Cladomorphus petropolisensis, a New Species of Stick Insect from the Atlantic Forest, Rio de Janeiro, Brazil †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Studied
2.2. COI Amplification and Sequencing
2.3. Phylogenetic Analyses and K2P Divergences
2.4. Morphological Study
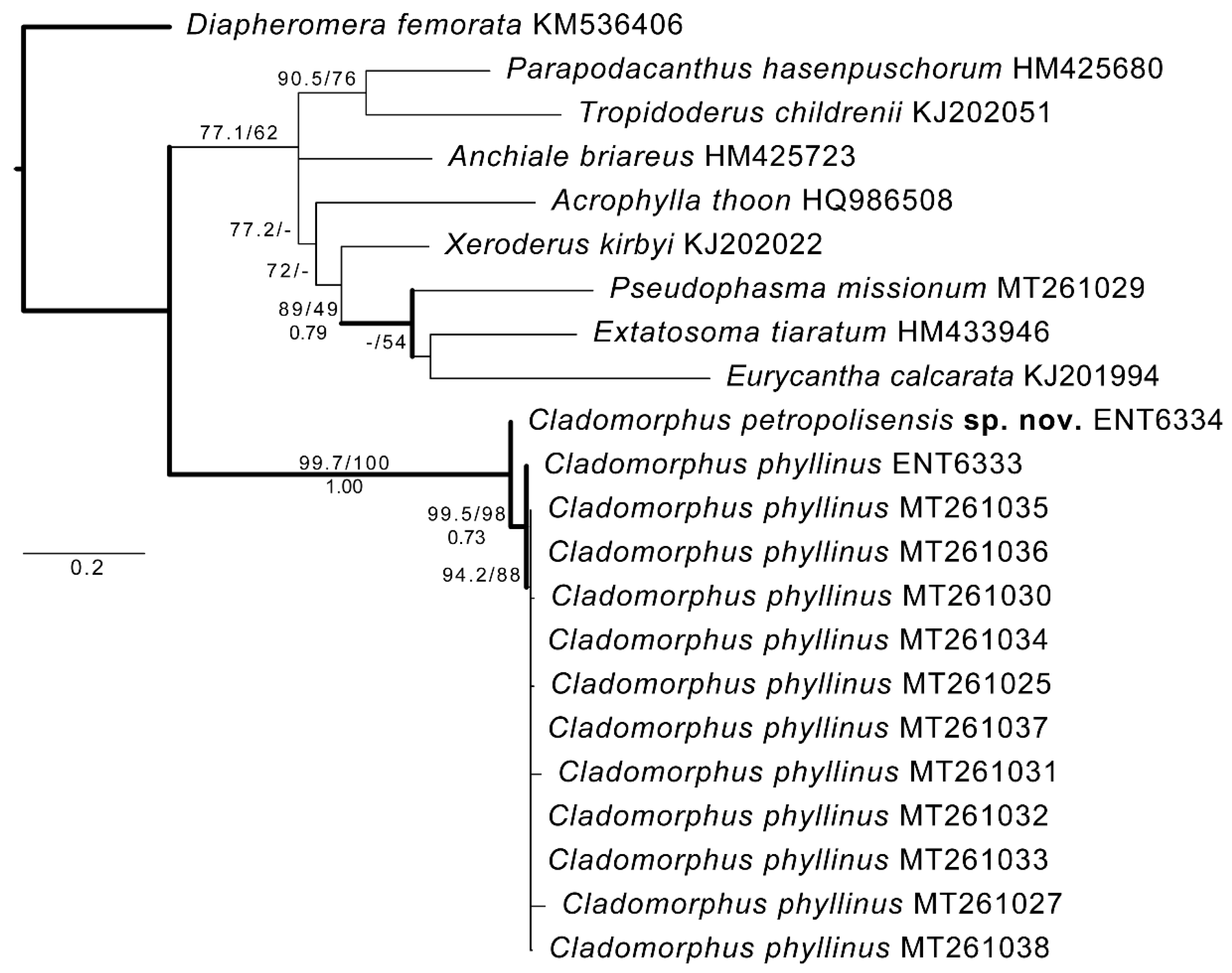
3. Results
3.1. COI Evidence
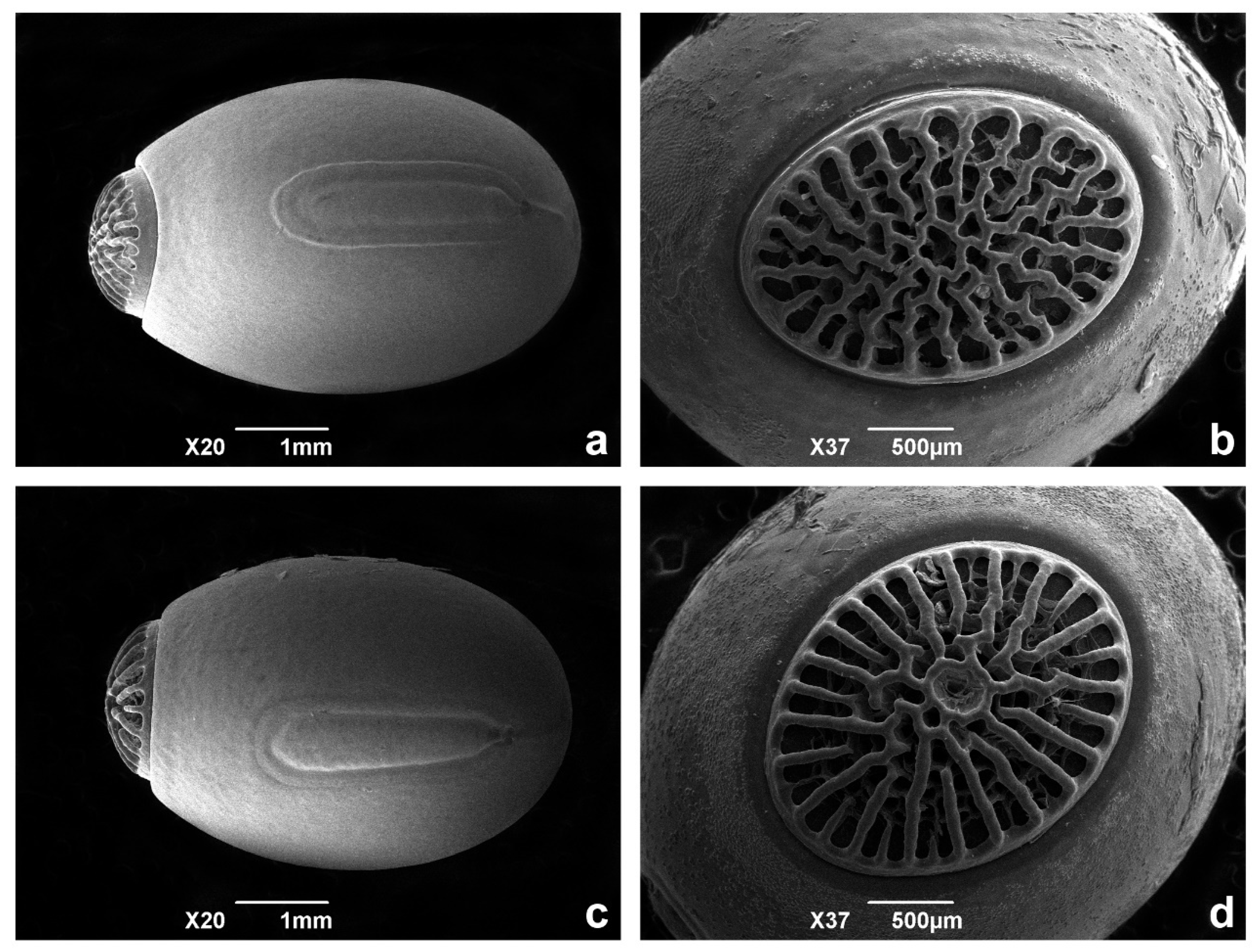
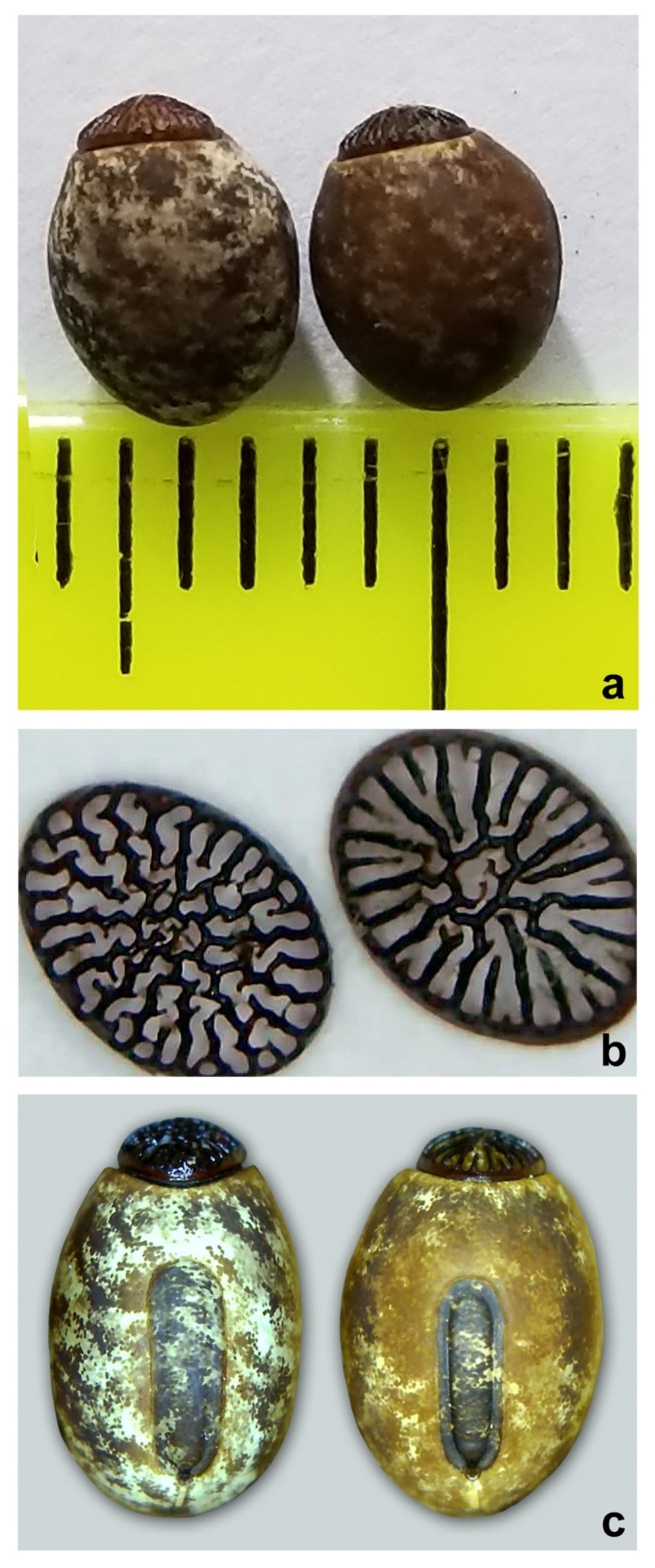
3.2. Morphological Evidence
3.3. Taxonomy
3.4. Additional Comparative Material
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradler, S.; Buckley, T.R. Biodiversity of Phasmatodea. In Insect Biodiversity: Science and Society; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 281–313. [Google Scholar] [CrossRef]
- Brock, P.D.; Büscher, T.; Baker, E. Phasmida Species File Online (Version 5.0/5.0). Available online: http://phasmida.speciesfile.org/ (accessed on 5 May 2022).
- Crispino, E.B.; Chiquetto-Machado, P.I.; Engelking, P.W.; Cancello, E.M. Contributions to the knowledge of Canuleius Stål (Phasmatodea: Heteronemiidae): Taxonomy, morphology and notes on the biology of two species. Zootaxa 2020, 4743, 511–535. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; Bradler, S.; Whiting, M.F. Evolution of oviposition techniques in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, e216. [Google Scholar] [CrossRef]
- Simon, S.; Letsch, H.; Bank, S.; Buckley, T.R.; Donath, A.; Liu, S.; Machida, R.; Meusemann, K.; Misof, B.; Podsiadlowski, L.; et al. Old World and New World Phasmatodea: Phylogenomics resolve the evolutionary history of stick and leaf insects. Front. Ecol. Evol. 2019, 7, 345. [Google Scholar] [CrossRef]
- Heleodoro, R.A.; Rafael, J.A. Phasmatidae in: Catálogo Taxonômico da Fauna do Brasil. PNUD. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/33855 (accessed on 5 May 2022).
- Baker, E. The worldwide status of phasmids (Insecta: Phasmida) as pests of agriculture and forestry, with ageneralised theory of phasmid outbreaks. Agric Food Secur. 2015, 4, 22. [Google Scholar] [CrossRef]
- Madeira-Ott, T.; Thyssen, P.J.; Costa, J. Phasmatodea (Arthropoda, Insecta) in Brazil: Status, New Record and Proposal for using molecular tools to assist in species identification. Neotrop. Entomol. 2020, 49, 916–922. [Google Scholar] [CrossRef]
- Chiquetto-Machado, P.I. Redescription of the Brazilian stick insect Pseudophasma cambridgei Kirby (Phasmatodea: Pseudophasmatidae), with first description of the female and egg. Austral Entomol. 2018, 57, 392–402. [Google Scholar] [CrossRef]
- Costa, J.; Torres, L.; Provance, W.D.; Brugnera, R.; Grazia, J. First report of predation by a stink bug (Supputius cincticeps Stål) on a walking-stick insect (Cladomorphus phyllinus Gray), with reflections on evolutionary mechanisms for camouflage. Paraná, Acta Biol. 2019, 48, 5–15. [Google Scholar] [CrossRef]
- Costa, J.; Chiquetto-Machado, P.I.; Gil-Santana, H. Predation strategies of Harpactor angulosus (Lepeletier & Serville, 1825) (Hemiptera: Reduviidae) on Cladomorphus phyllinus Gray, 1835 (Phasmatodea: Phasmatidae) in captivity. Rev. Chilena Ent. 2022, 48, 531–543. [Google Scholar] [CrossRef]
- Heleodoro, R.A.; Rafael, J.A. Is the Phasmatodea male genitalia useful for systematics? A case study in Creoxylus and Prexaspes (Insecta: Phasmatodea) from the Brazilian Amazon Basin. Zool. Anz. 2019, 278, 66–79. [Google Scholar] [CrossRef]
- Heleodoro, R.A.; Queiroz, L.; Rafael, J.A. Two new species of Periphloea Redtenbacher, 1906 (Insecta: Phasmatodea: Pseudophasmatidae) from the Brazilian Amazon Basin. Zootaxa 2021, 5047, 520–530. [Google Scholar] [CrossRef]
- Chiquetto-Machado, P.I.; Torres, L.; Costa, J. Bionomic notes on parthenogenetic females and a record of parasitism by Forcipomyia Meigen (Diptera: Ceratopogonidae) in the stick insect Cladoxerus cryphaleus (Westwood) (Phasmatodea: Phasmatidae). Rev. Bras. Entomol. 2020, 64, e20200086. [Google Scholar] [CrossRef]
- Chiquetto-Machado, P.I.; Morales, E.C.; Cancello, E.M. Taxonomic revision of Paraphasma Redtenbacher, 1906 (Phasmatodea, Pseudophasmatidae) based on phallic and external morphology. Zootaxa 2022, 5122, 1–80. [Google Scholar] [CrossRef] [PubMed]
- Crispino, E.; Egelking, P.W.; Ghirotto, V.M. Contributions to the knowledge of Ceroys (Miroceroys) Piza, 1936 (Phasmatodea: Heteronemiidae): Two new mossy stick insects from the Atlantic Forest of Brazil. Zootaxa 2022, 5134, 34–60. [Google Scholar] [CrossRef] [PubMed]
- Chiquetto-Machado, P.I.; Cancello, E.M. Cladistic analysis of Paraphasma (Phasmatodea: Pseudophasmatidae) highlights the importance of the phallic organ for phasmid systematics. Zool. J. Linn. Soc. 2021, 193, 158–198. [Google Scholar] [CrossRef]
- Ghirotto, V.M. Unmasking a master of camouflage: The rich morphology, taxonomy, and biology of the Brazilian stick insect Canuleius similis (Phasmatodea: Heteronemiidae), with general considerations on phasmid genitalia. Zool. Anz. 2021, 292, 30–57. [Google Scholar] [CrossRef]
- Heleodoro, R.A. The first two cases of antisymmetry in the male genitalia of Phasmatodea reveal a new species of Isagoras Stål, 1875 (Phasmatodea: Pseudophasmatidae: Xerosomatinae) from the Brazilian Atlantic Forest. Zool. Anz. 2022, 296, 161–178. [Google Scholar] [CrossRef]
- Araujo, F.F.; Garraffoni, A.R.S. Sinopse dos Phasmatodea (Insecta) Descritos para o Brasil. Entomobrasilis 2012, 5, 232–237. [Google Scholar] [CrossRef]
- Costa-Lima, A.M. Insetos do Brasil; Série didática; Escola Nacional de Agronomia: Rio de Janeiro, Brazil, 1938; 468p. [Google Scholar]
- Alvarenga, C.D.; Souza, H.R.; Giustolin, T.A.; Matrangolo, C.A.R.; Silva, J.F. Biologia de Cladomorphus phyllinus Gray (Phasmatodea: Phasmatidae) em folhas de goiabeira (Psidium guajava). EntomoBrasilis 2018, 11, 65–69. [Google Scholar] [CrossRef]
- Dorval, A.; Peres-Filho, O.; Moraes, C.S.P.; Berti-Filho, E. Biologia e Estudo Comportamental de Bacteria tuberculata Piza Jr., 1939 (Phasmatodea; Phasmatidae) em Folhas de Anjico (Piptenia spp.). Sci. For. 2003, 63, 150–157. Available online: http://www.ipef.br/publicacoes/scientia/nr63/cap12.pdf (accessed on 4 May 2022).
- Torres, L.; Benitez, H.A.; Costa, J. Longevity, fertility and average eggs viability of parthenogenetic females of Cladomorphus phyllinus Gray (Phasmatodea, Phasmatide). Entomobrasilis 2022, 15, e998. [Google Scholar] [CrossRef]
- Kumagai, A.F.; Fonseca, N.G. Uma nova espécie de Cladomorphus (Phasmatidae, Cladomorphinae) de Minas Gerais, Brasil. Rev. Bras. Entomol. 2009, 53, 41–44. [Google Scholar] [CrossRef][Green Version]
- Hennemann, F.H.; Conle, O.V.; Perez-Gelabert, D.E. Studies on Neotropical Phasmatodea XVI: Revision of Haplopodini Günther, 1953 (rev. stat.), with notes on the subfamily Cladomorphinae Bradley and Galil, 1977 and the descriptions of a new tribe, four new genera and nine new species (Phasmatodea: “Anareolatae”: Phasmatidae: Cladomorphinae). Zootaxa 2016, 4128, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Zoller, S.; Lutzoni, F. Bayes or bootstrap? A simulation study coparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 2003, 20, 255–266. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Hayat, M.A. Principle and Techniques of Scanning Electron Microscopy: Biological Aplications; John Wiley & Sons: Dallas, TX, USA, 1970; 370p. [Google Scholar]
- Monteiro, F.A.; Donnelly, M.J.; Beard, C.B.; Costa, J. Nested clade and phylogeographic analyses of the Chagas disease vector Triatoma brasiliensis in Northeast Brazil. Mol. Phylogenet. Evol. 2004, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, L.; Cuccodoro, G.; Chen, L.; Lu, C. Taxonomic note of Oberea fuscipennis (Chevrolat, 1852) based on morphological and DNA bar-code data (Coleoptera, Cerambycidae, Lamiinae). Zootaxa 2016, 4136, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Madeira, T.; Souza, C.M.; Cordeiro, J.; Thyssen, P.J. The use of DNAbarcode for identifying species of Oxysarcodexia Townsend (Diptera: Sarcophagidae): A preliminary survey. Acta Trop. 2016, 161, 73–78. [Google Scholar] [CrossRef]
- Sheffield, C.S.; Heron, J.; Gibbs, J.; Onuferko, T.M.; Oram, R.; Best, L.; Silva, N.; Dumesh, S.; Pindar, A.; Rowe, G. Contribution of DNA barcoding to the study of the bees (Hymenoptera: Apoidea) of Canada: Progress to date. Can. Entomol. 2017, 149, 736–754. [Google Scholar] [CrossRef]
- Vera, A.; Pastenes, L.; Veloso, C.; Méndez, M.A. Phylogenetic relation-ships in the genus Agathemera (Insecta: Phasmatodea) inferred fromthe genes 16S, COI and H3. Zool. J. Linn. Soc. 2012, 165, 63–72. [Google Scholar] [CrossRef]
- Bradler, S.; Robertson, J.A.; Whiting, M.F. A molecular phylogeny of Phasmatodea with emphasis on Necrosciinae, the most species-richsubfamily of stick insects. System. Entomol. 2014, 39, 205–222. [Google Scholar] [CrossRef]
- Tihelka, E.; Cai, C.; Giacomelli, M.; Pisani, D.; Donoghue, P.C. Integrated phylogenomic and fossil evidence of stick and leaf insects (Phasmatodea) reveal a Permian–Triassic co-origination with insectivores. R. Soc. Open Sci. 2020, 7, 201689. [Google Scholar] [CrossRef]
- Bank, S.; Buckley, T.R.; Büscher, T.H.; Bresseel, J.; Constant, J.; De Haan, M.; Dittmar, D.; Dräger, H.; Kahar, R.S.; Kang, A.; et al. Reconstructing the nonadaptive radiation of an ancient lineage of ground-dwelling stick insects (Phasmatodea: Heteropterygidae). Syst. Entomol. 2021, 46, 487–507. [Google Scholar] [CrossRef]
- Bank, S.; Cumming, R.T.; Li, Y.; Henze, K.; Le Tirant, S.; Bradler, S. A tree of leaves: Phylogeny and historical biogeography of the leaf insects (Phasmatodea: Phylliidae). Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef]
- Forni, G.; Martelossi, J.; Valero, P.; Hennemann, F.H.; Conle, O.; Luchetti, A.; Mantovani, B. Macroevolutionary analyses provide new evidence of phasmid wings evolution as a reversible process. Syst. Biol. 2022, 71, 1471–1486. [Google Scholar] [CrossRef]
- Gutierrez-Valencia, J.; Gutierrez, Y.; Dias, L.G. Species delimitation in the crypsis-defended and polymorphic stick insects of the genus Libethra (Phasmatodea, Diapheromeridae). Zool. Scr. 2017, 46, 693–705. [Google Scholar] [CrossRef]
- Glaw, F.; Hawlitschek, O.; Dunz, A.; Goldberg, J.; Bradler, S. When giant stick insects play with colors: Molecular phylogeny of the Achriopterini and description of two new splendid species (Phasmatodea: Achrioptera) from Madagascar. Front. Ecol. Evol. 2019, 7, 105. [Google Scholar] [CrossRef]
- Zompro, O. A key to the stick-insect genera of the ‘Anareolatae’ ofthe New World, with descriptions of several new taxa (Insecta: Phasmatodea). Stud. Neotrop. Fauna Environ. 2004, 39, 133–144. [Google Scholar] [CrossRef]
- Zompro, O. Phasmatodea. In Insetos do Brasil: Diversidade e Taxonomia; Holos Press: Ribeirão Preto, Brazil, 2012; pp. 289–306. [Google Scholar]
- Clark, J.T. The eggs of stick insects (Phasmida): A review with descriptions of the eggs of eleven species. Syst. Entomol. 1976, 1, 95–105. [Google Scholar] [CrossRef]
- Clark, J.T. A key to the eggs of stick and leaf insects (Phasmida). Syst. Entomol. 1979, 4, 325–331. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Mallet, J.R.S.; Takiya, D.M. Cladomorphus petropolisensis, a New Species of Stick Insect from the Atlantic Forest, Rio de Janeiro, Brazil. Animals 2022, 12, 2871. https://doi.org/10.3390/ani12202871
Costa J, Mallet JRS, Takiya DM. Cladomorphus petropolisensis, a New Species of Stick Insect from the Atlantic Forest, Rio de Janeiro, Brazil. Animals. 2022; 12(20):2871. https://doi.org/10.3390/ani12202871
Chicago/Turabian StyleCosta, Jane, Jacenir R. S. Mallet, and Daniela Maeda Takiya. 2022. "Cladomorphus petropolisensis, a New Species of Stick Insect from the Atlantic Forest, Rio de Janeiro, Brazil" Animals 12, no. 20: 2871. https://doi.org/10.3390/ani12202871
APA StyleCosta, J., Mallet, J. R. S., & Takiya, D. M. (2022). Cladomorphus petropolisensis, a New Species of Stick Insect from the Atlantic Forest, Rio de Janeiro, Brazil. Animals, 12(20), 2871. https://doi.org/10.3390/ani12202871








