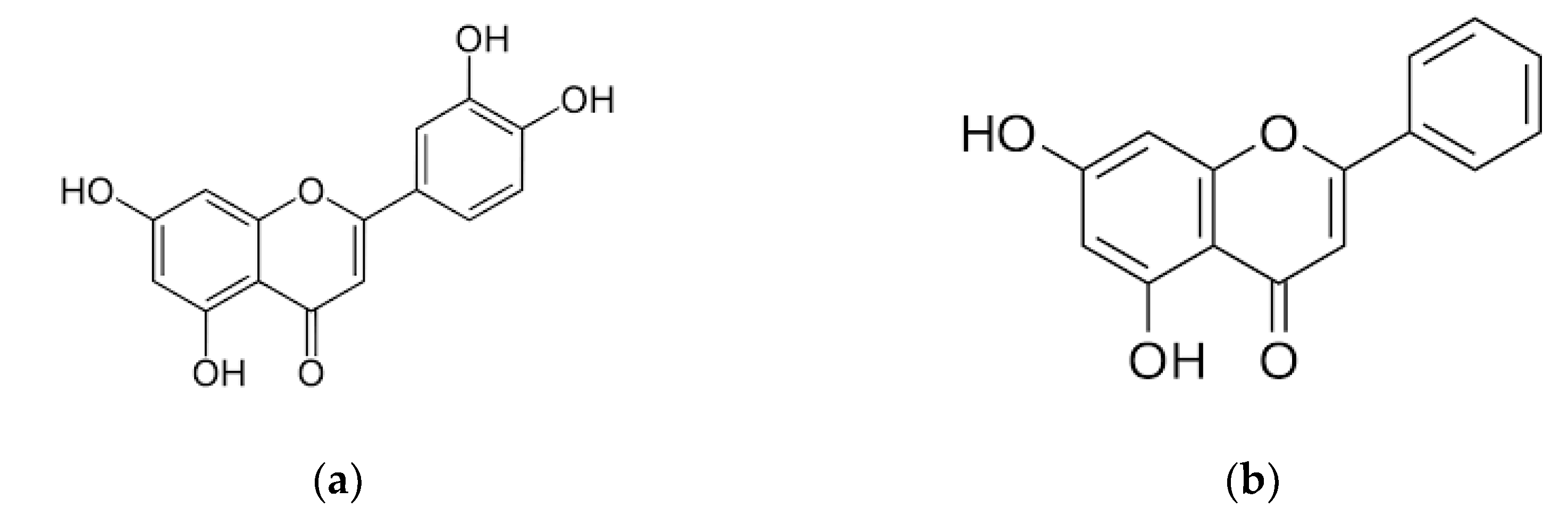
Luteolin and Chrysin Could Prevent E. coli Lipopolysaccharide-Ochratoxin A Combination-Caused Inflammation and Oxidative Stress in In Vitro Porcine Intestinal Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cells and Culturing Conditions
2.3. Treatments of IPEC-J2 Cells with OTA, LPS and Flavonoids LUT and CHR
2.4. Cell Viability Evaluation
2.5. Detection of Changes in the Redox Status of IPEC-J2 Cells
2.6. Determination of Proinflammatory Cytokine IL-6 and IL-8 Expression
2.7. Statistical Analysis
3. Results
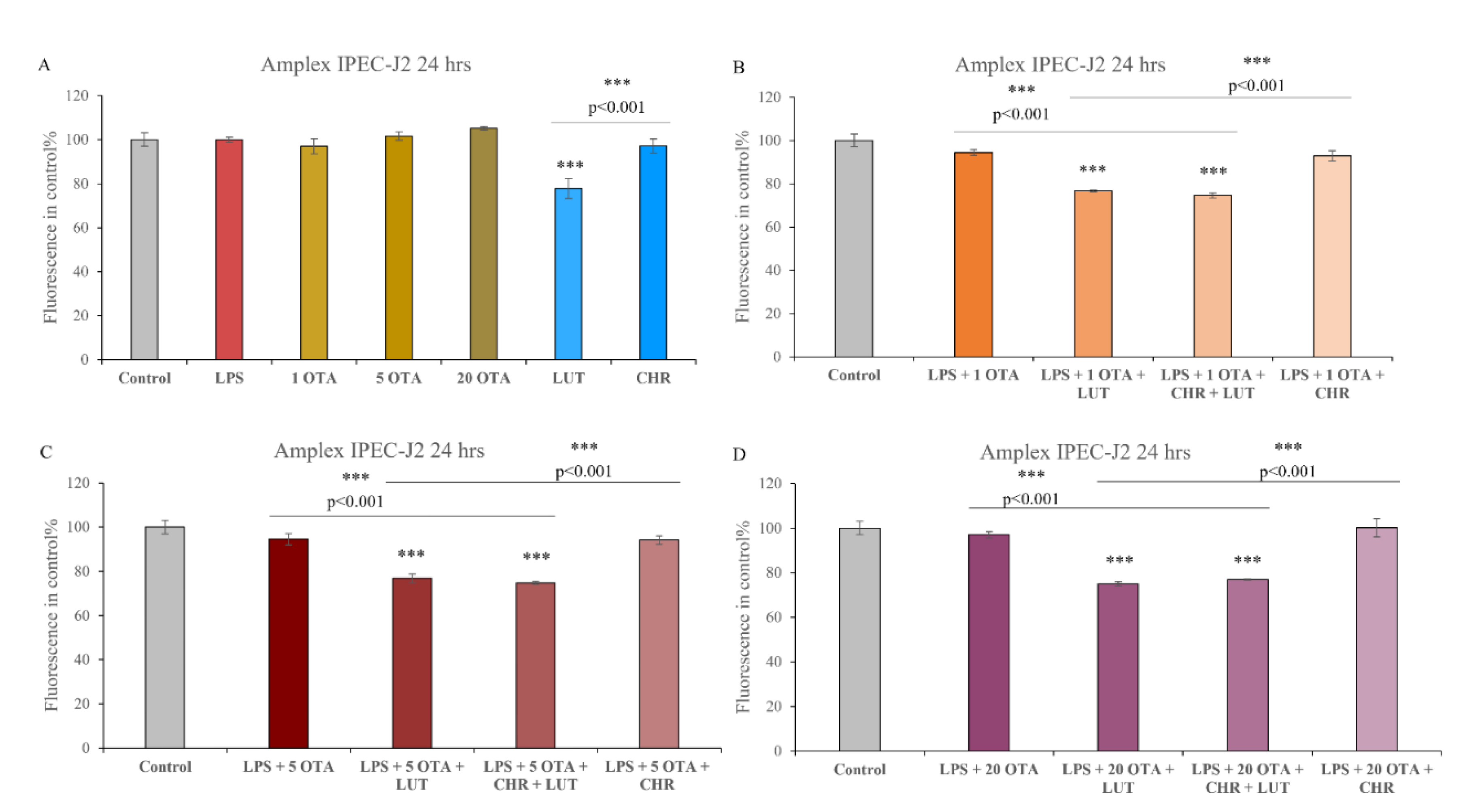
3.1. Cell Viability Assay on the IPEC-J2 Cell Line
3.2. Detection of EC Hydrogen-Peroxide Production
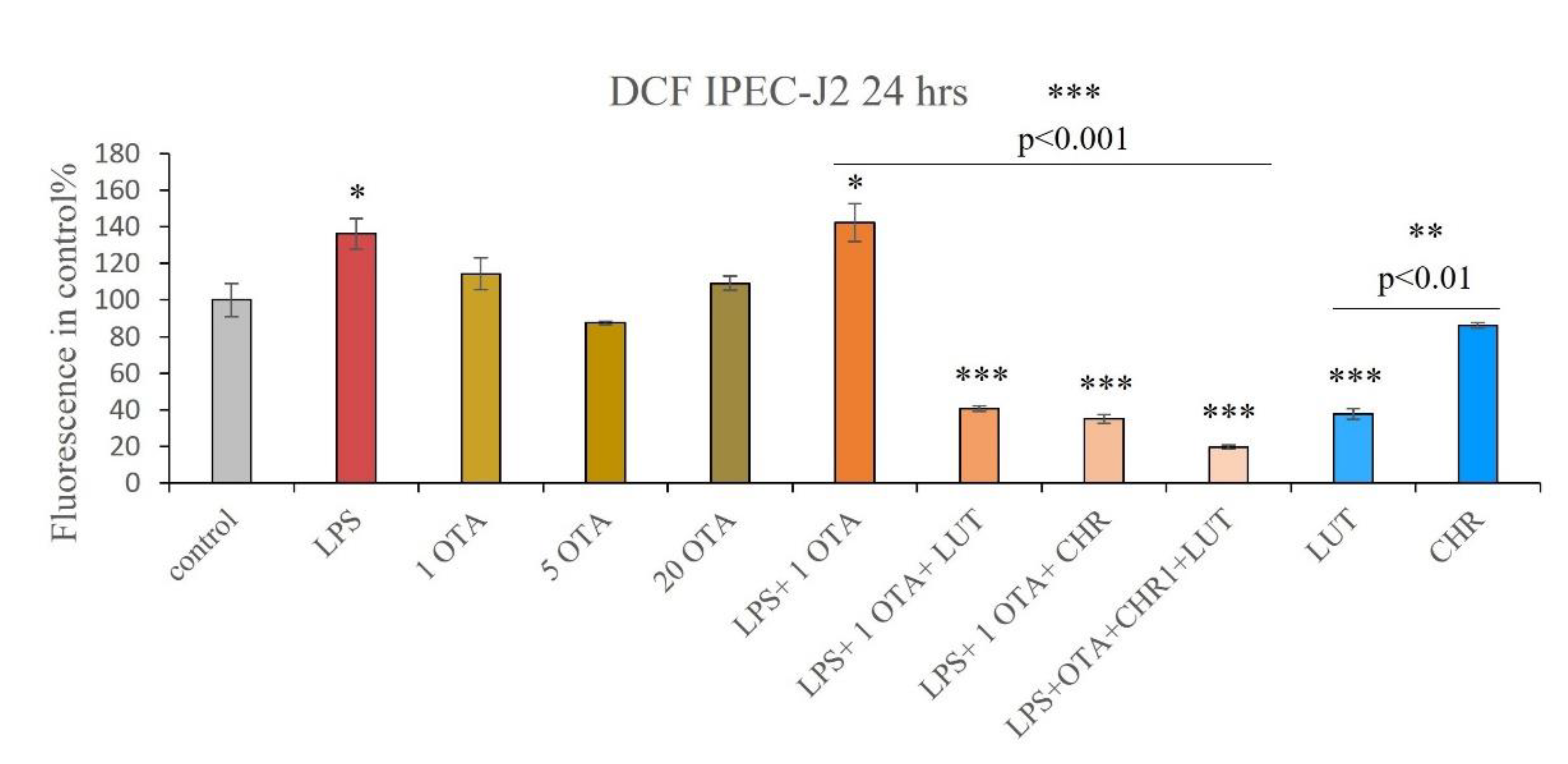
3.3. IC ROS Determination
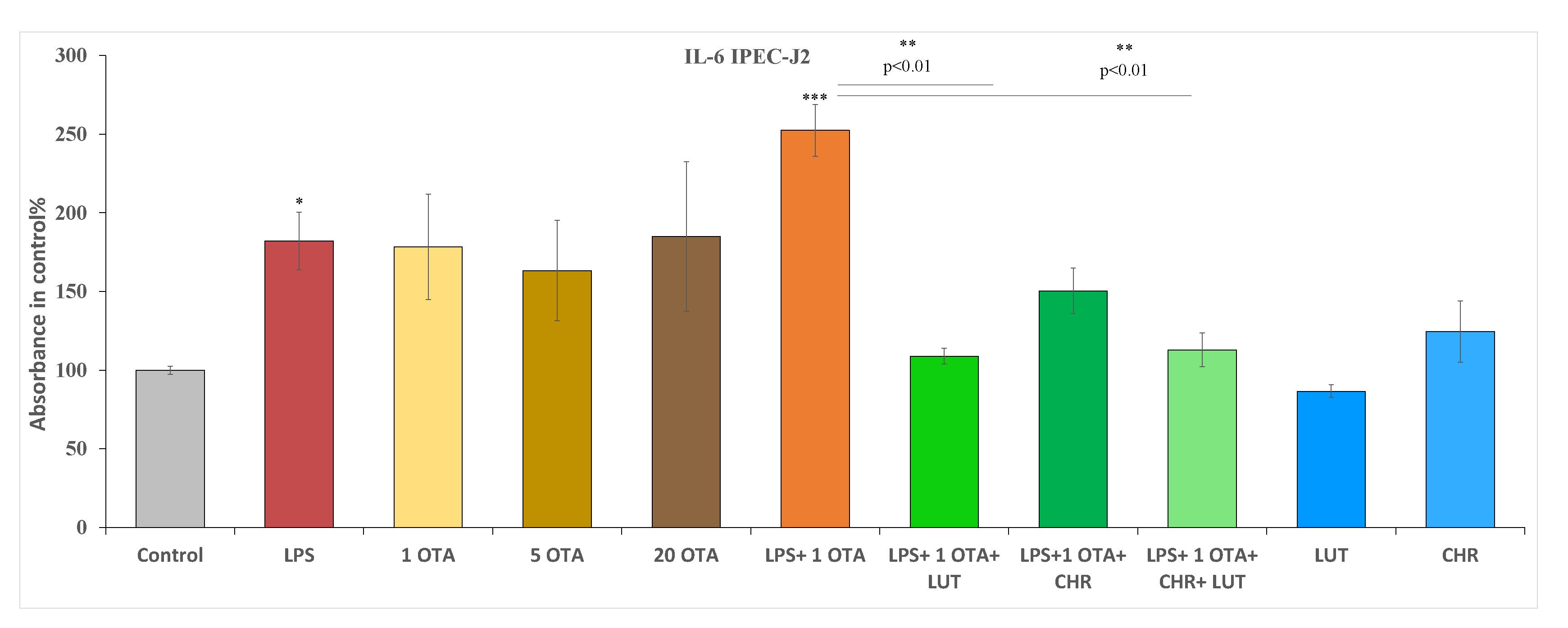
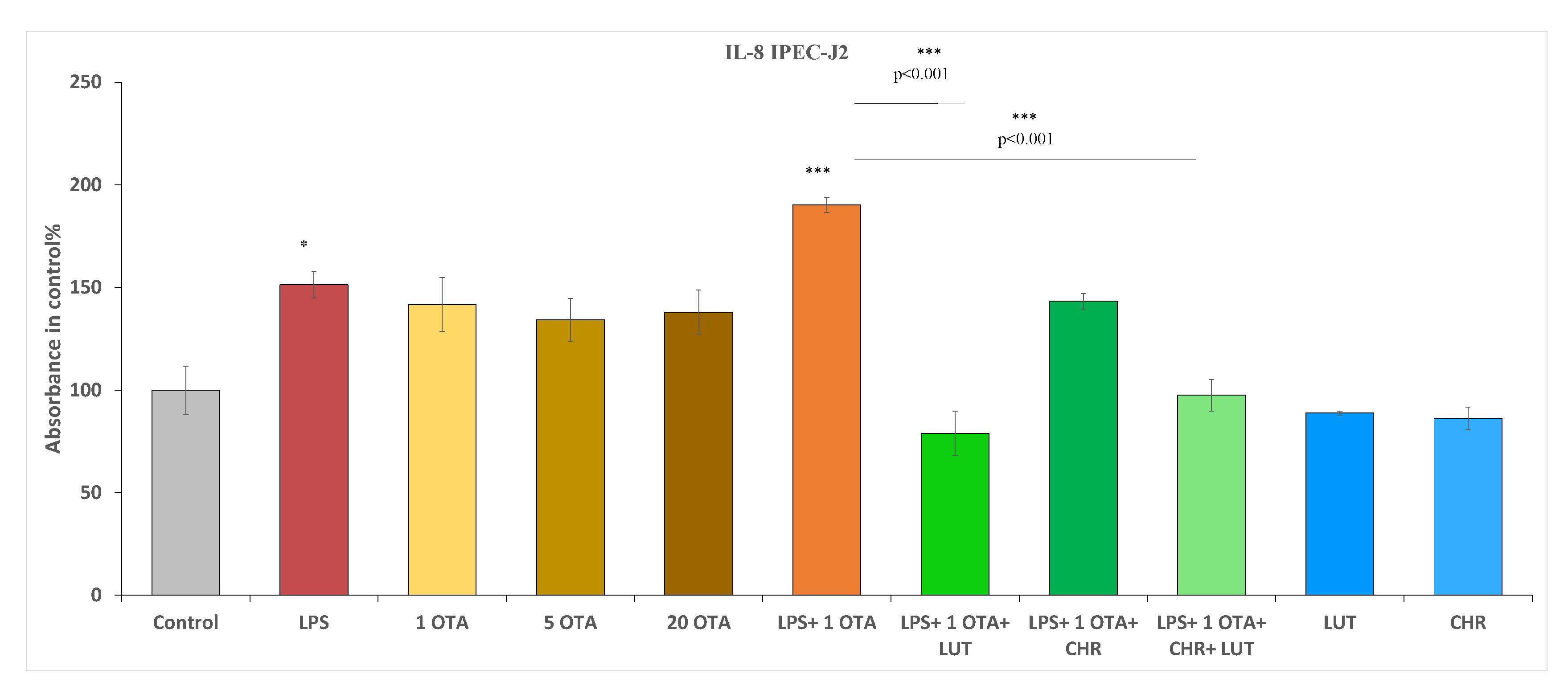
3.4. The Changes in IL-6 and IL-8 Levels after Exposure to OTA, LPS and the Selected Flavonoids in IPEC-J2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus Plantarum Inhibits Growth and Adhesion of Enterotoxigenic Escherichia Coli and Mediates Host Defense. Front. Microbiol. 2018, 9, 1364. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The Biological Stress of Early Weaned Piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia Coli in Postweaning Diarrhea in Pigs: An Update on Bacterial Types, Pathogenesis, and Prevention Strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Bailey, M. The Mucosal Immune System: Recent Developments and Future Directions in the Pig. Dev. Comp. Immunol. 2009, 33, 375–383. [Google Scholar] [CrossRef]
- Sun, Y.; Kim, S.W. Intestinal Challenge with Enterotoxigenic Escherichia Coli in Pigs, and Nutritional Intervention to Prevent Postweaning Diarrhea. Anim. Nutr. 2017, 3, 322–330. [Google Scholar] [CrossRef]
- Luppi, A. Swine Enteric Colibacillosis: Diagnosis, Therapy and Antimicrobial Resistance. Porcine Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a Flavonoid, as an Anticancer Agent: A Review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-Inflammatory Effects of Luteolin: A Review of in Vitro, in Vivo, and in Silico Studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Gras, M.A.; Palade, M.L.; Taranu, I. Comparative Effect of Ochratoxin A on Inflammation and Oxidative Stress Parameters in Gut and Kidney of Piglets. Regul. Toxicol. Pharmacol. 2017, 89, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, E.; Ziegler, K. Ochratoxin A from a Toxicological Perspective. J. Vet. Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef]
- el Khoury, A.; Atoui, A. Ochratoxin a: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef]
- Vlachou, M.; Pexara, A.; Solomakos, N.; Govaris, A. Ochratoxin A in Slaughtered Pigs and Pork Products. Toxins 2022, 14, 67. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Li, S.; Wu, C.; Wang, J.; Zheng, N. Modulation of Mucin (MUC2, MUC5AC and MUC5B) MRNA Expression and Protein Production and Secretion in Caco-2/HT29-MTX Co-Cultures Following Exposure to Individual and Combined Aflatoxin M1 and Ochratoxin A. Toxins 2019, 11, 132. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Gao, Y.-N.; Huang, S.-N.; Wang, J.-Q.; Zheng, N. Ex Vivo and In Vitro Studies Revealed Underlying Mechanisms of Immature Intestinal Inflammatory Responses Caused by Aflatoxin M1 Together with Ochratoxin A. Toxins 2022, 14, 173. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, N.; Chen, Y.; Fu, C.; Huang, K. OTA Induces Intestinal Epithelial Barrier Dysfunction and Tight Junction Disruption in IPEC-J2 Cells through ROS/Ca2+-Mediated MLCK Activation. Environ. Pollut. 2018, 242, 106–112. [Google Scholar] [CrossRef]
- Maresca, M.; Yahi, N.; Younès-Sakr, L.; Boyron, M.; Caporiccio, B.; Fantini, J. Both Direct and Indirect Effects Account for the Pro-Inflammatory Activity of Enteropathogenic Mycotoxins on the Human Intestinal Epithelium: Stimulation of Interleukin-8 Secretion, Potentiation of Interleukin-1beta Effect and Increase in the Transepithelial Passage of Commensal Bacteria. Toxicol. Appl. Pharmacol. 2008, 228, 84–92. [Google Scholar] [CrossRef]
- Farkas, O.; Mátis, G.; Pászti-Gere, E.; Palócz, O.; Kulcsár, A.; Petrilla, J.; Csikó, G.; Neogrády, Z.; Gálfi, P. Effects of Lactobacillus Plantarum 2142 and Sodium N-Butyrate in Lipopolysaccharide-Triggered Inflammation: Comparison of a Porcine Intestinal Epithelial Cell Line and Primary Hepatocyte Monocultures with a Porcine Enterohepatic Co-Culture System. J. Anim. Sci. 2014, 92, 3835–3845. [Google Scholar] [CrossRef]
- Palócz, O.; Pászti-Gere, E.; Gálfi, P.; Farkas, O. Chlorogenic Acid Combined with Lactobacillus Plantarum 2142 Reduced LPS-Induced Intestinal Inflammation and Oxidative Stress in IPEC-J2 Cells. PLoS ONE 2016, 11, e0166642. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Li, S.; Pi, D.; Zhu, H.; Hou, Y.; Shi, H.; Leng, W. Asparagine Attenuates Intestinal Injury, Improves Energy Status and Inhibits AMP-Activated Protein Kinase Signalling Pathways in Weaned Piglets Challenged with Escherichia Coli Lipopolysaccharide. Br. J. Nutr. 2015, 114, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary Supplementation of Aspartate Enhances Intestinal Integrity and Energy Status in Weanling Piglets after Lipopolysaccharide Challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, J.; Sun, Z.; Li, J.; Sun, W.; Mao, J.; Wang, Y. Protective Effects of Taurine on Growth Performance and Intestinal Epithelial Barrier Function in Weaned Piglets Challenged without or with Lipopolysaccharide. Anim. Prod. Sci. 2017, 58, 2011–2022. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Wu, G.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Luo, J.; Mao, X.; et al. Amelioration of Enterotoxigenic Escherichia Coli-Induced Intestinal Barrier Disruption by Low-Molecular-Weight Chitosan in Weaned Pigs Is Related to Suppressed Intestinal Inflammation and Apoptosis. Int. J. Mol. Sci. 2019, 20, E3485. [Google Scholar] [CrossRef]
- Sundaram, T.S.; Giromini, C.; Rebucci, R.; Baldi, A. Omega-3 Polyunsaturated Fatty Acids Counteract Inflammatory and Oxidative Damage of Non-Transformed Porcine Enterocytes. Animals 2020, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Frangiamone, M.; Cimbalo, A.; Alonso-Garrido, M.; Vila-Donat, P.; Manyes, L. In Vitro and in Vivo Evaluation of AFB1 and OTA-Toxicity through Immunofluorescence and Flow Cytometry Techniques: A Systematic Review. Food Chem. Toxicol. 2022, 160, 112798. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Zhai, N.; Chen, X.; Gan, F.; Li, H.; Huang, K. Ochratoxin A-Induced Apoptosis of IPEC-J2 Cells through ROS-Mediated Mitochondrial Permeability Transition Pore Opening Pathway. J. Agric. Food Chem. 2017, 65, 10630–10637. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex-32019R0006-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2019/6/oj (accessed on 29 August 2022).
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Geagea, A.G.; Jurjus, A.; et al. Nutrition, Oxidative Stress and Intestinal Dysbiosis: Influence of Diet on Gut Microbiota in Inflammatory Bowel Diseases. Biomed. Pap. 2016, 160, 461. [Google Scholar] [CrossRef]
- Iizuka, M.; Sasaki, K.; Hirai, Y.; Shindo, K.; Konno, S.; Itou, H.; Ohshima, S.; Horie, Y.; Watanabe, S. Morphogenic Protein Epimorphin Protects Intestinal Epithelial Cells from Oxidative Stress by the Activation of EGF Receptor and MEK/ERK, PI3 Kinase/Akt Signals. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G39–G52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kovács, D.; Karancsi, Z.; Farkas, O.; Jerzsele, Á. Antioxidant Activity of Flavonoids in LPS-Treated IPEC-J2 Porcine Intestinal Epithelial Cells and Their Antibacterial Effect against Bacteria of Swine Origin. Antioxidants 2020, 9, 1267. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; González-Arias, C.A.; Ramos, A.J.; Sanchis, V.; Fernández-Cruz, M.L. Cytotoxicity of the Mycotoxins Deoxynivalenol and Ochratoxin A on Caco-2 Cell Line in Presence of Resveratrol. Toxicol. In Vitro 2015, 29, 1639–1646. [Google Scholar] [CrossRef]
- Harris, G.K.; Qian, Y.; Leonard, S.S.; Sbarra, D.C.; Shi, X. Luteolin and Chrysin Differentially Inhibit Cyclooxygenase-2 Expression and Scavenge Reactive Oxygen Species but Similarly Inhibit Prostaglandin-E2 Formation in RAW 264.7 Cells. J. Nutr. 2006, 136, 1517–1521. [Google Scholar] [CrossRef]
- Bustos, P.S.; Deza-Ponzio, R.; Páez, P.L.; Cabrera, J.L.; Virgolini, M.B.; Ortega, M.G. Flavonoids as Protective Agents against Oxidative Stress Induced by Gentamicin in Systemic Circulation. Potent Protective Activity and Microbial Synergism of Luteolin. Food Chem. Toxicol. 2018, 118, 294–302. [Google Scholar] [CrossRef]
- Ramyaa, P.; Padma, V.V. Ochratoxin-Induced Toxicity, Oxidative Stress and Apoptosis Ameliorated by Quercetin—Modulation by Nrf2. Food Chem. Toxicol. 2013, 62, 205–216. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, C.; Li, X.; Zhou, S.; Hua, J.; Huang, J.; Li, Y.; Yang, K.; Zhang, P.; Zhang, Y.; et al. Luteolin Alleviates Ochratoxin A Induced Oxidative Stress by Regulating Nrf2 and HIF-1α Pathways in NRK-52E Rat Kidney Cells. Food Chem. Toxicol. 2020, 141, 111436. [Google Scholar] [CrossRef]
- Karancsi, Z.; Kovács, D.; Palkovicsné Pézsa, N.; Gálfi, P.; Jerzsele, Á.; Farkas, O. The Impact of Quercetin and Its Methylated Derivatives 3-o-Methylquercetin and Rhamnazin in Lipopolysaccharide-Induced Inflammation in Porcine Intestinal Cells. Antioxidants 2022, 11, 1265. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Barna, R.F.; Pászti, E.A.; Babiczky, Á.; Szóládi, Á.; Jerzsele, Á.; Gere, E.P. Beneficial Effects of Rosmarinic Acid on IPEC-J2 Cells Exposed to the Combination of Deoxynivalenol and T-2 Toxin. Mediat. Inflamm. 2020, 2020, 8880651. [Google Scholar] [CrossRef] [PubMed]
- Akira, S. TLR Signaling. In From Innate Immunity to Immunological Memory; Current Topics in Microbiology and Immunology; Pulendran, B., Ahmed, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–16. ISBN 978-3-540-32636-6. [Google Scholar]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Zhang, W.; Yang, Z.; Ding, B.; Zhu, H.; Liu, Y.; Qiu, Y.; Yin, Y.; Wu, G. Protective Effects of N-Acetylcysteine on Intestinal Functions of Piglets Challenged with Lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, S.; Xia, Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.; Zhang, H.; Zhang, M.; Ye, H.; et al. Ochratoxin A Induces Liver Inflammation: Involvement of Intestinal Microbiota. Microbiome 2019, 7, 151. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlert, A.; Palkovicsné Pézsa, N.; Móritz, A.V.; Jerzsele, Á.; Farkas, O.; Pászti-Gere, E. Luteolin and Chrysin Could Prevent E. coli Lipopolysaccharide-Ochratoxin A Combination-Caused Inflammation and Oxidative Stress in In Vitro Porcine Intestinal Model. Animals 2022, 12, 2747. https://doi.org/10.3390/ani12202747
Wohlert A, Palkovicsné Pézsa N, Móritz AV, Jerzsele Á, Farkas O, Pászti-Gere E. Luteolin and Chrysin Could Prevent E. coli Lipopolysaccharide-Ochratoxin A Combination-Caused Inflammation and Oxidative Stress in In Vitro Porcine Intestinal Model. Animals. 2022; 12(20):2747. https://doi.org/10.3390/ani12202747
Chicago/Turabian StyleWohlert, Annelie, Nikolett Palkovicsné Pézsa, Alma Virág Móritz, Ákos Jerzsele, Orsolya Farkas, and Erzsébet Pászti-Gere. 2022. "Luteolin and Chrysin Could Prevent E. coli Lipopolysaccharide-Ochratoxin A Combination-Caused Inflammation and Oxidative Stress in In Vitro Porcine Intestinal Model" Animals 12, no. 20: 2747. https://doi.org/10.3390/ani12202747
APA StyleWohlert, A., Palkovicsné Pézsa, N., Móritz, A. V., Jerzsele, Á., Farkas, O., & Pászti-Gere, E. (2022). Luteolin and Chrysin Could Prevent E. coli Lipopolysaccharide-Ochratoxin A Combination-Caused Inflammation and Oxidative Stress in In Vitro Porcine Intestinal Model. Animals, 12(20), 2747. https://doi.org/10.3390/ani12202747





