A Standard Procedure for In Vitro Digestion Using Rumen Fermenters: A Collaborative Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Standardization of the Machine-Rinsing Procedure for Filter Bags
2.2. Collaborative Study
3. Results and Discussion
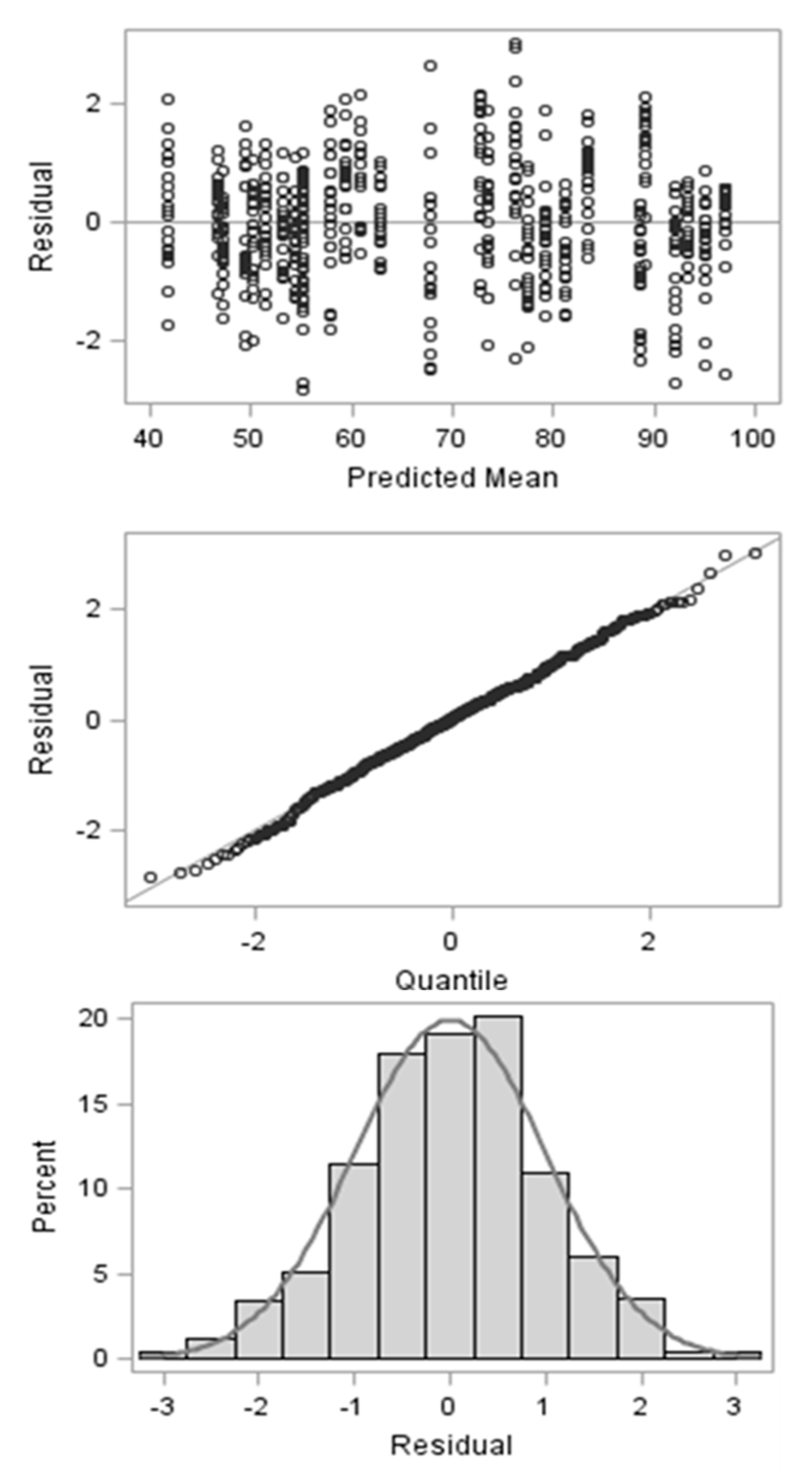
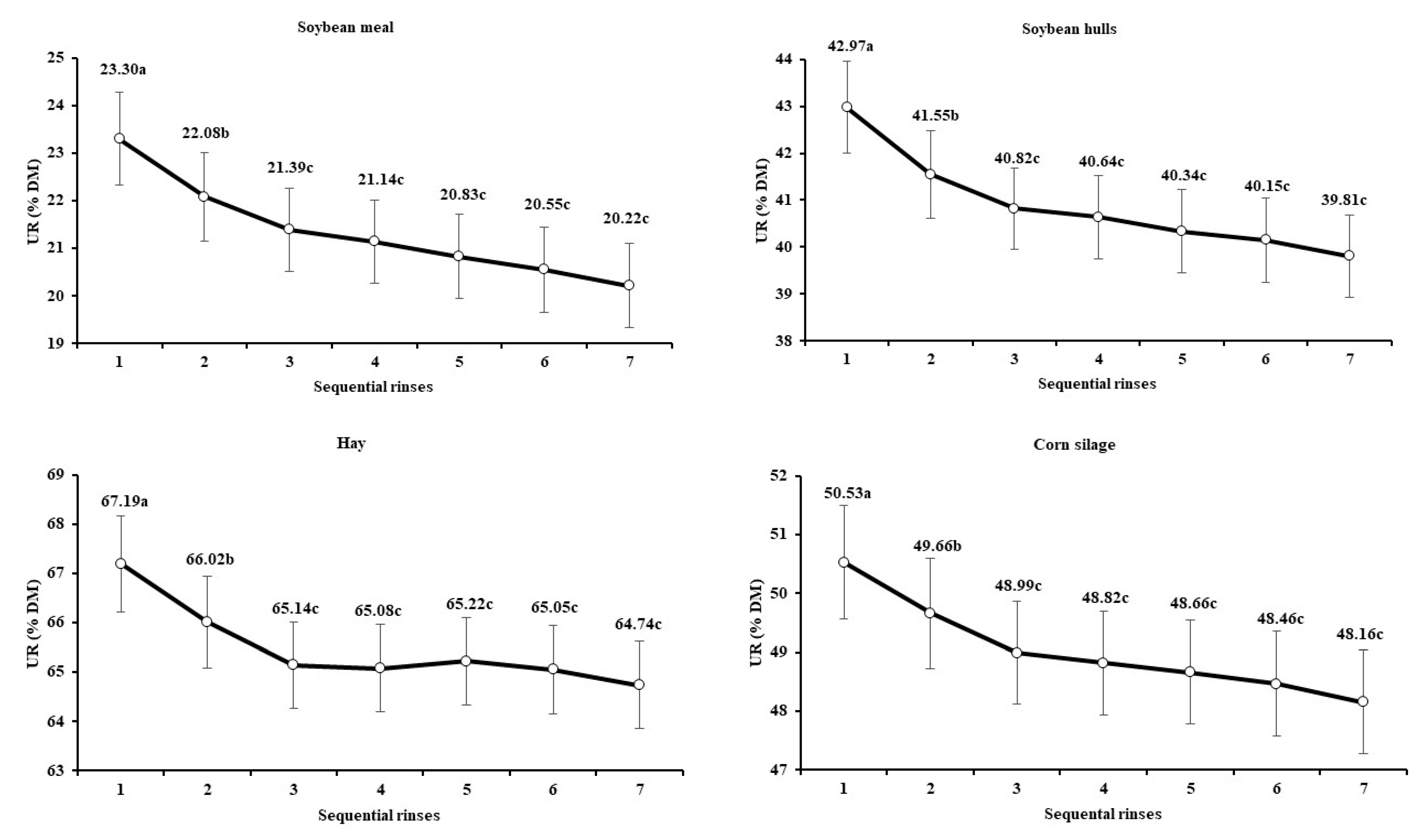
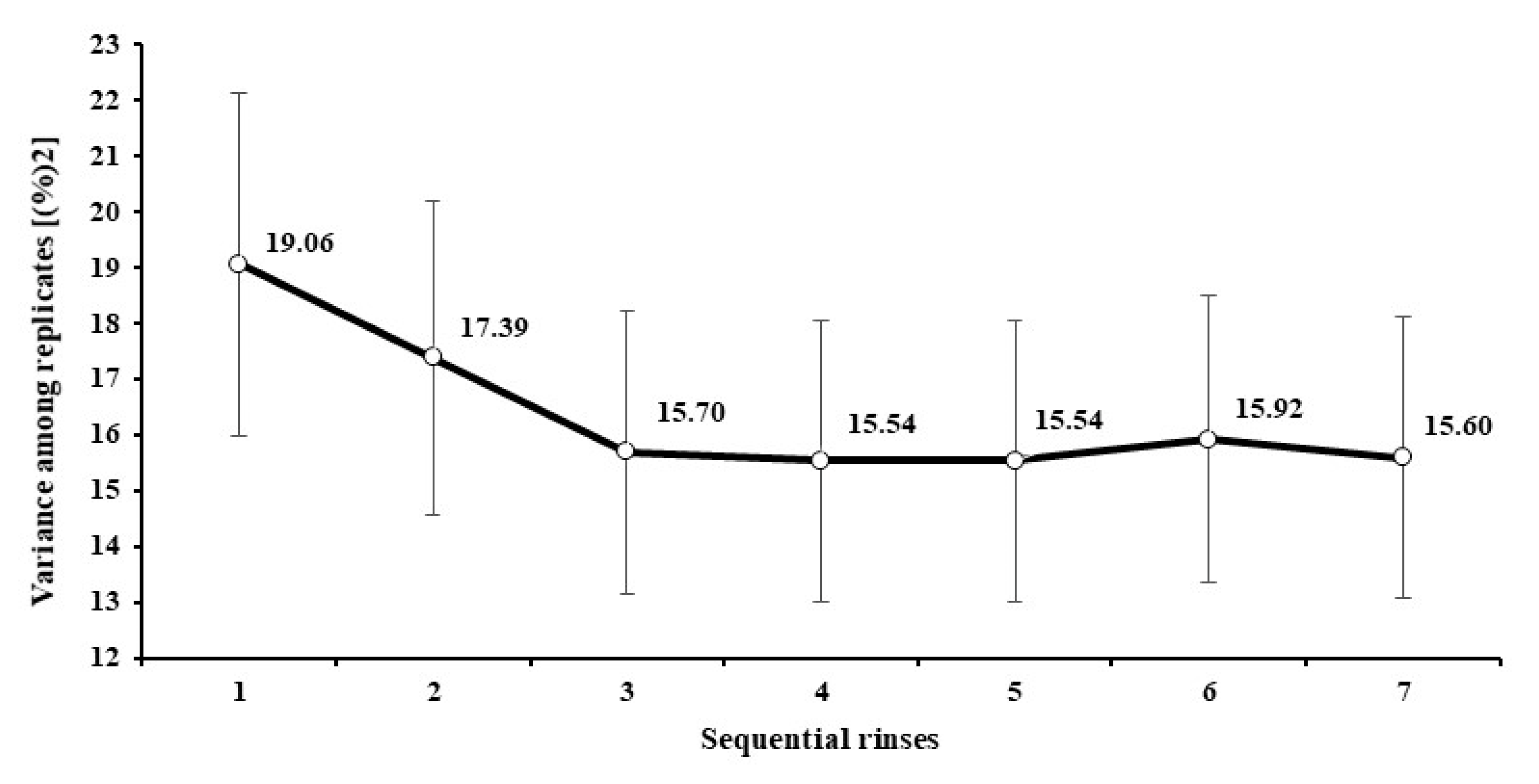
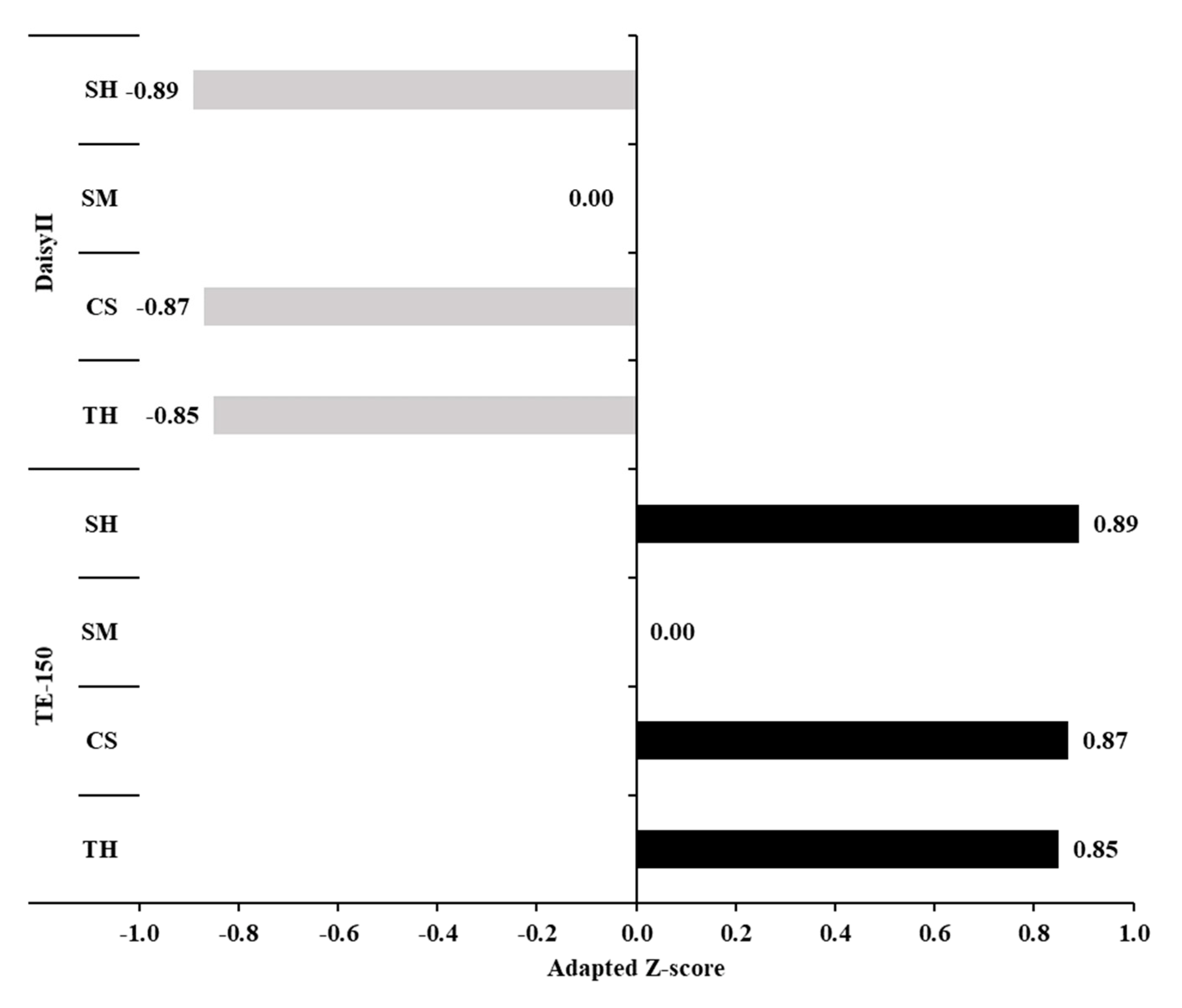
3.1. Standardization of the Machine-Rinsing Procedure for Filter Bags
3.2. Collaborative Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Silva, T.E.; Detmann, E.; Camacho, L.F.; Saliba, E.O.S.; Palma, M.N.N.; Valadares Filho, S.C. Comparação de métodos in vitro para quantificação da digestibilidade da matéria seca e da fibra em detergente neutro de forragens e concentrados. Arq. Bras. Med. Vet. Zootec. 2017, 69, 1635–1644. [Google Scholar] [CrossRef][Green Version]
- Codex Alimentarius Comission. Procedural Manual, 27th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; p. 248. [Google Scholar]
- Mertens, D.R. Challenges in measuring insoluble dietary fiber. J. Anim. Sci. 2003, 81, 3233–3249. [Google Scholar] [CrossRef] [PubMed]
- Adesogan, A.T. Effect of bag type on the apparent digestibility of feeds in ANKOM Daisy II incubators. Anim. Feed Sci. Technol. 2005, 119, 333–344. [Google Scholar] [CrossRef]
- Mould, F.L.; Kliem, K.E.; Morgan, R.; Mauricio, R.M. In vitro microbial inoculum: A review of its function and properties. Anim. Feed Sci. Technol. 2005, 123–124, 31–50. [Google Scholar] [CrossRef]
- Hall, M.B.; Mertens, D.R. In vitro fermentation vessel type and method alter fiber digestibility estimates. J. Dairy Sci. 2008, 91, 301–307. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of gas composition in headspace and bicarbonate concentrations in media on gas and methane production, degradability, and rumen fermentation using in vitro gas production techniques. J. Dairy Sci. 2013, 96, 4592–4600. [Google Scholar] [CrossRef]
- Strnad, I.; Makkar, H.P.S. Things to Know about the Ring Test; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; p. 4. [Google Scholar]
- Camacho, L.F.; Silva, T.E.; Palma, M.N.N.; Assunção, A.S.; Rodrigues, J.P.; Costa e Silva, L.F.; Detmann, E. Evaluation of buffer solutions and urea addition for estimating the in vitro digestibility of feeds. J. Anim. Sci. 2019, 97, 922–931. [Google Scholar] [CrossRef]
- Castro-Montoya, J.M.; Dickhoefer, U. The use of filter bags in combination with an in vitro system to evaluate forage degradation in mixed substrates. Anim. Feed Sci. Technol. 2019, 249, 46–53. [Google Scholar] [CrossRef]
- Valente, T.N.P.; Detmann, E.; Filho, S.C.V.; Da Cunha, M.; Queiroz, A.C.; Sampaio, C.B. In situ estimation of indigestible compounds contents in cattle feed and feces using bags made from different textiles. Rev. Bras. Zootecn. 2011, 40, 666–675. [Google Scholar] [CrossRef]
- Assunção, A.S.; Silva, T.E.; Quirino, D.; Franco, M.O.; Detmann, E. Variability among animals and incubation protocols for ruminant in situ degradation studies with tropical feeds. Animals 2022, 12, 1901. [Google Scholar] [CrossRef]
- Detmann, E.; e Silva, L.F.C.; Rocha, G.C.; Palma, M.N.N.; Rodrigues, J.P.P. Métodos Para Análise de Alimentos; Visconde do Rio Branco: Suprema, Brazil, 2021; p. 350. [Google Scholar]
- Machado, M.G.; Detmann, E.; Mantovani, H.C.; Filho, S.C.V.; Bento, C.B.P.; Marcondes, M.I.; Assunção, A.S. Evaluation of the length of adaptation period for changeover and crossover nutritional experiments with cattle fed tropical forage-based diets. Anim. Feed Sci. Technol. 2016, 222, 133–148. [Google Scholar] [CrossRef]
- Vanzant, E.S.; Cochran, R.C.; Titgemeyer, E.C. Standardization of in situ techniques for ruminant feedstuff evaluation. J. Anim. Sci. 1998, 76, 2717–2729. [Google Scholar] [CrossRef]
- Horwitz, W. Evaluation of analytical methods used for regulations of foods and drugs. Anal. Chem. 1982, 54, 67A–76A. [Google Scholar] [CrossRef]
- Horwitz, W.; Albert, R. The Horwitz ratio (HorRat): A useful index of method performance with respect to precision. J. AOAC Int. 2006, 89, 1095–1109. [Google Scholar] [CrossRef]
- Horwitz, W.; Albert, R.; Deustsch, M.J.; Thompson, J.N. Precision parameters of methods of analysis required for nutrition labelling. Part I. Major nutrients. J. AOAC Int. 1990, 73, 661–680. [Google Scholar] [CrossRef]
- Wernimont, G.T.; Spendley, W. Use of Statistics to Develop and Evaluate Analytical Methods; AOAC International: Gaithersburg, MD, USA, 1985; p. 183. [Google Scholar]
- De Boer, G.; Murphy, J.J.; Kennelly, J.J. A modified method for determination of in situ rumen degradation of feedstuffs. Can. J. Anim. Sci. 1987, 67, 93–102. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Fritz, J.O.; Cochran, R.C.; Rooney, W.L.; Bolsen, K.K. Protein degradation in response to spontaneous heating in alfalfa hay by in situ and ficin methods. J. Dairy Sci. 1997, 80, 700–713. [Google Scholar] [CrossRef]
- Paine, C.A.; Crawshaw, R.; Barber, W.P. A complete exchange method for the in sacco estimation of rumen degradability on a routine basis. In Forage Protein in Ruminant Animal Production; Thompson, D.J., Beever, D.E., Gunn, R.G., Eds.; British Society of Animal Production: Cambridge, UK, 1982; pp. 177–178. [Google Scholar] [CrossRef]
- American Oil Chemists‘ Society-AOCS. Collaborative Studies Procedures (AOCS Procedure M 4-86); AOCS: Urbana, IL, USA, 2009; p. 9. [Google Scholar]
- Youden, W.J.; Steiner, E.H. Statistical Manual of the Association of Official Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 1975; p. 88. [Google Scholar]
- Thiex, N.; Anderson, S.; Gildemeister, B. Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/Submersion Method): Collaborative study. J. AOAC Int. 2003, 86, 888–898. [Google Scholar] [CrossRef]
- Van Soest, P.J. The Nutritional Ecology of the Ruminant, 2nd ed.; Cornell Univeristy Press: Ithaca, NY, USA, 1994; p. 476. [Google Scholar]
- Pell, A.N.; Schofield, P. Computerized monitoring of gas production to measure forage digestion in vitro. J. Dairy Sci. 1993, 76, 1063–1073. [Google Scholar] [CrossRef]
- Cornou, C.; Storm, I.M.L.D.; Hindrichsen, I.K.; Worgan, H.; Bakewell, E.; Yáñez-Ruiz, D.R.; Abecia, L.; Tagliapietra, F.; Cattani, M.; Ritz, C.; et al. A ring test of a wireless in vitro gas production system. Anim. Prod. Sci. 2013, 53, 585–592. [Google Scholar] [CrossRef]
- Van Laar, H.; Van Straalen, W.M.; Van Gelder, A.H.; De Boever, J.L.; D’Heer, B.; Vedder, H.; Kroes, R.; De Bot, P.; Van Hees, J.; Cone, J.W. Repeatability and reproducibility of an automated gas production technique. Anim. Feed Sci. Technol. 2006, 127, 133–150. [Google Scholar] [CrossRef]
- Chui, Q.S.H.; Barros, C.B.; Silva, T.D. Parâmetros r e R obtidos de programa interlaboratorial-como usá-los. Química Nova 2009, 32, 2209–2213. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society–AOCS. Determination of Precision of Analytical Methods (AOCS Procedure M 1-92); AOCS: Urbana, IL, USA, 2017; p. 3. [Google Scholar]

| Feed | Dry Matter 1 | Crude Protein 2 | Neutral Detergent Fibre 2 |
|---|---|---|---|
| Tifton 85 hay | 90.6 | 6.68 | 74.5 |
| Corn silage | 24.9 | 6.23 | 50.1 |
| Soybean meal | 88.4 | 47.9 | 24.0 |
| Soybean hulls | 88.0 | 15.4 | 66.4 |
| Feed 1,2 | ||||
|---|---|---|---|---|
| Laboratory | Tifton 85 Hay | Corn Silage | Soybean Meal | Soybean Hulls |
| 1 | 49.1 ± 0.70 | 58.0 ± 0.96 | 89.0 ± 0.89 | 79.6 ± 1.14 |
| 2 | 42.4 ± 0.85 | 48.5 ± 0.94 | 86.3 ± 0.61 | 65.3 ± 1.20 |
| 3 | 45.9 ± 0.57 3 | 51.4 ± 0.76 | 94.2 ± 0.59 | 73.5 ± 0.87 |
| 4 | 56.2 ± 0.57 | 63.1 ± 0.46 | 97.4 ± 0.64 | 79.6 ± 0.61 |
| 5 | 47.8 ± 0.49 | 53.2 ± 0.54 | 85.1 ± 0.78 | 76.5 ± 0.91 |
| 6 | 51.6 ± 0.66 | 61.5 ± 0.67 | 93.2 ± 0.41 | 75.4 ± 0.74 |
| 7 | 52.6 ± 0.59 | 64.0 ± 0.65 4 | 93.4 ± 0.76 3 | 78.3 ± 0.76 |
| Overall | 49.4 ± 0.43 | 57.1 ± 0.56 | 91.2 ± 0.43 | 75.4 ± 0.52 |
| Feed | |||||
|---|---|---|---|---|---|
| Laboratory | Tifton 85 Hay | Corn Silage | Soybean Meal | Soybean Hulls | Sum 1 |
| 1 | 6 | 5 | 5 | 2.5 | 18.5 |
| 2 | 5 | 6 | 6 | 7 | 24.0 |
| 3 | 3 | 4 | 2 | 1 | 10.0 |
| 4 | 1 | 2 | 1 | 2.5 | 6.5 |
| 5 | 7 | 7 | 7 | 5 | 26.0 |
| 6 | 4 | 3 | 4 | 6 | 17.0 |
| 7 | 2 | 1 | 3 | 4 | 10.0 |
| Feed | |||||
|---|---|---|---|---|---|
| Overall | Tifton 85 Hay | Corn Silage | Soybean Meal | Soybean Hulls | |
| Variance components [(%)2] | |||||
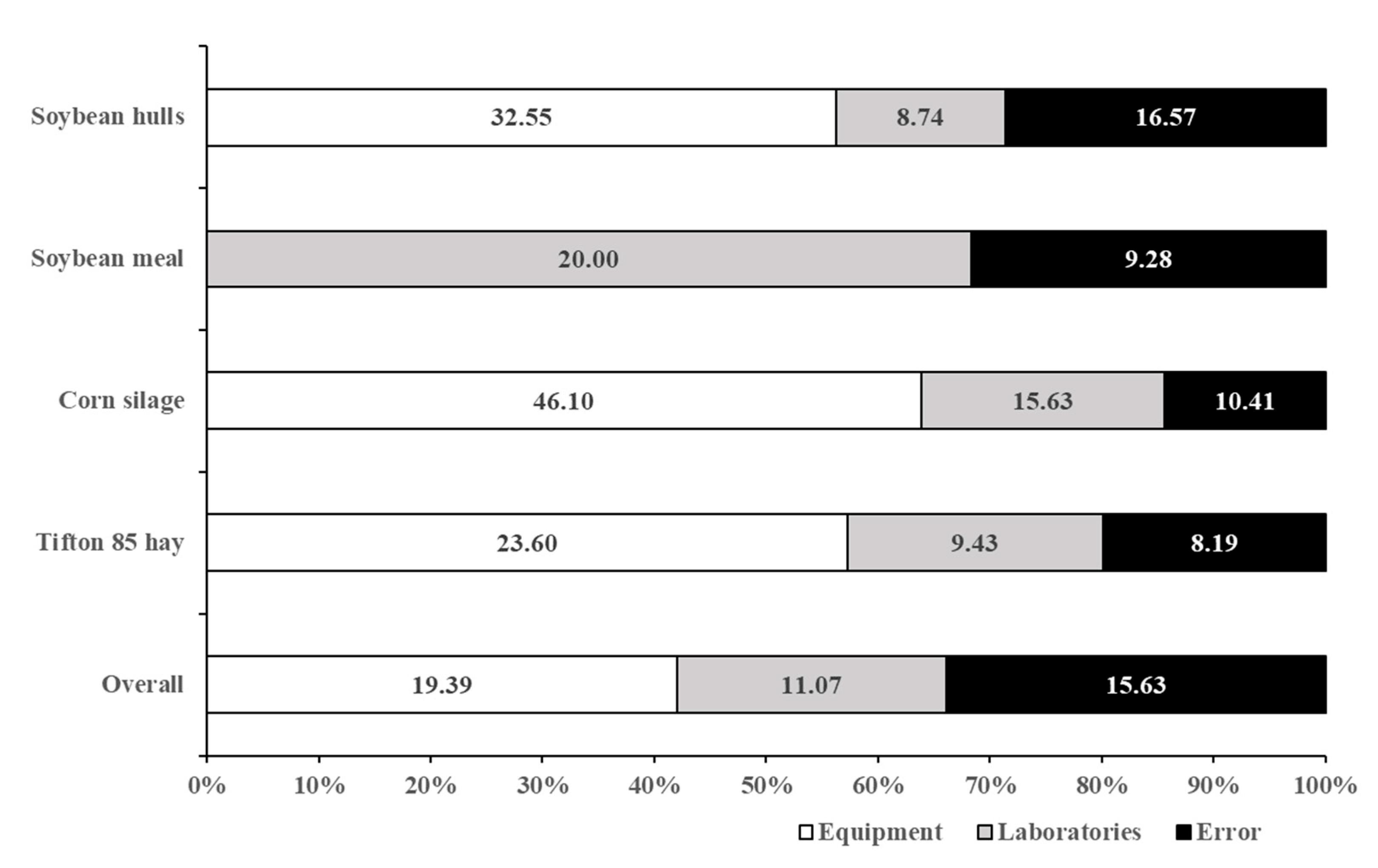
| Laboratories | 11.07 | 9.43 | 15.63 | 20.00 | 8.74 |
| Error | 15.63 | 8.19 | 10.41 | 9.28 | 16.57 |
| Technical indicators 1 | |||||
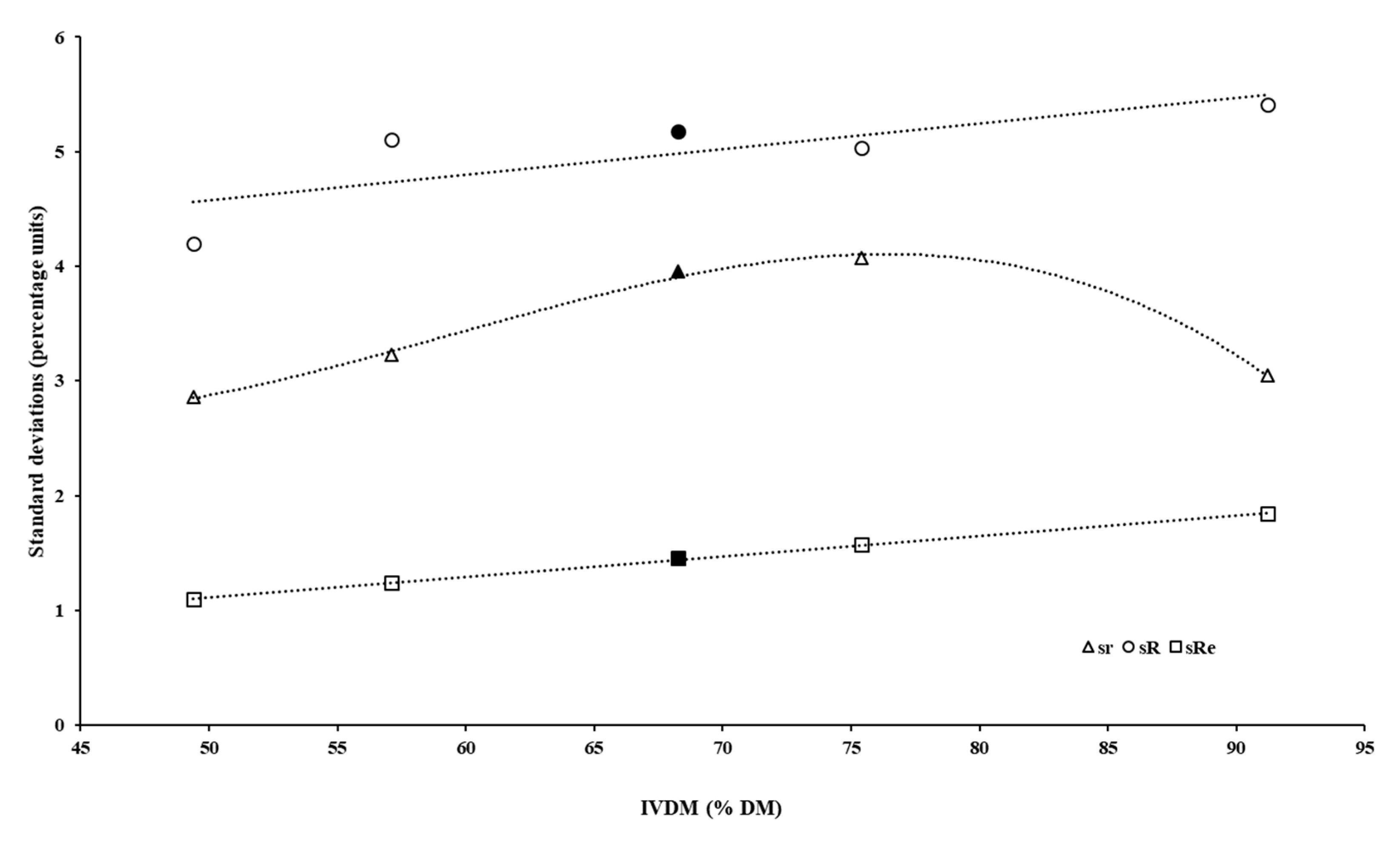
| aIVDMD (%) | 68.3 | 49.4 | 57.1 | 91.2 | 75.4 |
| sr | 3.95 | 2.86 | 3.23 | 3.04 | 4.07 |
| r (%) | 5.79 | 5.79 | 5.65 | 3.34 | 5.40 |
| sR | 5.17 | 4.20 | 5.10 | 5.41 | 5.03 |
| R (%) | 7.57 | 8.50 | 8.94 | 5.93 | 6.67 |
| r/R | 0.76 | 0.68 | 0.63 | 0.56 | 0.80 |
| sRe | 1.45 | 1.10 | 1.24 | 1.85 | 1.57 |
| Re (%) | 2.12 | 2.22 | 2.18 | 2.02 | 2.09 |
| HorRat | 3.57 | 3.83 | 4.10 | 2.94 | 3.19 |
| RL | - | 11.8 | 14.3 | 15.1 | 14.1 |
| Δmax 2 | - | 8.0 (68) | 10.5 (73) | 12.0 (79) | 7.5 (53) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho, L.F.; da Silva, T.E.; Rodrigues, J.P.P.; Franco, M.d.O.; Detmann, E. A Standard Procedure for In Vitro Digestion Using Rumen Fermenters: A Collaborative Study. Animals 2022, 12, 2842. https://doi.org/10.3390/ani12202842
Camacho LF, da Silva TE, Rodrigues JPP, Franco MdO, Detmann E. A Standard Procedure for In Vitro Digestion Using Rumen Fermenters: A Collaborative Study. Animals. 2022; 12(20):2842. https://doi.org/10.3390/ani12202842
Chicago/Turabian StyleCamacho, Larissa Frota, Tadeu Eder da Silva, João Paulo Pacheco Rodrigues, Marcia de Oliveira Franco, and Edenio Detmann. 2022. "A Standard Procedure for In Vitro Digestion Using Rumen Fermenters: A Collaborative Study" Animals 12, no. 20: 2842. https://doi.org/10.3390/ani12202842
APA StyleCamacho, L. F., da Silva, T. E., Rodrigues, J. P. P., Franco, M. d. O., & Detmann, E. (2022). A Standard Procedure for In Vitro Digestion Using Rumen Fermenters: A Collaborative Study. Animals, 12(20), 2842. https://doi.org/10.3390/ani12202842






