New Occurrences of the Tiger Shark (Galeocerdo cuvier) (Carcharhinidae) off the Coast of Rio de Janeiro, Southeastern Brazil: Seasonality Indications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
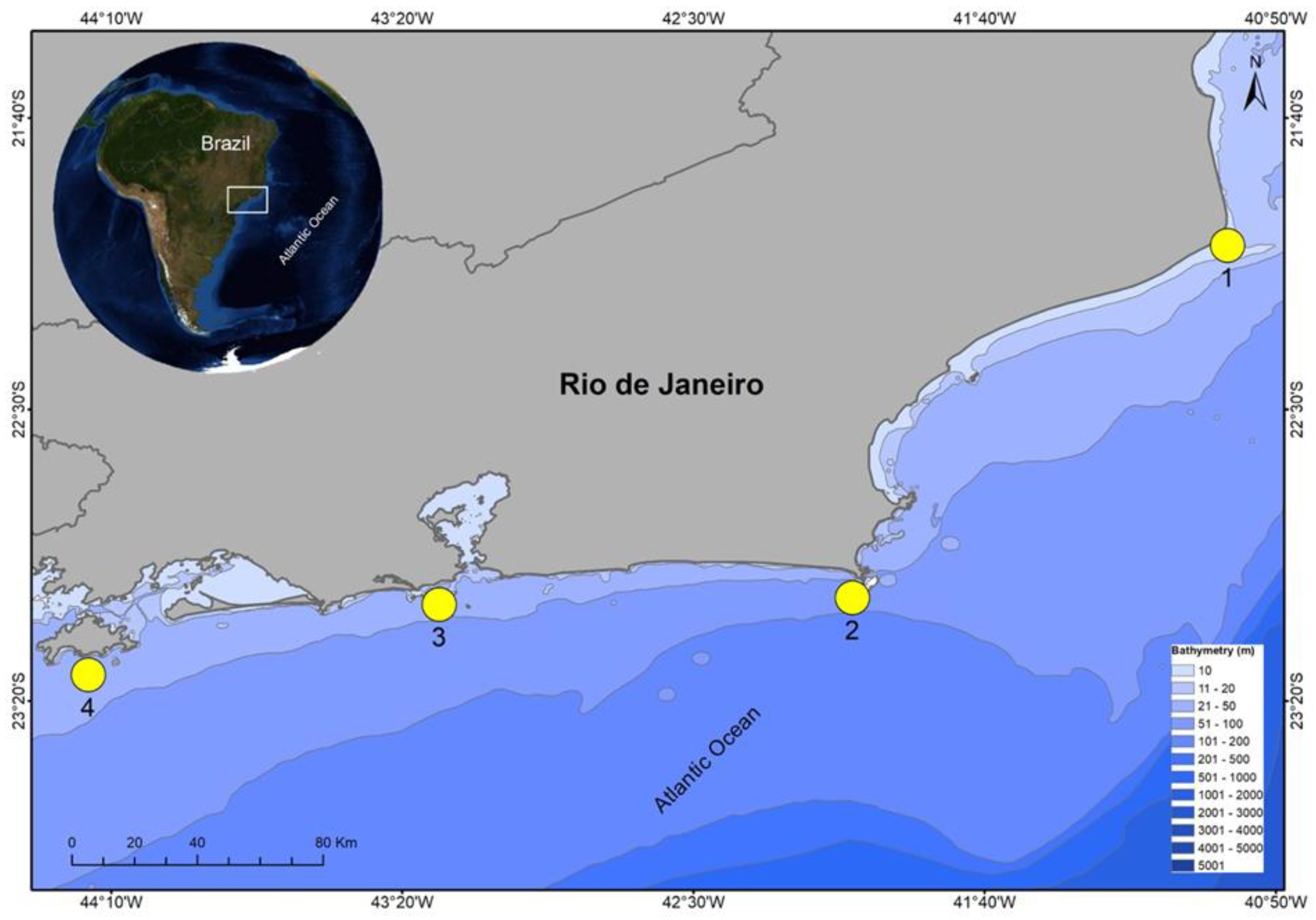
2.1. Study Area
2.2. Data Sampling
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammerschlag, N.; Schmitz, O.J.; Flecker, A.S.; Lafferty, K.D.; Sih, A.; Atwood, T.B.; Gallagher, A.J.; Irschick, D.J.; Skubel, R.; Cooke, S.J. Ecosystem function and services of aquatic predators in the anthropocene. Trends Ecol. Evol. 2019, 34, 369–383. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Dulvy, N.K. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Simpfendorfer, C.A.; Dulvy, N.K. Bright spots of sustainable shark fishing. Curr. Biol. 2017, 27, 97–98. [Google Scholar] [CrossRef] [Green Version]
- Hauser-Davis, R.A.; Amorim-Lopes, C.; Araujo, N.L.F.; Rebouças, M.; Gomes, R.A.; Rocha, R.C.C.; Dos Santos, L.N. On mobulid rays and metals: Metal content for the first Mobulamobular record for the state of Rio de Janeiro, Brazil and a review on metal ecotoxicology assessments for the Manta and Mobula genera. Mar. Pollut. Bull. 2021, 168, 112472. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Pollock, C.M. Extinction risk and conservation of the world’s sharks and rays. Elife 2014, 3, e00590. [Google Scholar] [CrossRef] [Green Version]
- Booth, H.; Squires, D.; Milner-Gulland, E.J. The neglected complexities of shark fisheries, and priorities for holistic risk-based management. Ocean. Coast. Manag. 2019, 182, 104994. [Google Scholar] [CrossRef]
- Dent, F.; Clarke, S. State of the Global Market for Shark Products; FAO Fisheries and Aquaculture Technical Paper 590; FAO: Rome, Italy, 2015. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture: Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Ferrette, B.L.S.; Domingues, R.R.; Ussami, L.H.F.; Moraes, L.; Magalhães, C.O.; Amorim, A.F.; Hilsdorf, A.W.S.; Oliveira, C.; Foresti, F.; Mendonça, F.F. DNA-based species identification of shark finning seizures in Southwest Atlantic: Implications for wildlife trade surveillance and law enforcement. Biodivers. Conserv. 2019, 28, 4007–4025. [Google Scholar] [CrossRef]
- Ferretti, F.; Jacoby, D.M.P.; Pfleger, M.O.; White, T.D.; Dent, F.; Micheli, F.; Rosenberg, A.A.; Crowder, L.B.; Block, B.A. Shark fin trade bans and sustainable shark fisheries. Conserv. Lett. 2020, 13, e12708. [Google Scholar] [CrossRef] [Green Version]
- Pincinato, R.B.M.; Gasalla, M.A.; Garlock, T.; Anderson, J.L. Market incentives for shark fisheries. Mar. Policy 2022, 139, 105031. [Google Scholar] [CrossRef]
- Freire, K.M.F.; Almeida, Z.S.; Amador, J.R.E.T.; Aragão, J.A.; Araújo, A.R.R.; Ávila-da-Silva, A.O.; Bentes, B.; Carneiro, M.H.; Chiquieri, J.; Fernandes, C.A.F.; et al. Reconstruction of marine commercial landings for the Brazilian industrial and artisanal fisheries from 1950 to 2015. Front. Mar. Sci. 2021, 8, 659110. [Google Scholar] [CrossRef]
- Cruz, M.; Szynwelski, B.E.; Ochotorena de Freitas, T.R. Biodiversity on sale: The shark meat market threatens elasmobranchs in Brazil. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 3437–3450. [Google Scholar] [CrossRef]
- ICMBio. Livro Vermelho Da Fauna Brasileira Ameaçada de Extinção: Volume VI—Peixes; Instituto Chico Mendes de Conservação da Biodiversidade/Ministério do Meio Ambiente: Brasília, Brazil, 2018. Available online: https://www.Icmbio.gov.br/portal/images/stories/comunicacao/publicacoes/publicacoesdiversas/livro_vermelho_2018_vol6.pdf (accessed on 12 July 2022).
- Hazin, F.H.V.; Wanderley Júnior, J.A.D.M.; Mattos, S.M.G.D. Distribuição e abundância relativa de tubarões no litoral do Estado de Pernambuco, Brasil. Arquivos de Ciências do Mar 2002, 33, 33–42. [Google Scholar]
- Araujo, N.L.F.; Lopes, C.A.; Brito, V.B.; Santos, L.N.D.; Barbosa Filho, M.L.V.; Amaral, C.R.L.D.; Hauser-Davis, R.A. Artisanally landed elasmobranchs along the coast of Rio de Janeiro, Brazil. Bol. Do Laboratório De Hidrobiol. 2020, 30, 33–53. [Google Scholar] [CrossRef]
- Rupp, A.; Bornatowski, H. Food web model to assess the fishing impacts and ecological role of elasmobranchs in a coastal ecosystem of Southern Brazil. Environ. Biol. Fishes 2021, 104, 905–921. [Google Scholar] [CrossRef]
- IUCN. International Union for Conservation of Nature Red List. The IUCN Red List of Threatened Species 2018. Available online: https://www.iucnredlist.org/species/39378/2913541 (accessed on 12 July 2022).
- Brown, C.J.; Roff, G. Life-history traits inform population trends assessing the conservation status of a declining tiger shark population. Biol. Conserv. 2019, 239, 108230. [Google Scholar] [CrossRef]
- Lucifora, L.O.; García, V.B.; Menni, R.C.; Worm, B. Spatial patterns in the diversity of sharks, rays, and chimaeras (Chondrichthyes) in the Southwest Atlantic. Biodivers. Conserv. 2012, 2, 407–419. [Google Scholar] [CrossRef]
- Ministério da Pesca e Aquicultura. Pesca Artesanal; Ministério da Pesca e Aquicultura: Brasília, Brazil, 2014. Available online: http://www.mpa.gov.br/pesca/artesanal (accessed on 12 July 2022).
- Silva-Júnior, L.C.D.; Andrade, A.C.D.; Vianna, M. Caracterização de uma pescaria de pequena escala em uma área de importância ecológica para elasmobrânquios, no Recreio dos Bandeirantes, Rio de Janeiro. Arq. De Ciências Do Mar. 2008, 41, 47–57. [Google Scholar]
- Tomás, A.R.G.; Gomes, U.L.; Ferreira, B.P. Distribuição temporal dos elasmobrânquios na pesca de pequena escala de Barra de Guaratiba, Rio De Janeiro, Brasil. Bol. Do Inst. De Pesca 2018, 36, 317–324. [Google Scholar]
- Afonso, A.S.; Hazin, F.H.V.; Barreto, R.R.; Santana, F.M.; Lessa, R.P. Extraordinary growth in tiger sharks Galeocerdo cuvier from the South Atlantic Ocean. J. Fish Biol. 2012, 81, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.A.; Fowler, S.; Compagno, L. Sharks of the World: A Fully Illustrated Guide; Wild Nature Press: Plymouth, UK, 2013; 528p. [Google Scholar]
- Ferreira, L.C.; Thums, M.; Heithaus, M.R.; Barnett, A.; Abrantes, K.G.; Holmes, B.J.; Zamora, L.M.; Frisch, A.J.; Pepperell, J.G.; Burkholder, D.; et al. The trophic role of a large marine predator, the tiger shark Galeocerdo Cuvier. Sci. Rep. 2017, 7, 7641. [Google Scholar] [CrossRef] [Green Version]
- Bornatowski, H.; Wedekin, L.L.; Heithaus, M.R.; Marcondes, M.C.C.; Rossi-Santos, M.R. Shark scavenging and predation on cetaceans at Abrolhos Bank, eastern Brazil. J. Mar. Biol. Assoc. U. K. 2012, 92, 1767–1772. [Google Scholar] [CrossRef]
- Dicken, M.L.; Hussey, N.E.; Christiansen, H.N.; Smale, M.J.; Nkabi, N.; Cliff, G.; Wintner, S.P. Diet and trophic ecology of the tiger shark (Galeocerdo cuvier) from South African waters. PLoS ONE 2017, 12, e0177897. [Google Scholar] [CrossRef] [PubMed]
- Miranda, F.R.; Santos, P.M.; Rodrigues, M.T.; Cumplido, R.; Kersul, M.G. Consumption of a maned sloth (Bradypus torquatus Illiger, 1811) by a tiger shark (Galeocerdo cuvier Péron & LeSueur, 1822) in southeastern Brazil. Notas Sobre Mamíferos Sudam. 2021, 3, 1–7. [Google Scholar]
- Randall, J.E. Review of the biology of the tiger shark (Galeocerdo cuvier). Aust. J. Mar. Freshw. Res. 1992, 43, 21–31. [Google Scholar] [CrossRef]
- Branstetter, S.; Musick, J.A.; Colvocoresses, J.A. A comparison of the age and growth of the tiger shark, Galeocerdo cuvieri, from off Virginia and from the Northwestern Gulf of Mexico. Fish. Bull. 1987, 85, 269–279. [Google Scholar]
- Trindade-Santos, I.; Freire, K.M.F. Analysis of reproductive patterns of fishes from three Large Marine Ecosystems. Front. Mar. Sci. 2015, 2, 38. [Google Scholar] [CrossRef]
- INEA. 2021. Available online: http://www.inea.rj.gov.br/biodiversidade-territorio/gerenciamento-costeiro/ (accessed on 12 July 2022).
- FIPERJ. Entidades do Setor. Colônia; Fundação Instituto de Pesca do Estado do Rio de Janeiro: Rio de Janeiro, Brazil, 2019. Available online: http://www.fiperj.rj.gov.br/index.php/entidade (accessed on 12 July 2022).
- Santos, S.R.; Macedo, M.L.C.; Maciel, T.R.; Souza, G.B.G.; Almeida, L.S.; Gadig, O.B.F.; Vianna, M. A tale that never loses in the telling: Considerations for the shifting ethnobaseline based on artisanal fisher records from the southwestern Atlantic. Ethnobiol. Conserv. 2022, 11, 3. [Google Scholar]
- Rosas, F.C.W.; Capistrano, L.C.; Di Beneditto, A.P.; Ramos, R. Hydruga leptonyx recovered from the stmoach of a tiger shark captured off the Rio de Janeiro coast, Brazil. Mammalia 1992, 56, 153–155. [Google Scholar]
- Gomes, U.L.; Signori, C.N.; Gadig, O.B.F.; Santos, U.R.S. Guia para Identificação de Tubarões e Raias do Rio de Janeiro, 1st ed.; Technical Books Editora: Rio de Janeiro, Brasil, 2010; 234p. [Google Scholar]
- Gomes, U.L.; Santos, H.R.S.; Gadig, O.B.F.; Signorini, C.N.; Vicente, M.M. Guia para identificação dos tubarões, raias e quimeras do Estado Rio de Janeiro (Chondrichthyes: Elasmobranchii e Holocephali). Revista Nordestina de Biologia 2019, 27, 171–368. [Google Scholar]
- Lopes, M.S.; Bertucci, T.C.P.; Rapagnã, L.; Tubino, R.D.A.; Monteiro-Neto, C.; Tomas, A.R.G.; Aguilera Socorro, O. The path towards endangered species: Prehistoric fisheries in Southeastern Brazil. PLoS ONE 2016, 11, e0154476. [Google Scholar] [CrossRef] [Green Version]
- Lopes, E.Q.; dos Santos, B.D.P.; de Faria, I.C.; de Lima, T.G.; de Lucca, D.S.Q. Registros de ocorrências de tubarões tigres (Galeocerdo cuvier, Peron e Lesueur, 1822) no litoral de Peruíbe-São Paulo-Brasil, APA-CIP e Unidades de Conservação do Mosaico Jureia Itatins-SP. Braz. J. Anim. Environ. Res. 2020, 3, 4270–4282. [Google Scholar] [CrossRef]
- Lopes, M.S.; Grouard, S.; Gaspar, M.D.; Sabadini-Santos, E.; Bailon, S.; Aguilera, O. Middle Holocene marine and land-tetrapod biodiversity recovered from Galeão shell mound, Guanabara Bay, Brasil. Quat. Int. 2022, 610, 80–96. [Google Scholar] [CrossRef]
- Teixeira, T.P.; Pinto, B.C.; Terra, B.D.F.; Estiliano, E.O.; Gracia, D.; Araújo, F.G. Diversidade das assembléias de peixes nas quatro unidades geográficas do rio Paraíba do Sul. Iheringia: Série Zoologia 2005, 95, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Valentin, J.L. The Cabo Frio Upwelling System, Brazil. In Coastal Marine Ecosystems of Latin America; Seeliger, U., Kjerfve, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 97–105. [Google Scholar]
- Leduc, A.O.; De Carvalho, F.H.; Hussey, N.E.; Reis-Filho, J.A.; Longo, G.O.; Lopes, P.F. Local ecological knowledge to assist conservation status assessments in data poor contexts: A case study with the threatened sharks of the Brazilian Northeast. Biodivers. Conserv. 2021, 30, 819–845. [Google Scholar] [CrossRef]
- FIPERJ. Relatório. 2016. Available online: http://www.fiperj.rj.gov.br/fiperj_imagens/arquivos/revistarelatorios2015.pdf (accessed on 12 July 2022).
- Da Silva, D.R.; Paranhos, R.; Vianna, M. Spatial patterns of distribution and the influence of seasonal and abiotic factors on demersal ichthyofauna in an estuarine tropical bay. J. Fish Biol. 2016, 89, 821–846. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.V.T.; Lima, A.P.; Maciel, T.R.; Santos, S.R.B.; Vianna, M. A baía de Guanabara é um ambiente importante para a conservação neotropical? Uma abordagem ictiológica. Diversidade e Gestão 2018, 2, 76–89. [Google Scholar]
- Silva, F.G.; Vianna, M. Diet and reproductive aspects of the endangered butterfly ray Gymnura altavela raising the discussion of a possible nursery area in a highly impacted environment. Braz. J. Oceanogr. 2018, 66, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Heupel, M.R.; Carlson, J.K.; Simpfendorfer, C.A. Shark nursery areas: Concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 2007, 337, 287–297. [Google Scholar] [CrossRef]
- Santos, P.R.; Balanin, S.; Gadig, O.B.; Garrone-Neto, D. The historical and contemporary knowledge on the elasmobranchs of Cananeia and adjacent waters, a coastal marine hotspot of southeastern Brazil. Reg. Stud. Mar. Sci. 2022, 51, 102224. [Google Scholar] [CrossRef]
- Afonso, A.S.; Andrade, H.A.; Hazin, F.H. Structure and dynamics of the shark assemblage off Recife, northeastern Brazil. PLoS ONE 2014, 9, e102369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajemian, M.J.; Drymon, J.M.; Hammerschlag, N.; Wells, R.J.D.; Street, G.; Falterman, B.; Mckinney, J.A.; Drigers, W.B., III; Hoffmayer, E.R.; Fischer, C.; et al. Movement patterns and habitat use of tiger sharks (Galeocerdo cuvier) across ontogeny in the Gulf of Mexico. PLoS ONE 2020, 15, e0234868. [Google Scholar] [CrossRef] [PubMed]
- Heithaus, M.; Dill, L.; Marshall, G.; Buhleier, B. Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar. Biol. 2002, 140, 237–248. [Google Scholar]
- EBC. 2021. Available online: https://agenciabrasil.ebc.com.br/economia/noticia/2021-08/em-11-anos-arrecadacao-de-royalties-de-petroleo-no-rio-subiu-225 (accessed on 12 July 2022).
- Holland, K.N.; Anderson, J.M.; Coffey, D.M.; Holmes, B.J.; Meyer, C.G.; Royer, M.A. A Perspective on Future tiger shark Research. Front. Mar. Sci. 2019, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Fistarol, G.O.; Coutinho, F.H.; Moreira, A.P.B.; Venas, T.; Ca´novas, A.; de Paula, S.E.M.; Coutinho, R.; de Moura, R.L.; Valentin, J.L.; Tenenbaum, D.R.; et al. Environmental and sanitary conditions of Guanabara Bay, Rio de Janeiro. Front. Microbiol. 2015, 6, 1232. [Google Scholar] [CrossRef] [Green Version]
- Amorim-Lopes, C.; Willmer, I.Q.; Araujo, N.L.; de SPereira, L.H.S.; Monteiro, F.; Rocha, R.C.; Saint’Pierre, T.D.; dos Santos, L.N.; Siciliano, S.; Vianna, M.; et al. Mercury screening in highly consumed sharpnose sharks (Rhizoprionodon lalandii and R. porosus) caught artisanally in southeastern Brazil. Elem. Sci. Anthr. 2020, 8, 22. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Pereira, C.F.; Pinto, F.; Torres, J.P.M.; Malm, O.; Vianna, M. Mercury contamination in the recently described Brazilian white-tail dogfish Squalus albicaudus (Squalidae, Chondrichthyes). Chemosphere 2020, 250, 126228. [Google Scholar] [CrossRef]
- Maciel, O.L.C.; Willmer Isabel, Q.; Saintpierre, T.D.; Machado, W.T.V.; Siciliano, S.; Hauser-Davis, R.A. Arsenic contamination in widely consumed Caribbean sharpnose sharks in southeastern Brazil: Baseline data and concerns regarding fisheries resources. Mar. Pollut. Bull. 2021, 172, 112905. [Google Scholar] [CrossRef]
- Willmer, I.Q.; Wosnick, N.; Rocha, R.C.C.; Saintpierre, T.D.; Vianna, M.; Hauser-Davis, R.A. First report on metal and metalloid contamination of Ampullae of Lorenzini in sharks: A case study employing the Brazilian Sharpnose Shark Rhizoprionodonlalandii from Southeastern Brazil as an ecotoxicological model. Mar. Pollut. Bull. 2022, 179, 113671. [Google Scholar] [CrossRef]
- Paiva, L.; Vannuci-Silva, M.; Correa, B.; Santos-Neto, E.; Vianna, M.; Lailson-Brito, J.L. Additional pressure to a threatened species: High persistent organic pollutant concentrations in the tropical estuarine batoid Gymnuraaltavela. Bull. Environ. Contam. Toxicol. 2021, 107, 37–44. [Google Scholar] [CrossRef]
- Whitney, N.M.; Crow, G.L. Reproductive biology of the tiger shark (Galeocerdo cuvier) in Hawaii. Mar. Biol. 2007, 151, 63–70. [Google Scholar] [CrossRef]
- Lyons, K.; Adams, D.H. Maternal offloading of organochlorine contaminants in the yolk-sac placental scalloped hammerhead shark (Sphyrna lewini). Ecotoxicology 2015, 24, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Consales, G.; Marsili, L. Assessment of the conservation status of Chondrichthyans: Underestimation of the pollution threat. Eur. Zool. J. 2021, 88, 165–180. [Google Scholar] [CrossRef]
- Cooke, S.J.; Bergman, J.N.; Madliger, C.L.; Cramp, R.L.; Beardall, J.; Burness, G.; Chown, S.L. One hundred research questions in conservation physiology for generating actionable evidence to inform conservation policy and practice. Conserv. Physiol. 2021, 9, coab009. [Google Scholar] [CrossRef] [PubMed]
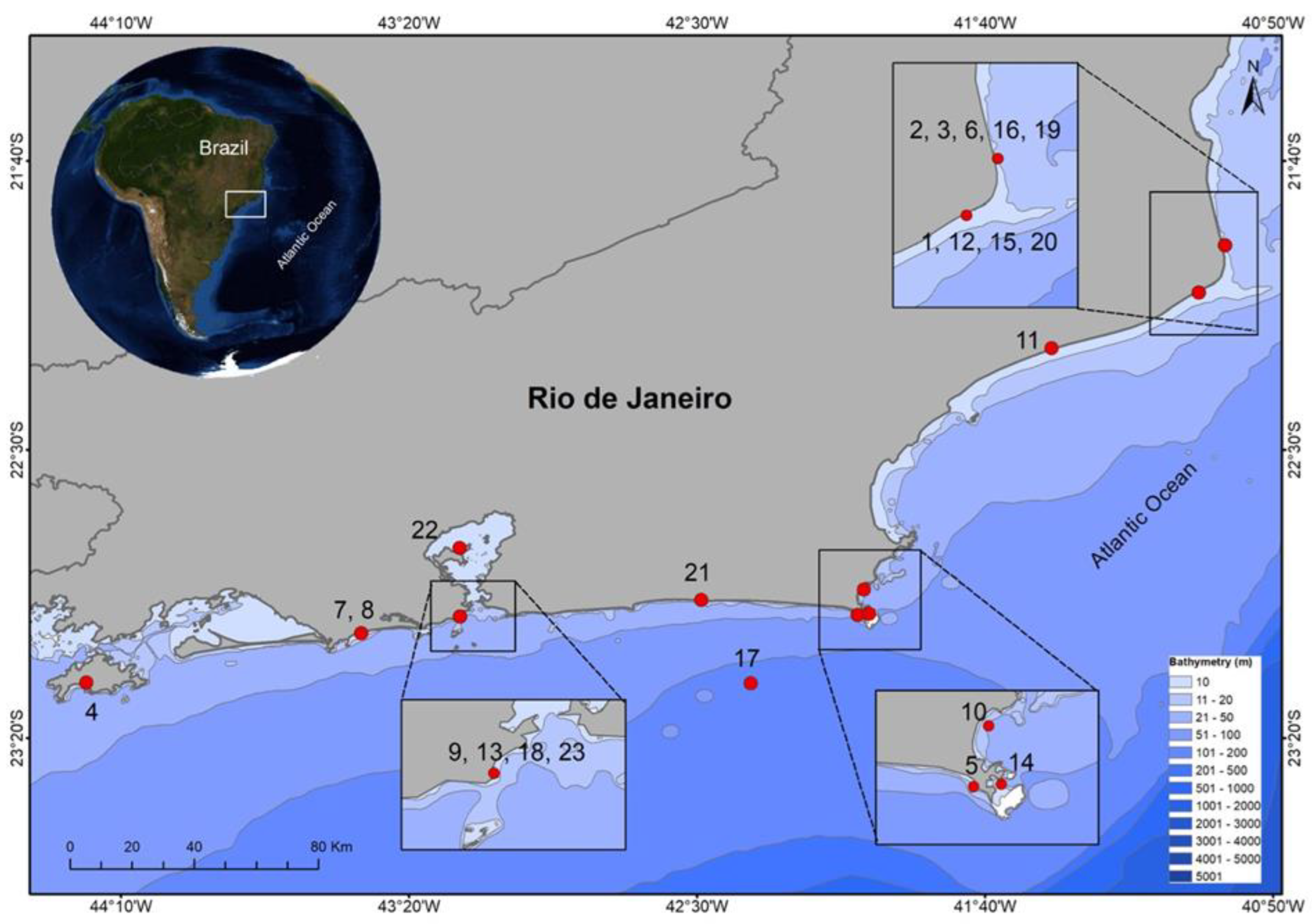

| Study ID | Month/Year | Sex | TL (cm) | TW (kg) | Source |
|---|---|---|---|---|---|
| 1 | Not informed/1985 | - | 161 | 20 | Santos et al. (2022a) |
| 2 | Not informed/1990 | - | 170 | - | Santos et al. (2022a) |
| 3 | Not informed/2000 | - | 338 | 220 | Current study |
| 4 | January/2002 | - | 300 | - | Current study |
| 5 | January/2003 * | - | 300 | - | Current study |
| 6 | Not informed/2003 | - | 393 | 360 | Santos et al. (2022a) |
| 7 | Not informed/2004 | F | 80 | - | Silva-Junior et al. (2008) |
| 8 | Not informed/2005 | - | - | - | Silva-Junior et al. (2008) |
| 9 | July/2013 | - | 150 | 40 | Current study |
| 10 | August/2013 | F | 186 | 103 | Current study |
| 11 | February/2016 | - | 280 | 220 | Current study |
| 12 | December/2016 | - | 250 | 350 | Current study |
| 13 | April/2018 | F | 89 | - | Araujo et al. (2020) |
| 14 | July/2018 | F | 160 | 23 | Current study |
| 15 | Not informed/2018 | - | 243 | 76 | Current study |
| 16 | February/2019 | M | 250 | 200 | Current study |
| 17 | March/2019 | M | 335 | 418 | Miranda et al. (2021) |
| 18 | September/2019 | F | 150 | - | Araujo et al. (2020) |
| 19 | February/2020 | - | 350 | 400 | Current study |
| 20 | December/2021 | F | 350 | 300 | Current study |
| 21 | January/2022 * | - | 250 | 200 | Current study |
| 22 | June/2022 | F | 180 | 75 | Current study |
| 23 | July/2022 ** | F | - | - | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aximoff, I.; Cumplido, R.; Rodrigues, M.T.; de Melo, U.G.; Fagundes Netto, E.B.; Santos, S.R.; Hauser-Davis, R.A. New Occurrences of the Tiger Shark (Galeocerdo cuvier) (Carcharhinidae) off the Coast of Rio de Janeiro, Southeastern Brazil: Seasonality Indications. Animals 2022, 12, 2774. https://doi.org/10.3390/ani12202774
Aximoff I, Cumplido R, Rodrigues MT, de Melo UG, Fagundes Netto EB, Santos SR, Hauser-Davis RA. New Occurrences of the Tiger Shark (Galeocerdo cuvier) (Carcharhinidae) off the Coast of Rio de Janeiro, Southeastern Brazil: Seasonality Indications. Animals. 2022; 12(20):2774. https://doi.org/10.3390/ani12202774
Chicago/Turabian StyleAximoff, Izar, Rodrigo Cumplido, Marcelo Tardelli Rodrigues, Ubirajara Gonçalves de Melo, Eduardo Barros Fagundes Netto, Sérgio Ricardo Santos, and Rachel Ann Hauser-Davis. 2022. "New Occurrences of the Tiger Shark (Galeocerdo cuvier) (Carcharhinidae) off the Coast of Rio de Janeiro, Southeastern Brazil: Seasonality Indications" Animals 12, no. 20: 2774. https://doi.org/10.3390/ani12202774
APA StyleAximoff, I., Cumplido, R., Rodrigues, M. T., de Melo, U. G., Fagundes Netto, E. B., Santos, S. R., & Hauser-Davis, R. A. (2022). New Occurrences of the Tiger Shark (Galeocerdo cuvier) (Carcharhinidae) off the Coast of Rio de Janeiro, Southeastern Brazil: Seasonality Indications. Animals, 12(20), 2774. https://doi.org/10.3390/ani12202774






