Rate and Fertility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dacheux, J.L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2013, 147, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G. The epididymis, cytoplasmic droplets and male fertility. Asian J. Androl. 2011, 13, 130–138. [Google Scholar] [CrossRef]
- Waberski, D.; Meding, S.; Dirksen, G.; Weitze, K.; Leiding, C.; Hahn, R. Fertility of long-term stored boar semen: Influence of extender (Androhep and Kiev), storage time and plasma droplets in the semen. Anim. Reprod. Sci. 1994, 36, 145–151. [Google Scholar] [CrossRef]
- Gadea, J.; Sellés, E.; Marco, M.A. The predictive value of porcine seminal parameters on fertility outcome under commercial conditions. Reprod. Domest. Anim. 2004, 39, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lovercamp, K.W.; Safranski, T.J.; Fischer, K.A.; Manandhar, G.; Sutovsky, M.; Herring, W.; Sutovsky, P. High resolution light microscopic evaluation of boar semen quality sperm cytoplasmic droplet retention in relationship with boar fertility parameters. Arch. Androl. 2007, 53, 219–228. [Google Scholar] [CrossRef]
- Schulze, M.; Rüdiger, K.; Müller, K.; Jung, M.; Well, C.; Reissmann, M. Development of an in vitro index to characterize fertilizing capacity of boar ejaculates. Anim. Reprod. Sci. 2013, 140, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef]
- García-Vázquez, F.A.; Hernández-Caravaca, I.; Matás, C.; Soriano-Úbeda, C.; Abril-Sánchez, S.; Izquierdo-Rico, M.J. Morphological study of boar sperm during their passage through the female genital tract. J. Reprod. Dev. 2015, 61, 407–413. [Google Scholar] [CrossRef]
- Henning, H.; Luther, A.-M.; Höfner, L.; Waberski, D. Compensability of an enhanced incidence of spermatozoa with cytoplasmic droplets in boar semen for use in artificial insemination: A single cell approach. Under review.
- Saacke, R.G.; Dalton, J.C.; Nadir, S.; Nebel, R.L.; Bame, J.H. Relationship of seminal traits and insemination time to fertilization rate and embryo quality. Anim. Reprod. Sci. 2000, 60–61, 663–677. [Google Scholar] [CrossRef]
- Amann, R.P.; Saacke, R.G.; Barbato, G.F.; Waberski, D. Measuring Male-to-Male Differences in Fertility or Effects of Semen Treatments. Annu. Rev. Anim. Biosci. 2018, 6, 255–286. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Friedrich, J.; Drommer, W.; Bicker, G.; Waberski, D.; Töpfer-Petersen, E. Kinetic characterization of the changes in protein tyrosine phosphorylation of membranes, cytosolic Ca2+ concentration and viability in boar sperm populations selected by binding to oviductal epithelial cells. Reproduction 2001, 122, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Castillo-Martín, M.; Bonet, S.; Briz, M.D. Direct binding of boar ejaculate and epididymal spermatozoa to porcine epididymal epithelial cells is also needed to maintain sperm survival in in vitro co-culture. Anim. Reprod. Sci. 2012, 131, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Broekhuijse, M.L.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Broekhuijse, M.L.; Feitsma, H.; Gadella, B.M. Field data analysis of boar semen quality. Reprod. Domest. Anim. 2011, 46 (Suppl. 2), 59–63. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Mohammadpour, F.; Schröter, F.; Jakop, U.; Hönicke, H.; Hasenfuss, T.; Henne, H.; Schön, J.; Müller, K. Suitability of semen stress tests for predicting fertilizing capacity of boar ejaculates. Theriogenology 2021, 176, 73–81. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for the Least-Square estimation of non-linear parameters. SIAM J. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Silva, P.F.; Gadella, B.M. New assays for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze–thawing. Theriogenology 2005, 63, 458–469. [Google Scholar] [CrossRef]
- Flesch, F.M.; Brouwers, J.F.; Nievelstein, P.F.; Verkleij, A.J.; van Golde, L.M.; Colenbrander, B.; Gadella, B.M. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J. Cell Sci. 2001, 114, 3543–3555. [Google Scholar] [CrossRef]
- Henning, H.; Luther, A.M.; Waberski, D. A High incidence of sperm with cytoplasmic droplets affects the response to bicarbonate in preserved boar semen. Animals 2021, 11, 2570. [Google Scholar] [CrossRef]
- Den Daas, J.H.; De Jong, G.; Lansbergen, L.M.; Van Wagtendonk-De Leeuw, A.M. The relationship between the number of spermatozoa inseminated and the reproductive efficiency of individual dairy bulls. J. Dairy Sci. 1998, 81, 1714–1723. [Google Scholar] [CrossRef]
- Roca, J.; Broekhuijse, M.L.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar differences in artificial insemination outcomes: Can they be minimized? Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 48–55. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, I.; del Olmo, D.; Sijses, L.; Martinez-Alborcia, M.J.; Cuello, C.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Differences in the ability of spermatozoa from individual boar ejaculates to withstand different semen-processing techniques. Anim. Reprod. Sci. 2012, 132, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Buder, S.; Rüdiger, K.; Beyerbach, M.; Waberski, D. Influences on semen traits used for selection of young AI boars. Anim. Reprod. Sci. 2014, 148, 164–170. [Google Scholar] [CrossRef]
- Banaszewska, D.; Biesiada-Drzazga, B.; Andraszek, K. Frequency of cytoplasmic droplets depends on the breed and age of insemination boars. Folia Biol. 2015, 63, 9–18. [Google Scholar] [CrossRef] [PubMed]
| Sperm Quality Characteristics | Day 1 | Mean | SD | Min | Median | Max |
|---|---|---|---|---|---|---|
| Semen volume, mL | 0 | 214.9 | 72.7 | 20.0 | 205.0 | 408.0 |
| Sperm concentration, 109/mL | 0 | 0.313 | 0.122 | 0.119 | 0.284 | 0.874 |
| Sperm output, 109 | 0 | 58.6 | 18.4 | 9.8 | 57.4 | 119.9 |
| Dose volume, mL | 1 | 87.6 | 1.5 | 83.4 | 87.5 | 92.5 |
| Total sperm number per dose, 109 | 1 | 2.24 | 0.48 | 1.15 | 2.24 | 5.06 |
| Mitochondrial active sperm, % | 2 | 76.2 | 6.6 | 53.9 | 76.8 | 88.2 |
| Membrane intact sperm, % | 2 | 78.0 | 6.5 | 55.9 | 78.5 | 89.7 |
| Morphologically normal sperm, % | 3 | 79.4 | 13.1 | 30.5 | 81.5 | 99.0 |
| Cytoplasmic droplet rate, % | 3 | 5.0 | 6.4 | 0 | 2.5 | 52.0 |
| Total sperm motility, % | 3 | 81.7 | 15.3 | 15.5 | 87.7 | 96.9 |
| Cold-resistant sperm, % | 3 | 51.9 | 16.4 | 18.8 | 50.6 | 91.9 |
| Heat-resistant sperm, % | 7 | 52.1 | 23.2 | 8.2 | 58.6 | 84.9 |
| Parameter | Mean | SD | Min | Median | Max |
|---|---|---|---|---|---|
| Parity | 4.2 | 2.4 | 1.0 | 4.0 | 13.0 |
| Weaning-to-estrus interval, d | 6.0 | 5.8 | 2.0 | 4.0 | 54.0 |
| Gestation length, d | 115.7 | 1.6 | 79.0 | 116.0 | 120.0 |
| Farrowing rate, % | 94.9 | 7.1 | 50.0 | 100.0 | 100.0 |
| Lactation period, d | 26.0 | 4.2 | 0 | 27.0 | 48.0 |
| Total number of piglets born, n | 15.4 | 5.3 | 0 | 16.0 | 29.0 |
| Number of live born piglets, n | 14.7 | 3.7 | 1.0 | 15.0 | 27.0 |
| Number of stillborn piglets, n | 1.5 | 2.0 | 0 | 1.0 | 18.0 |
| Number of piglets weaned per litter, n | 11.3 | 2.6 | 0 | 12.0 | 34.0 |
| Farrowing interval, d | 149.1 | 6.6 | 127.0 | 147.0 | 204.0 |
| (a) | |||||
| CD Group | Sows (n) | Parity | Lactation Period (d) | Weaning-to-Estrus Interval (d) | Gestation Length (d) |
| A | 1235 | 4 (2–6) | 27 (25–27) a | 4 (4–5) a | 116 (115–117) |
| B | 125 | 4 (2–6) | 27 (26–27) a | 4 (4–5) a | 116 (115–116) |
| C | 137 | 4 (2–6) | 27 (26–28) a | 5 (4–5) a | 115 (115–116) |
| p-value | 0.114 | 0.039 | 0.036 | 0.117 | |
| (b) | |||||
| CD Group | Sows (n) | Farrowing Rate (%) | Total Number of Piglets Born | Number of Live Born Piglets | Number of StillbornPiglets |
| A | 1235 | 96.0 (90.9–100) a | 16 (13–18) | 15 (13–17) | 1 (0–2) a |
| B | 125 | 94.4 (88.9–100) a | 17 (14–19) | 15.5 (13–17) | 1 (0–3) a |
| C | 137 | 100(93.8–100) b | 16 (14–19) | 15 (13–17) | 1 (0–2) a |
| p-value | <0.001 | 0.205 | 0.806 | 0.044 | |
| (a) | |||||
| CD Group | Sows (n) | Total Sperm Number per Dose (109) | Total Sperm Motility (%) | Morphologically Normal Sperm (%) | |
| A | 1235 | 2.28 (1.95–2.56) a | 87.3 (80.5–91.1) a | 85.5 (74.5–91.5) a | |
| B | 125 | 2.20 (2.00–2.35) a,b | 88.9 (82.1–92.2) a | 70.5 (61.5–75.3) b | |
| C | 137 | 2.08 (1.77–2.24) b | 91.7 (84.3–93.4) b | 63.5 (60.3–70.5) b | |
| p-value | <0.001 | <0.001 | 0.002 | ||
| (b) | |||||
| CD Group | Sows (n) | Mitochondrial Active Sperm (%) | Membrane Intact Sperm (%) | Heat-Resistant Sperm (%) | Cold-Resistant Sperm (%) |
| A | 1235 | 77.0 (71.8–81.9) a | 78.5 (74.1–83.0) | 56.7 (31.0–72.4) a | 48.7 (38.6–66.1) a |
| B | 125 | 76.7 (73.6–79.8) a | 79.9 (73.8–81.2) | 62.5 (48.1–75.7) b | 52.0 (39.1–60.4) a |
| C | 137 | 75.1 (71.4–79.3) a | 76.9 (73.3–81.3) | 60.8 (50.0–72.7) b | 60.5 (49.6–71.5) b |
| p-value | 0.019 | 0.466 | <0.001 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, M.; Waberski, D. Compensability of Enhanced Cytoplasmic Droplet Rates in Boar Semen: Insights of a Retrospective Field Study. Animals 2022, 12, 2892. https://doi.org/10.3390/ani12202892
Schulze M, Waberski D. Compensability of Enhanced Cytoplasmic Droplet Rates in Boar Semen: Insights of a Retrospective Field Study. Animals. 2022; 12(20):2892. https://doi.org/10.3390/ani12202892
Chicago/Turabian StyleSchulze, Martin, and Dagmar Waberski. 2022. "Compensability of Enhanced Cytoplasmic Droplet Rates in Boar Semen: Insights of a Retrospective Field Study" Animals 12, no. 20: 2892. https://doi.org/10.3390/ani12202892
APA StyleSchulze, M., & Waberski, D. (2022). Compensability of Enhanced Cytoplasmic Droplet Rates in Boar Semen: Insights of a Retrospective Field Study. Animals, 12(20), 2892. https://doi.org/10.3390/ani12202892






