Transmission of Zoonotic Diseases in the Daily Life of Ancient Pompeii and Herculaneum (79 CE, Italy): A Review of Animal–Human–Environment Interactions through Biological, Historical and Archaeological Sources
Abstract
Simple Summary
Abstract
1. Introduction
2. Zoonotic Diseases in Ancient Times: Biological, Archaeological and Literary Evidence
3. Historical Background of the Roman Cities of Pompeii and Herculaneum
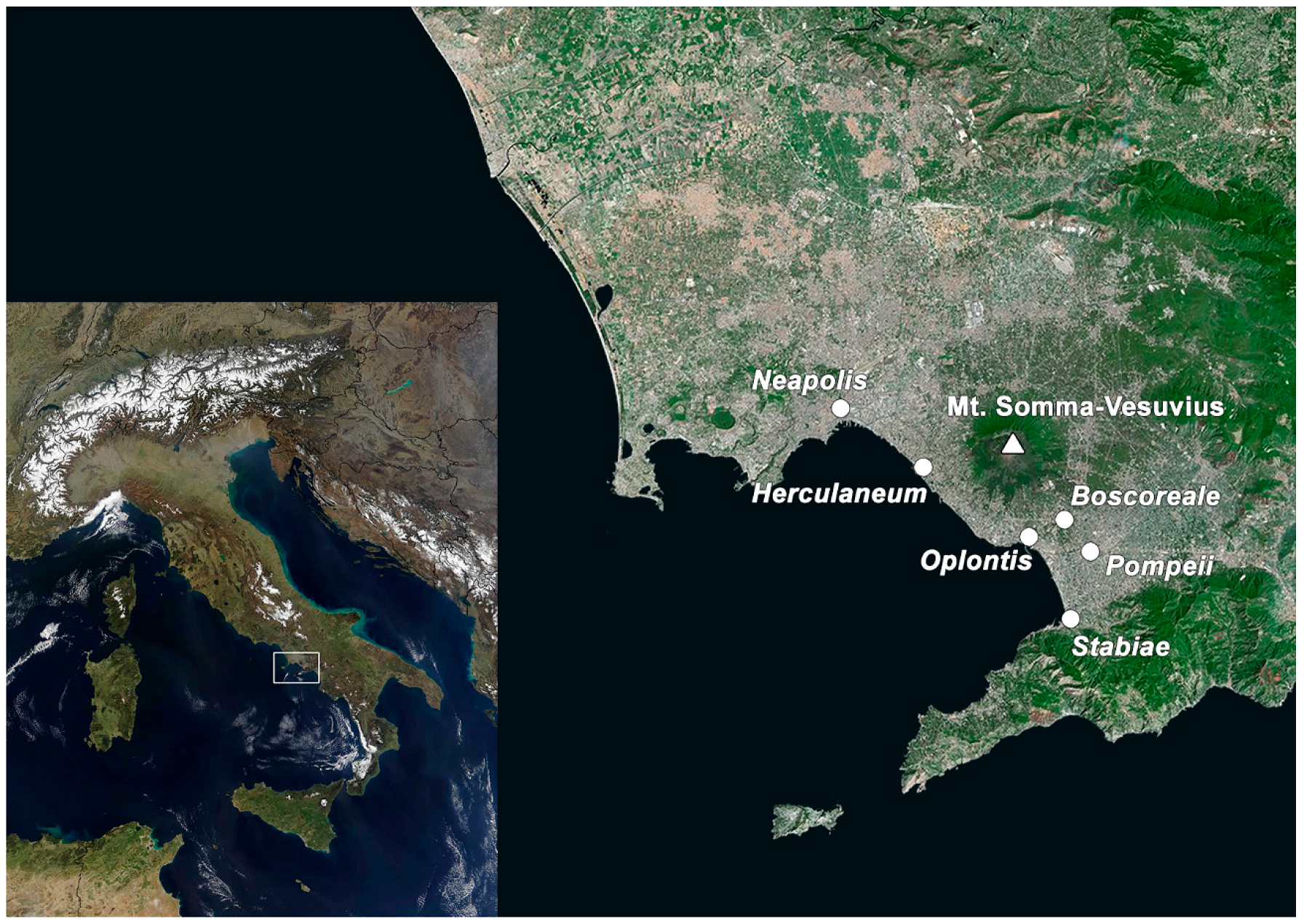
3.1. Geographical Setting
3.2. The Eruption of Mount Somma–Vesuvius in 79 CE
3.3. Investigation and Interpretation of the Different Information Sources
4. Animal–Human–Environment Interaction in the Daily Life at Pompeii and Herculaneum
4.1. Population Density and Housing
4.2. Trade Routes
4.3. Livestock, Wild, Exotic and Household Animals
4.3.1. Exotic Animals
4.3.2. Livestock
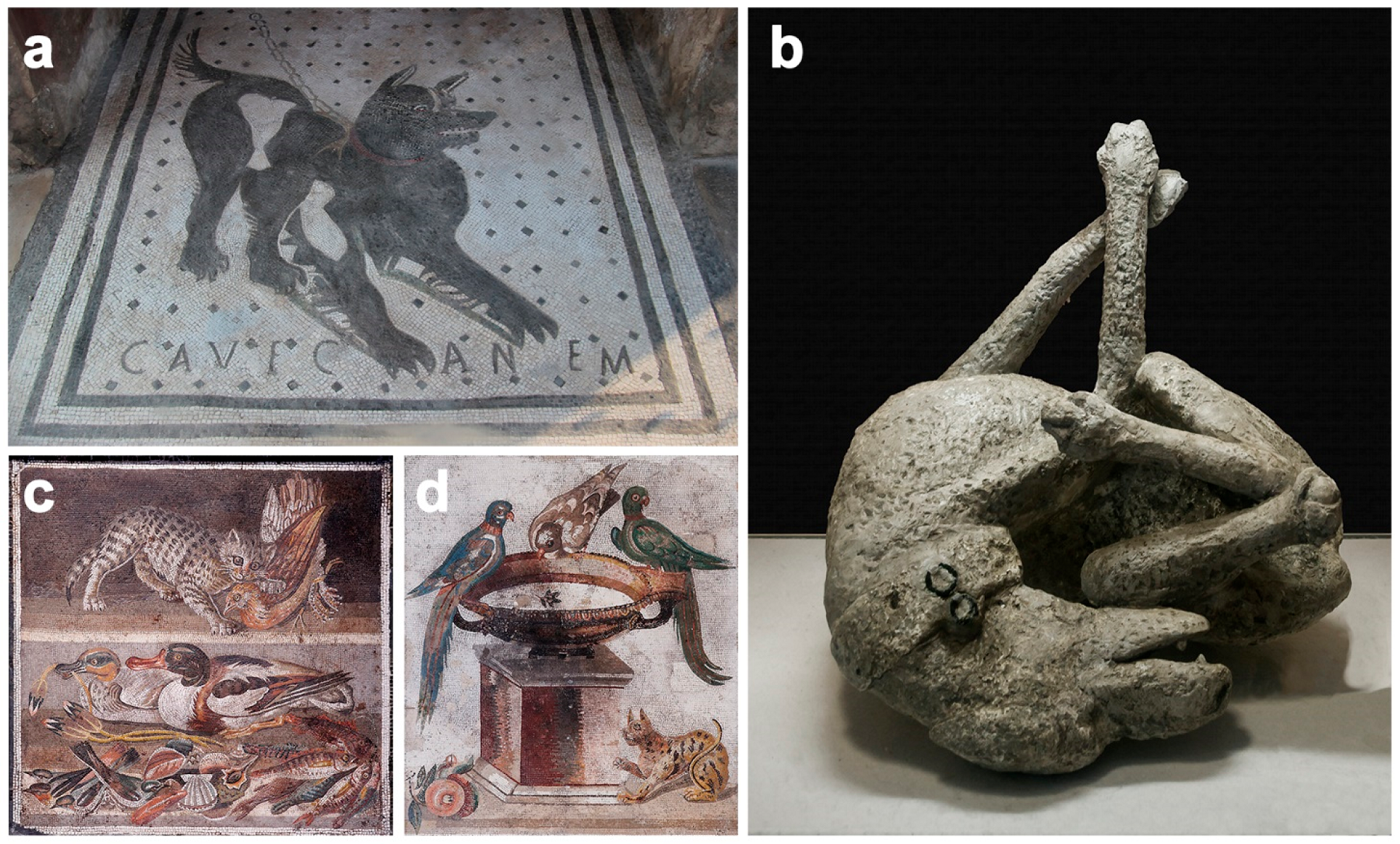
4.3.3. Household Animals
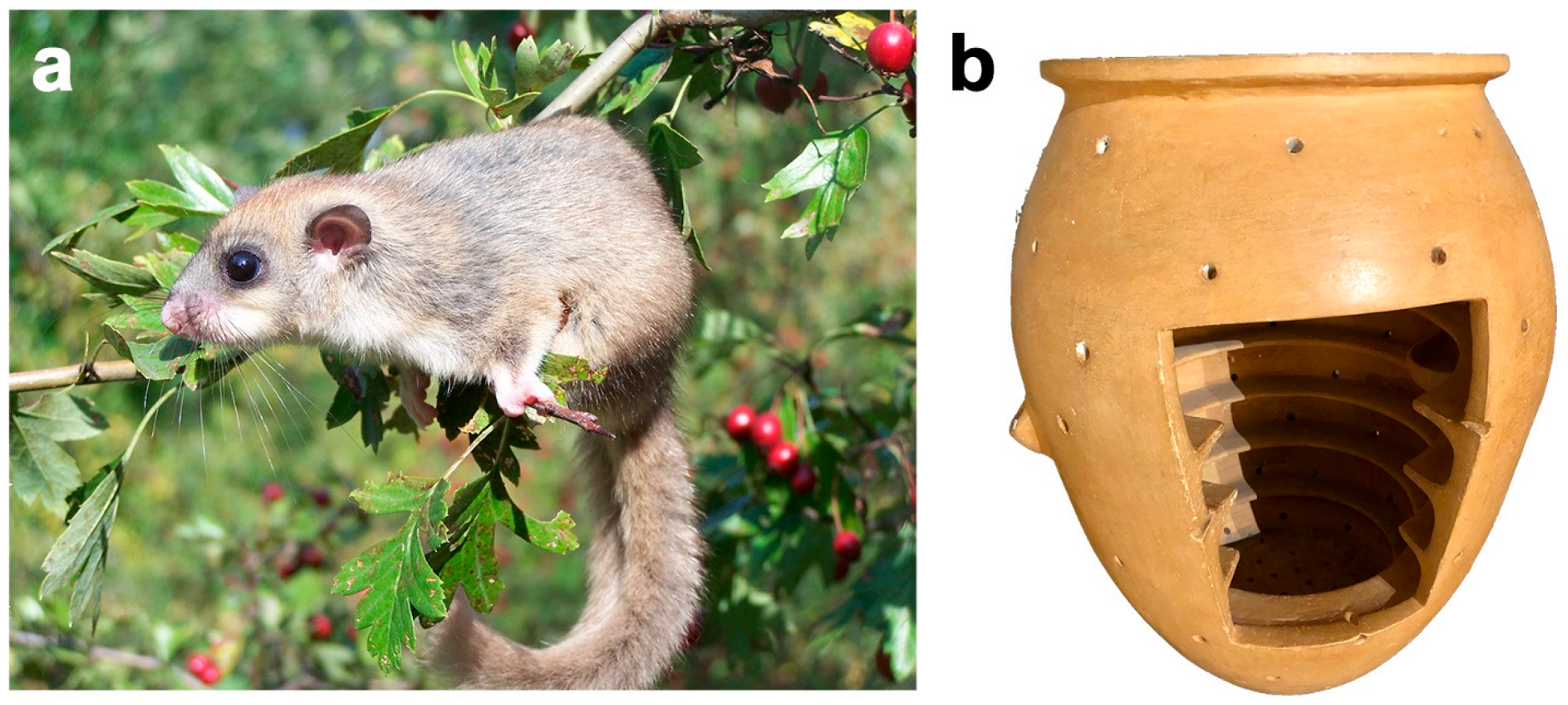
4.3.4. Wild Animals
4.4. Occupational and Work-Related Zoonotic Diseases
4.4.1. Agriculture
4.4.2. Livestock Farming
4.4.3. Textile Industry
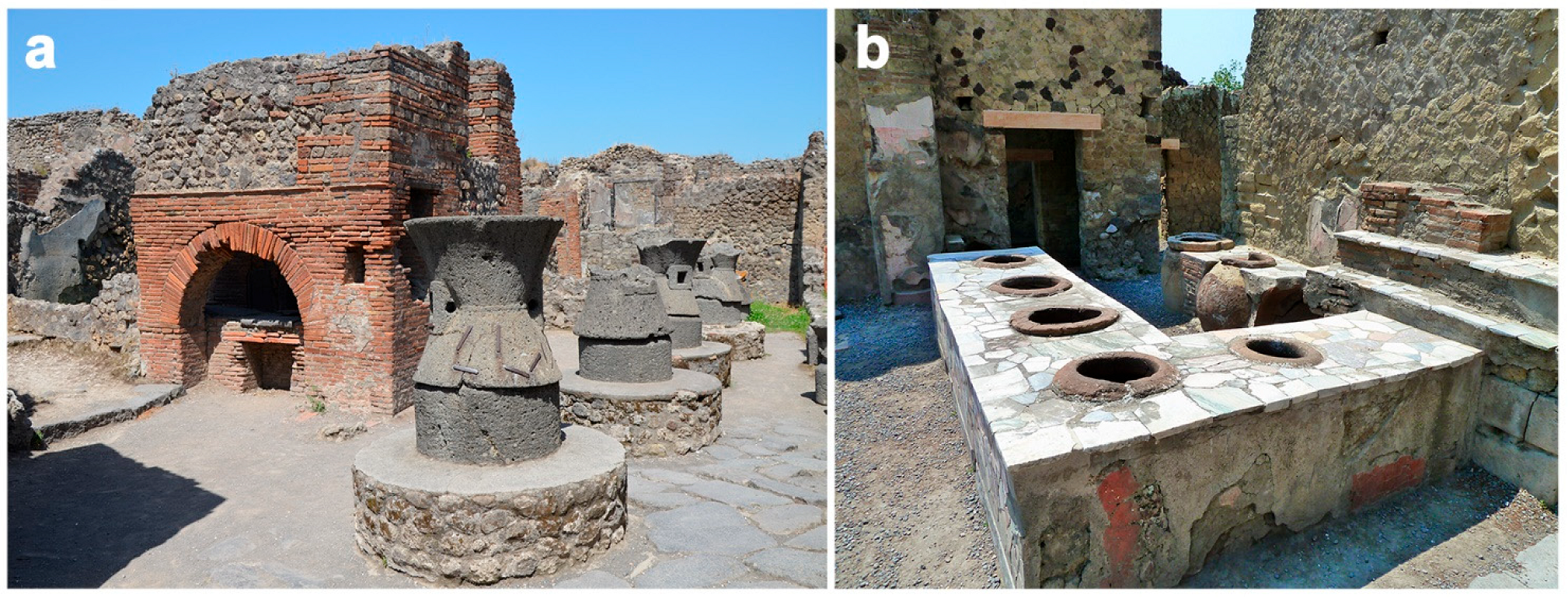
4.4.4. Storage and Grain Processing, and Bakeries (Pistrina)
4.4.5. Foodborne Zoonotic Diseases in Taverns (Thermopolia, Cauponae, Popinae) and Home
4.5. Latrines, Sewers and Baths (Thermae)
4.6. Rituals
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). The Control of Neglected Zoonotic Diseases: A Route to Poverty Alleviation. In Report of a Joint WHO/DFID-AHP Meeting with the Participation of FAO and OIE; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Fong, I.W. Animals and Mechanisms of Disease Transmission. In Emerging Zoonoses: A Worldwide Perspective; Fong, I.W., Ed.; Springer: Toronto, ON, Canada, 2017; pp. 15–38. [Google Scholar] [CrossRef]
- United Nations Industrial Development Organization (UNIDO). Coronavirus: The Economic Impact. Available online: https://www.unido.org/stories/coronavirus-economic-impact (accessed on 12 January 2020).
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 19 November 2021).
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef] [PubMed]
- Parry, J. Breaches of safety regulations are probable cause of recent SARS outbreak, WHO says. BMJ 2004, 328, 1222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lam, T.T.; Jia, N.; Zhang, Y.W.; Shum, M.H.; Jiang, J.F.; Zhu, H.C.; Tong, Y.G.; Shi, Y.X.; Ni, X.B.; Liao, Y.S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Damborg, P.; Broens, E.M.; Chomel, B.B.; Guenther, S.; Pasmans, F.; Wagenaar, J.A.; Weese, J.S.; Wieler, L.H.; Windahl, U.; Vanrompay, D.; et al. Bacterial zoonoses transmitted by household pets: State-of-the-art and future perspectives for targeted research and policy actions. J. Comp. Pathol. 2016, 155, S27–S40. [Google Scholar] [CrossRef]
- Ganière, J.P. Risk of zoonoses by animal bites and scratches. Rev. Prat. 2019, 69, 320–323. [Google Scholar]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- De Graaf, M.; Beck, R.; Caccio, S.M.; Duim, B.; Fraaij, P.L.; Le Guyader, F.S.; Lecuit, M.; Le Pendu, J.; de Witt, E.; Schultsz, C. Sustained fecal-oral human-to-human transmission following a zoonotic event. Curr. Opin. Virol. 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Tauxe, R.V. Emerging foodborne diseases: An evolving public health challenge. Emerg. Infect. Dis. 1997, 3, 425–434. [Google Scholar] [CrossRef]
- Foil, L.D.; Gorham, J.R. Mechanical Transmission of Disease Agents by Arthropods. In Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods; Eldridge, B.F., Edman, J.D., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 461–514. [Google Scholar] [CrossRef]
- Khan, M.A.H.N.A. Important vector-borne diseases with their zoonotic potential: Present situation and future perspective. Bangladesh J. Vet. Med. 2015, 13, 1–14. [Google Scholar] [CrossRef]
- Bierque, E.; Thibeaux, R.; Girault, D.; Soupé-Gilbert, M.E.; Goarant, C. A systematic review of Leptospira in water and soil environments. PLoS ONE 2020, 15, e0227055. [Google Scholar] [CrossRef]
- Slifko, T.R.; Smith, H.V.; Rose, J.B. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 2000, 30, 1379–1393. [Google Scholar] [CrossRef]
- Houldcroft, C.J.; Underdown, S.J. Neanderthal genomics suggests a Pleistocene time frame for the first epidemiologic transition. Am. J. Phys. Anthropol. 2016, 160, 379–388. [Google Scholar] [CrossRef]
- Bartosiewicz, L.; Gál, E. Inflammatory Diseases and Bone. In Shuffling Nags, Lame Ducks: The Archaeology of Animal Disease; Bartosiewicz, L., Gál, E., Eds.; Oxbow Books: Oxford, UK, 2013; pp. 91–104. [Google Scholar]
- Buzic, I.; Giuffra, V. The paleopathological evidence on the origins of human tuberculosis: A review. J. Prev. Med. Hyg. 2020, 61, E3–E8. [Google Scholar] [CrossRef]
- Bendrey, R.; Cassidy, J.P.; Fournié, G.; Merrett, D.C.; Oakes, R.H.A.; Taylor, G.M. Approaching ancient disease from a One Health perspective: Interdisciplinary review for the investigation of zoonotic brucellosis. Int. J. Osteoarchaeol. 2020, 30, 99–108. [Google Scholar] [CrossRef]
- D’Anastasio, R.; Zipfel, B.; Moggi-Cecchi, J.; Stanyon, R.; Capasso, L. Possible brucellosis in an early hominin skeleton from Sterkfontein, South Africa. PLoS ONE 2009, 4, e6439. [Google Scholar] [CrossRef]
- Dow, G.K.; Reed, C.G. The origins of sedentism: Climate, population, and technology. J. Econ. Behav. Organ. 2015, 119, 56–71. [Google Scholar] [CrossRef]
- Chessa, B.; Pereira, F.; Arnaud, F.; Amorim, A.; Goyache, F.; Mainland, I.; Kao, R.R.; Pemberton, J.M.; Beraldi, D.; Steer, M.; et al. Revealing the history of sheep domestication using retrovirus integrations. Science 2009, 324, 532–536. [Google Scholar] [CrossRef]
- Zeder, M.A. The domestication of animals. J. Anthropol. Res. 2012, 68, 161–190. [Google Scholar] [CrossRef]
- Namdar, L.; Vardi, J.; Paz, Y.; Sapir-Hen, L. Variation in economic specialization as revealed through the study of Pottery Neolithic faunal assemblages from the southern Levant. Archaeol. Anthropol. Sci. 2021, 13, 207. [Google Scholar] [CrossRef]
- Evershed, R.P.; Payne, S.; Sherratt, A.G.; Copley, M.S.; Coolidge, J.; Urem-Kotsu, D.; Kotsakis, K.; Özdoğan, M.; Özdoğan, A.E.; Nieuwenhuyse, O.; et al. Earliest date for milk use in the Near East and Southeastern Europe linked to cattle herding. Nature 2008, 455, 528–531. [Google Scholar] [CrossRef]
- Greenfield, H.J. A Reconsideration of the Secondary Products Revolution: 20 Years of Research in the Central Balkans. In The Zooarchaeology of Milk and Fats, Proceedings of the 9th ICAZ Conference, Durham, UK, 23–28 August 2002; Mulville, J., Outram, A., Eds.; Oxbow Books: Oxford, UK, 2005; pp. 14–31. [Google Scholar]
- Greenfield, H.J. The secondary products revolution: The past, the present and the future. World Archaeol. 2010, 42, 29–54. [Google Scholar] [CrossRef]
- Greenfield, H.J.; Arnold, E.R. Go(a) t milk? New perspectives on the zooarchaeological evidence for the earliest intensification of dairying in south eastern Europe. World Archaeol. 2015, 47, 792–818. [Google Scholar] [CrossRef]
- Sherratt, A. Plough and Pastoralism: Aspects of the Secondary Products Revolution. In Pattern of the Past: Studies in Honour of David Clarke; Hodder, I., Isaac, G., Hammond, N., Eds.; Cambridge University Press: Cambridge, UK, 1981; pp. 261–306. [Google Scholar]
- Abedellaah, B.A.; Elkadragy, M.; Sharsher, A.; Rashed, R.; Elbaz, H.T. Veterinary surgery and gynecology in the ancient Egypt. Assiut Vet. Med. J. 2019, 65, 129–134. [Google Scholar]
- Griffith, F.L. The Petrie Papyri: Hieratic Papyri from Kahun and Gurob (Principally of the Middle Kingdom); Bernard Quaritch: London, UK, 1898. [Google Scholar]
- Bryan, C.P. Ancient Egyptian Medicine: The Papyrus Ebers; Ares Publishers: Chicago, IL, USA, 1974. [Google Scholar]
- Dirckx, J.H. Virgil on anthrax. Am. J. Dermatopathol. 1981, 3, 191–196. [Google Scholar] [CrossRef]
- Dirckx, J.H. Pestilence narratives in classical literature: A study in creative imitation: I. Homer, Sophocles, Thucydides, and Lucretius. Am. J. Dermatopathol. 2000, 22, 197–202. [Google Scholar] [CrossRef]
- Dirckx, J.H. Pestilence narratives in classical literature: A study in creative imitation. II. Virgil, Ovid, Seneca, and Silius Italicus. Am. J. Dermatopathol. 2000, 22, 459–464. [Google Scholar] [CrossRef]
- Shoja, M.M.; Tubbs, R.S.; Ghabili, K.; Griessenauer, C.J.; Balch, M.W.; Cuceu, M. The Roman Empire legacy of Galen (129–200 AD). Childs Nerv. Syst. 2015, 31, 1–5. [Google Scholar] [CrossRef]
- Zasada, A.A. Detection and identification of Bacillus anthracis: From conventional to molecular microbiology methods. Microorganisms 2020, 8, 125. [Google Scholar] [CrossRef]
- Capasso, L. I Fuggiaschi di Ercolano: Paleobiologia Delle Vittime Dell’eruzione Vesuviana del 79 d.C; “L’Erma” di Bretschneider: Rome, Italy, 2001. [Google Scholar]
- Sigurdsson, H. The Environmental and Geomorphological Context of the Volcano. In The World of Pompeii; Dobbins, J.J., Foss, P.W., Eds.; Routledge: New York, NY, USA, 2007; pp. 43–62. [Google Scholar]
- Discœudres, J.-P. History and Historical Sources. In The World of Pompeii; Dobbins, J.J., Foss, P.W., Eds.; Routledge: New York, NY, USA, 2007; pp. 9–27. [Google Scholar]
- Flohr, M.; Wilson, A. The Economy of Pompeii; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Pesando, F.; Guidobaldi, M.P. Guide Archeologiche Laterza: Pompei, Oplontis, Ercolano, Stabiae; Laterza: Bari, Italy, 2018. [Google Scholar]
- Cooley, A.E.; Cooley, M.G.L. Pompeii and Herculaneum: A Sourcebook; Routledge: New York, NY, USA, 2014. [Google Scholar]
- Stefani, G. La vera data dell’eruzione. Archeo 2006, 260, 10–13. [Google Scholar]
- Rolandi, G.; Paone, A.; Di Lascio, M.; Stefani, G. The 79 AD eruption of Somma: The relationship between the date of the eruption and the southeast tephra dispersion. J. Volcanol. Geotherm. Res. 2007, 169, 87–98. [Google Scholar] [CrossRef]
- Lirer, L.; Pescatore, T.; Booth, B.; Walker, G.P.L. Two Plinian pumice-fall deposits from Somma-Vesuvius, Italy. Geol. Soc. Am. Bull. 1973, 84, 759–772. [Google Scholar] [CrossRef]
- Sheridan, M.F.; Barberi, F.; Rosi, M.; Santacroce, R. A model of Plinian eruptions of Vesuvius. Nature 1981, 289, 282–285. [Google Scholar] [CrossRef]
- Sigurdsson, H.; Cashdollar, S.; Sparks, S.R.J. The eruption of Vesuvius in A.D. 79: Reconstruction from historical and volcanological evidence. Am. J. Archaeol. 1982, 86, 39–51. [Google Scholar] [CrossRef]
- De Carolis, E.; Patricelli, G.; Ciarallo, A. Rinvenimenti di corpi nell’area urbana di Pompei. Riv. Studi Pompeiani 1998, 9, 75–123. [Google Scholar]
- Petrone, P.; Pucci, P.; Vergara, A.; Amoresano, A.; Birolo, L.; Pane, F.; Sirano, F.; Niola, M.; Buccelli, C.; Grazioano, V. A hypothesis of sudden body fluid vaporization in the 79 AD victims of Vesuvius. PLoS ONE 2018, 13, e0203210. [Google Scholar] [CrossRef]
- Schmidt, C.W.; Oakley, E.; D’Anastasio, R.; Brower, R.; Remy, A.; Viciano, J. Herculaneum. In The Analysis of Burned Human Remains, 2nd ed.; Schmidt, C.W., Symes, S.A., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 149–161. [Google Scholar]
- Petrone, P. The Herculaneum victims of the 79 AD Vesuvius eruption: A review. J. Anthropol. Sci. 2019, 97, 69–89. [Google Scholar] [CrossRef]
- Beard, M. Pompeii: The life of a Roman Town; Profile Books: London, UK, 2008. [Google Scholar]
- De Carolis, E. Il Mobile a Pompei ed Ercolano: Letti, Tavoli, Sedie e Armadi; “L’Erma” di Bretschneider: Rome, Italy, 2007. [Google Scholar]
- Dyer, T.H. Pompeii: Its History, Buildings, and Antiquities; Bell & Daldy: London, UK, 1867. [Google Scholar]
- Vulpes, B. Illustrazione di Tutti gli Strumenti Chirurgici Scavati in Ercolano e Pompei e che ora Conservansi nel Reale Museo Borbonico di Napoli; Stamperia Reale: Naples, Italy, 1847. [Google Scholar]
- Wallace-Hadrill, A. Houses and Society in Pompeii and Herculaneum; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Capasso, L. Bacteria in two-millennia-old cheese, and related epizoonoses in Roman populations. J. Infect. 2002, 45, 122–127. [Google Scholar] [CrossRef]
- Capasso, L. Infectious diseases and eating habits at Herculaneum (1st century AD, southern Italy). Int. J. Osteoarchaeol. 2007, 17, 350–357. [Google Scholar] [CrossRef]
- D’Anastasio, R.; Capasso, L. Microbiological food contamination and status of health at Herculaneum (1st century AD, southern Italy). Eur. J. Inflamm. 2007, 5, 165–169. [Google Scholar] [CrossRef]
- Nicholson, R.; Robinson, J.; Robinson, M.; Rowan, E. From the waters to the plate to the latrine: Fish and seafood from the Cardo V sewer, Herculaneum. J. Marit. Archaeol. 2018, 13, 263–284. [Google Scholar] [CrossRef]
- Rowan, E. Roman Diet and Nutrition in the Vesuvian Region: A Study of the Bioarchaeological Remains from the Cardo V Sewer at Herculaneum. Ph.D. Thesis, University of Oxford, Oxford, UK, 2014. [Google Scholar]
- Bisel, S.C. Nutrition in first century Herculaneum. Anthropologie 1988, 26, 61–66. [Google Scholar]
- Bisel, S.C. The human skeletons of Herculaneum. Int. J. Anthropol. 1991, 6, 1–20. [Google Scholar] [CrossRef]
- De Carolis, E.; Patricelli, G. Rinvenimenti di corpi umani nel suburbio pompeiano e nei siti di Ercolano e Stabia. Riv. Studi Pompeiani 2013, 24, 11–32. [Google Scholar]
- Lazer, E. Resurrecting Pompeii; Routledge: Abingdon, UK, 2009. [Google Scholar]
- Torino, M.; Rognini, M.; Fornaciari, G. Dental fluorosis in ancient Herculaneum. Lancet 1995, 345, 1306. [Google Scholar] [CrossRef]
- Viciano, J.; D’Anastasio, R.; Capasso, L. Timing of maxillofacial–oral injuries in an individual of the ancient city of Herculaneum (79 AD, Naples, Italy): A case report. Dent. Traumatol. 2015, 31, 215–227. [Google Scholar] [CrossRef]
- Viciano, J.; Remigio, M.; D’Anastasio, R.; Capasso, L. Anatomical variations of the foramen transversarium of cervical vertebrae from the ancient population of Herculaneum (79 CE; Naples, Italy). Homo Int. Z. Vgl. Forsch. Menschen 2021, 72, 61–85. [Google Scholar] [CrossRef]
- Tanga, C.; Viciano, J.; Monza, F.; D’Anastasio, R.; Capasso, L. Dental paleopathology seen through historical, archaeological and biological sources in ancient Herculaneum (79 AD, Italy). Med. Hist. 2020, 4, e2020007. [Google Scholar]
- Wallace, R.E. An Introduction to Wall Inscriptions from Pompeii and Herculaneum; Bolchazy-Carducci Publishers: Wauconda, IL, USA, 2005. [Google Scholar]
- Mitchell, P.D. Human parasites in the Roman World: Health consequences of conquering an empire. Parasitology 2017, 144, 48–58. [Google Scholar] [CrossRef]
- Retief, F.P.; Cilliers, L. Epidemics of the Roman Empire, 27 BC–AD 476. S. Afr. Med. J. 2000, 90, 267–272. [Google Scholar]
- Mitchell, P.D. A Better Understanding of Sanitation and Health in the Past. In Sanitation, Latrines and Intestinal Parasites in Past Populations; Mitchell, P.D., Ed.; Ashgate: Farnham, UK, 2015; pp. 229–233. [Google Scholar]
- Oerlemans, A.P.A.; Tacoma, L.E. Three great killers. Infectious diseases and patterns of mortality in Imperial Rome. Anc. Soc. 2014, 44, 213–241. [Google Scholar]
- Wallace-Hadrill, A. Imperial Rome: A city of immigrants? Acta Archaeol. Artium Hist. Pertin. 2016, 290, 53–72. [Google Scholar] [CrossRef]
- Taylor, C. A Tale of Two Cities: The Efficacy of Ancient and Medieval Sanitation Methods. In Sanitation, Latrines and Intestinal Parasites in Past Populations; Mitchell, P.D., Ed.; Ashgate: Farnham, UK, 2015; pp. 69–97. [Google Scholar]
- Briones, V.; Pérez-Sancho, M. Was ancient Rome the perfect environment for zoonoses transmission? Travel Med. Infect. Dis. 2020, 38, 101740. [Google Scholar] [CrossRef]
- Aldrete, G.S. Daily Life in the Roman City: Roma, Pompeii, and Ostia; Greenwood: London, UK, 2004. [Google Scholar]
- O’Gorman, K. Discovering commercial hospitality in Ancient Rome. Hosp. Rev. 2007, 9, 44–52. [Google Scholar]
- Ruiz de Arbulo, J.; Gris, F. Los Negocios de Hostelería en Pompeya: Cauponae, Hospitia et Stabula. In Emptor et Mercator: Spazi e Rappresentazioni del Commercio Romano; Santoro, S., Ed.; Edipuglia: Bari, Italy, 2017; pp. 153–176. [Google Scholar]
- Scobie, A. Slums, sanitation, and mortality in the Roman world. Klio 1986, 68, 399–433. [Google Scholar] [CrossRef]
- Vidal, J.M. Utilitas Frente a Venustas: Viviendas Populares de la Antigua Roma. In De la Estructura Doméstica al Espacio Social: Lecturas Arqueológicas del uso Social del Espacio; Gutiérrez, S., Grau, I., Eds.; Publicaciones de la Universidad de Alicante: Alicante, Spain, 2013; pp. 127–140. [Google Scholar]
- Hartnett, J. The Roman Street: Urban Life and Society in Pompeii, Herculaneum, and Rome; Cambridge University Press: New York, NY, USA, 2017. [Google Scholar]
- Matz, D. Daily Life of the Ancient Romans; Greenwood: London, UK, 2002. [Google Scholar]
- Hanson, J.W.; Ortman, S.G. A systematic method for estimating the populations of Greek and Roman settlements. J. Rom. Archaeol. 2017, 30, 301–324. [Google Scholar] [CrossRef]
- Clarke, W. Pompeii; Charles Knight: London, UK, 1836; Volume II. [Google Scholar]
- Nissin, L. Beds. In The Encyclopedia of Ancient History; Wiley Online Library: New York, NY, USA, 2017; pp. 1–2. [Google Scholar] [CrossRef]
- Kline, A.S. Virgil. Georgics: Book III. Livestock Farming. Available online: https://www.poetryintranslation.com/PITBR/Latin/VirgilGeorgicsIII.php (accessed on 7 February 2021).
- Flintoff, E. The Noric cattle plague. Quad. Urbinati Cult. Class. 1983, 13, 85–111. [Google Scholar] [CrossRef]
- Hull, T.G. Disease Transmitted from Animals to Man, 5th ed.; Charles, C., Ed.; Thomas: Springfield, IL, USA, 1963; pp. 82–125. [Google Scholar]
- Cucchi, T.; Vigne, J.D.; Auffray, J.C. First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the Western Mediterranean: A zooarchaeological revision of subfossil occurrences. Biol. J. Linn. Soc. 2005, 84, 429–444. [Google Scholar] [CrossRef]
- Vantassel, S.M.; Hyngstrom, S.E.; Ferraro, D.M. Controlling House Mice; NebGuide, University of Nebraska-Lincoln Extension, Institute of Agriculture and Natural Resources: Lincoln, NE, USA, 2012. [Google Scholar]
- Dahmana, H.; Granjon, L.; Diagne, C.; Davoust, B.; Fenollar, F.; Mediannikov, O. Rodents as hosts of pathogens and related zoonotic disease risk. Pathogens 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Himsworth, C.G.; Parsons, K.L.; Jardine, C.; Patrick, D.M. Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic. Dis. 2013, 13, 349–359. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.H.; Abdelgadir, M.; AlRashidi, M. Ectoparasites burden of House mouse (Mus musculus linnaeus, 1758) from Hai’l region, Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 2238–2244. [Google Scholar] [CrossRef]
- Holt, E.; Palazzo, S. The role of rodents in the disease ecology of the Roman city. Archaeol. Rev. Camb. 2013, 28, 132–154. [Google Scholar]
- Fulford, M.; Wallace-Hadrill, A. Towards a history of pre-Roman Pompeii: Excavations beneath the House of Amarantus (I.9.11–12), 1995–1998. Pap. Br. Sch. Rome 1999, 67, 37–144. [Google Scholar] [CrossRef]
- McCormick, M. Rats, communications, and plague: Toward an ecological history. J. Interdiscip. Hist. 2003, 34, 1–25. [Google Scholar] [CrossRef]
- Panti-May, J.A.; De Andrade, R.R.C.; Gurubel-González, Y.; Palomo-Arjona, E.; Sodá-Tamayo, L.; Meza-Sulú, J.; Ramírez-Sierra, M.; Dumonteil, E.; Vidal-Martínez, V.M.; Machaín-Williams, C. A survey of zoonotic pathogens carried by house mouse and black rat populations in Yucatan, Mexico. Epidemiol. Infect. 2017, 145, 2287–2295. [Google Scholar] [CrossRef]
- Ervynck, A. Sedentism or Urbanism? On the Origin of the Commensal Black Rat (Rattus Rattus). In Bones and the Man: Studies in Honour of Don Brothwell; Dobney, K., O’Connor, T., Eds.; Oxbow Books: Oxford, UK, 2002; pp. 95–109. [Google Scholar]
- Von den Driesch, A.; Boessneck, J. A Roman cat skeleton from Quseir on the Red Sea Coast. J. Archaeol. Sci. 1983, 10, 205–211. [Google Scholar] [CrossRef]
- Ruffino, L.; Vidal, E. Early colonization of Mediterranean islands by Rattus rattus: A review of zooarchaeological data. Biol. Invasions 2010, 12, 2389–2394. [Google Scholar] [CrossRef]
- Toškan, B.; Kryštufek, B. Noteworthy rodent records from the Upper Pleistocene and Holocene of Slovenia. Mammalia 2006, 70, 98–105. [Google Scholar] [CrossRef]
- Armitage, P.; West, B.; Steedman, K. New evidence of black rat in Roman London. Lond. Archaeol. 1984, 4, 375–383. [Google Scholar]
- Audoin-Rouzeau, F.; Vigne, J.D. La colonisation de l’Europe par le rat noir (Rattus rattus). Rev. Paleobiol. 1994, 13, 125–145. [Google Scholar]
- Kovács, Z.E. Dispersal history of an invasive rodent in Hungary—Subfossil finds of Rattus rattus. Acta Zool. Acad. Sci. Hung. 2012, 58, 379–394. [Google Scholar]
- Rackham, J. Rattus rattus: The introduction of the black rat into Britain. Antiquity 1979, 53, 112–120. [Google Scholar] [CrossRef]
- Arthur, P. Le Città Vesuviane: Problemi e Prospettive Nello Studio Dell’Ecologia Umana Nell’Antichità. In Ercolano 1738–1988: 250 Anni di Ricerca Archeologica, Atti del Convegno Internazionale Ravello-Ercolano-Napoli-Pompei, 30 Ottobre–5 Novembre 1988; Dell’Orto, L.F., Ed.; “L’Erma” di Bretschneider: Rome, Italy, 1993; pp. 193–199. [Google Scholar]
- Hirst, L.F. The Conquest of Plague: A Study of the Evolution of Epidemiology; Oxford University Press: Oxford, UK, 1953. [Google Scholar]
- MacKinnon, M. Pack Animals, Pets, Pests, and Other Non-Human Beings. In The Cambridge Companion to Ancient Rome; Erdkamp, P., Ed.; Cambridge University Press: Cambridge, UK, 2013; pp. 110–128. [Google Scholar] [CrossRef]
- Franco, C. Animals. In The World through Roman Eyes: Anthropological Approaches to Ancient Cultures; Bettini, M., Short, W.M., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 275–298. [Google Scholar]
- Lo Giudice, C. L’impiego degli animali negli spettacoli romani: Venatio e damnatio ad bestias. Italies 2008, 12, 361–395. [Google Scholar] [CrossRef]
- Toynbee, J.M.C. Animals in Roman Life and Art; Cornell University Press: New York, NY, USA, 1973. [Google Scholar]
- Beltran-Rizo, E. Gloria et favor populi: Los ludi venatorii en las editiones de Q. Fabio Memio Símaco. Ludica 2003, 9, 55–75. [Google Scholar]
- Malossini, F. Gli allevamenti animali nel fondo rustico dell’antica Roma. Atti Dell’accademia Roveretana Degli Agiati Ser. IX 2011, I, 145–215. [Google Scholar]
- MacKinnon, M. Cattle ‘breed’ variation and improvement in Roman Italy: Connecting the zooarchaeological and ancient textual evidence. World Archaeol. 2010, 42, 55–73. [Google Scholar] [CrossRef]
- King, A. Diet in the Roman world: A regional inter-site comparison of the mammal bones. J. Rom. Archaeol. 1999, 12, 168–202. [Google Scholar] [CrossRef]
- MacKinnon, M. High on the hog: Linking zooarchaeological, literary, and artistic data for pig breeds in Roman Italy. Am. J. Archaeol. 2001, 105, 649–673. [Google Scholar] [CrossRef]
- Chuang, R. The Acquisition of Domestic Equids in Roman Britain: The Identification of Domestic Equids and Case Study with Isotopic Analyses. Ph.D. Dissertation, University of Southampton, Southampton, UK, 2016. [Google Scholar]
- Genovese, A.; Cocca, T. Internal organization of an equine stable at Pompei. Anthropozoologica 2000, 31, 119–123. [Google Scholar]
- National Geographic. Horses Found in Pompeii May Have Been Harnessed to Flee Eruption. Available online: https://www.nationalgeographic.com/science/article/pompeii-horse-civita-giuliana-archaeology-science (accessed on 20 November 2021).
- Cipollaro, M. Strengthening ancient mtDNA equid sequences from Pompeii. J. Cell. Biochem. 2011, 112, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Cipollaro, M.; Di Bernardo, G. DNA from equine remains buried by the 79 A.D. Vesuvius eruption. Rend. Lincei Sci. Fis. Nat. 2004, 15, 151–157. [Google Scholar] [CrossRef]
- Di Bernardo, G.; Galderisi, U.; Del Gaudio, S.; D’Aniello, A.; Lanave, C.; De Robertis, M.T.; Cascino, A.; Cipollaro, M. Genetic characterization of Pompeii and Herculaneum Equidae buried by Vesuvius in 79 AD. J. Cell. Physiol. 2004, 199, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Gurney, S.M.R. Revisiting ancient mtDNA Equid sequences. J. Cell. Biochem. 2010, 111, 1080–1081. [Google Scholar] [CrossRef]
- MacKinnon, M. Meat and Other Animal Products. In The Routledge Handbook of Diet and Nutrition in the Roman World; Erdkamp, P., Holleran, C., Eds.; Routledge: New York, NY, USA, 2019; pp. 150–162. [Google Scholar]
- Manca, P.; Farina, V.; Gadau, S.; Lepore, G.; Genovese, A.; Zedda, M. Phenotypic features of the domestic pigs bred in the Roman settlements of Pompeii and Caralis. Ital. J. Anat. Embryol. 2004, 109, 105–114. [Google Scholar]
- García, L.G. Danni di Guerra a Pompei: Una Dolorosa Vicenda Quasi Dimenticata; “L’Erma” di Bretschrneider: Rome, Italy, 2006. [Google Scholar]
- Bochain, H.L. The Economy of Beekeeping: Examining an Overlooked Industry of the Ancient World. Master’s Thesis, University of Georgia, Athens, Greece, 2017. [Google Scholar]
- Crane, E. Beekeeping in the world of ancient Rome. Bee World 1994, 75, 118–134. [Google Scholar] [CrossRef]
- Kron, G. Reconstructing the Techniques and Potential Productivity of Roman Aquaculture in the Light of Recent Research and Practice. In Atlante Tematico di Topografia Antica; Suppl. 16; Quilici, L., Quilici Gigli, S., Eds.; “L’Erma” di Bretschneider: Rome, Italy, 2008; pp. 175–185. [Google Scholar]
- Lafon, X. Villa Maritima. In Recherches sur les Villas Littorales de L’italie Romaine; École Française de Rome: Rome, Italy, 2001. [Google Scholar]
- Kron, G. Ancient Fishing and Fish Farming. In Oxford Handbook of Animals in Classical Thought and Life; Campbell, G.L., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 267–280. [Google Scholar]
- Colonelli, G. Uso alimentare dei ghiri (Famiglia Myoxidae) nella storia antica e contemporanea. Antrocom 2007, 3, 69–76. [Google Scholar]
- Beerden, K. A conspicuous meal: Fattening dormice, snails and thrushes in the Roman world. Petit Propos. Culin. 2010, 90, 79–98. [Google Scholar]
- Beerden, K. Roman dolia and the fattening of dormice. Class. World 2012, 105, 227–235. [Google Scholar] [CrossRef]
- MacKinnon, M. Fauna of the Ancient Mediterranean World. In Oxford Handbook of Animals in Classical Thought and Life; Campbell, G.L., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 156–179. [Google Scholar]
- Lazenby, F.D. Greek and Roman household pets. Class. J. 1949, 44, 245–252. [Google Scholar]
- Zedda, M.; Manca, P.; Chisu, V.; Gadau, S.; Lepore, G.; Genovese, A.; Farina, V. Ancient Pompeian dogs—Morphological and morphometric evidence for different canine populations. Anat. Histol. Embryol. 2006, 35, 319–324. [Google Scholar] [CrossRef]
- MacKinnon, M. ‘Sick as a dog’: Zooarchaeological evidence for pet dog health and welfare in the Roman world. World Archaeol. 2010, 42, 290–309. [Google Scholar] [CrossRef]
- de Sandes-Moyer, K. The Dog in Roman Peasant Life. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2013. [Google Scholar]
- Akhand, A.J. The Roman Dogma of Animal Breeding: “Bark” Aeological Findings Reveal the Effects of Selective Pressures on Roman Dogs. Bachelor’s Thesis, University of Mary Washington, Fredericksburg, VA, USA, 2020. [Google Scholar]
- Starnone, V. Feritas in urbe: Marziale di fronte all’irriducibile potenza della natura. Maia 2015, 67, 99–123. [Google Scholar]
- Pearse, R. Martial, Epigrams. Book 1. Bohn’s Classical Library. 1897. Available online: http://www.tertullian.org/fathers/martial_epigrams_book01.htm (accessed on 19 April 2021).
- Lentacker, A.; De Cupere, B. Domestication of the cat and reflections on the scarcity of finds in archaeological contexts. Colloq. D’histoire Connaiss. Zool. 1994, 5, 69–78. [Google Scholar]
- Faure, E.; Kitchener, A.C. An archaeological and historical review of the relationships between felids and people. Anthrozoös 2009, 22, 221–238. [Google Scholar] [CrossRef]
- Jashemski, W.F. Evidence of Flora and Fauna in the Gardens and Cultivated Land Destroyed by Vesuvius in A.D. 79. In Volcanism and Fossil Biotas; Lockley, M.G., Rice, A., Eds.; Geological Society of America: Boulder, CO, USA, 1990; pp. 113–125. [Google Scholar]
- Kennedy, A. Leaving Rome: Alienation from and Attachment to the City in Augustan Literature. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2013. [Google Scholar]
- Stenseth, N.C.; Leirs, H.; Skonhoft, A.; Davis, S.A.; Pech, R.P.; Andreassen, H.P.; Singleton, G.R.; Lima, M.; Machang’u, R.S.; Makundi, R.H.; et al. Mice, rats, and people: The bio-economics of agricultural rodent pests. Front. Ecol. Environ. 2003, 1, 367–375. [Google Scholar] [CrossRef]
- Gubler, D.J.; Reiter, P.; Ebi, K.L.; Yap, W.; Nasci, R.; Pat, J.A. Climate variability and change in the United States: Potential impacts on vector-and rodent-borne diseases. Environ. Health Perspect. 2001, 109, 223–233. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- Bauerfeind, R.; Von Graevenitz, A.; Kimmig, P.; Schiefer, H.G.; Schwarz, T.; Slenczka, W.; Zahner, H. Zoonoses: Infectious Diseases Transmissible between Animals and Humans; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Conover, M.R.; Vail, R.M. Human Diseases from Wildlife; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bradley, P. Cities of Vesuvius: Pompeii and Herculaneum, 2nd ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Cook, B.; Ferrant, J. Occupational Zoonoses. OSHWiki. Available online: http://oshwiki.eu/index.php?title=Occupational_zoonoses&oldid=247270 (accessed on 23 July 2021).
- Batelli, G. Zoonoses as occupational diseases. Vet. Ital. 2008, 44, 601–609. [Google Scholar]
- Erdkamp, P. The Grain Market in the Roman Empire: A Social, Political and Economic Study; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Machline, V.C.; Machline, C. Urban sanitation and the use of human waste as manure in ancient Rome. In Proceedings of the 4th International Conference on Industrial and Hazardous Waste Management, Chania, Greece, 2–5 September 2014. [Google Scholar]
- Taylor, C. The disposal of human waste: A comparison between ancient Rome and medieval London. Past Imperfect 2005, 11, 53–72. [Google Scholar] [CrossRef]
- Viskari, E.L.; Grobler, G.; Karimäki, K.; Gorbatova, A.; Vilpas, R.; Lehtoranta, S. Nitrogen recovery with source separation of human urine—Preliminary results of its fertiliser potential and use in agriculture. Front. Sustain. Food Syst. 2018, 2, 32. [Google Scholar] [CrossRef]
- Anonymous. Of Husbandry, in Twelve Books: And His Book Concerning Trees; Translated into English, with illustrations from Pliny, Cato, Varro, Palladius, and other ancient and modern authors; A. Millar: London, UK, 1745. [Google Scholar]
- Semple, E.C. Ancient Mediterranean agriculture: Part II. Manuring and seed selection. Agric. Hist. 1928, 2, 129–156. [Google Scholar]
- Koloski-Ostrow, A.O. Pinpointing Behaviors, Attitudes, and Ideals for Roman Toilets. In The Archaeology of Sanitation in Roman Italy: Toilets, Sewers, and Water Systems; Koloski-Ostrow, A.O., Ed.; University of North Caroline Press: Chapel Hill, NC, USA, 2015; pp. 84–101. [Google Scholar]
- Farmer, V.A. Roman Farm Management: The Treatises of Cato and Varro Done to English, with Notes of Modern Instances; Macmillan: New York, NY, USA, 1918. [Google Scholar]
- Mitchell, P.D. Human Parasites in Medieval Europe: Lifestyle, Sanitation and Medical Treatment. In Advances in Parasitology; De Baets, K., Littlewood, D.T., Eds.; Academic Press: London, UK, 2015; pp. 389–420. [Google Scholar] [CrossRef]
- Williams, F.S.; Arnold-Foster, T.; Yeh, H.Y.; Ledger, M.L.; Baeten, J.; Poblome, J.; Mitchell, P.D. Intestinal parasites from the 2nd–5th century AD latrine in the Roman Baths at Sagalassos (Turkey). Int. J. Paleopathol. 2017, 19, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lorén, L.L. Zoonosis laborales: Riesgos de exposición a agentes biológicos en ganadería. Segur. Salud Trab. 2009, 55, 42–47. [Google Scholar]
- Curtis, R.I. Archaeological evidence for economic life at Pompeii: A survey. Class. Outlook 1980, 57, 98–102. [Google Scholar]
- Borgard, P.; Brun, J.P.; Leguilloux, M.; Tuffreau-Libre, M. Le produzioni artigianali a Pompei: Ricerche condotte dal Centre Jean Bérard. Riv. Studi Pompeiani 2003, 14, 9–29. [Google Scholar]
- Mau, A. Pompeii: Its Life and Art; MacMillan & Co.: New York, NY, USA, 1902. [Google Scholar]
- Febriana, S.A.; Jungbauer, F.; Soebono, H.; Coeenrads, P.J. Occupational allergic contact dermatitis and patch test results of leather workers at two Indonesian tanneries. Contact Dermat. 2012, 67, 277–283. [Google Scholar] [CrossRef]
- Oruko, R.O.; Odiyo, J.O.; Edokpayi, J.N. The Role of Leather Microbes in Human Health. In Role of Microbes in Human Health and Diseases; Chauhan, N.S., Ed.; Open Access Peer-Reviewed Chapter; IntechOpen: Rijeka, Croatia, 2019; pp. 243–371. [Google Scholar] [CrossRef]
- Pirson, F. Shops and Industries. In The World of Pompeii; Dobbins, J.J., Foss, P.W., Eds.; Routledge: New York, NY, USA, 2007; pp. 467–473. [Google Scholar]
- Bradley, M. ‘It all comes out in the wash’: Looking harder at the Roman fullonica. J. Rom. Archaeol. 2002, 15, 20–44. [Google Scholar] [CrossRef]
- Witty, M. Ancient Roman urine chemistry. Acta Archaeol. 2016, 87, 179–191. [Google Scholar] [CrossRef]
- Katz, J.T. Egnatius’ dental fricatives (Catullus 39.20). Class. Philol. 2000, 95, 338–348. [Google Scholar] [CrossRef]
- Grant, M. Cities of Vesuvius: Pompeii & Herculaneum; Penguin Books: Harmondsworth, UK, 1976. [Google Scholar]
- Flohr, M. Beyond Smell: The Sensory Landscape of the Roman Fullonica. In Senses of the Empire: Multisensory Approaches to Roman Culture; Betts, E., Ed.; Routledge: New York, NY, USA, 2017; pp. 39–53. [Google Scholar]
- Brown, M. Grain, pulses and olives: An attempt toward a quantitative approach to diet in ancient Rome. J. Wash. Acad. Sci. 2011, 97, 1–24. [Google Scholar]
- Heinrich, F. Cereals and Bread. In The Routledge Handbook of Diet and Nutrition in the Roman World; Erdkamp, P., Holleran, C., Eds.; Routledge: London, UK, 2019; pp. 101–115. [Google Scholar]
- Heinrich, F.; Hansen, A.M. Pulses. In The Routledge Handbook of Diet and Nutrition in the Roman World; Erdkamp, P., Holleran, C., Eds.; Routledge: London, UK, 2019; pp. 116–128. [Google Scholar]
- Purcell, N. The way we used to eat: Diet, community, and history at Rome. Am. J. Philol. 2003, 124, 329–358. [Google Scholar] [CrossRef]
- Garnsey, P. Famine and Food Supply in the Graeco-Roman World: Responses to Risk and Crisis; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Holleran, C. Market Regulation and Intervention in the Urban Food Supply. In The Routledge Handbook of Diet and Nutrition in the Roman World; Erdkamp, P., Holleran, C., Eds.; Routledge: London, UK, 2019; pp. 283–295. [Google Scholar]
- Rickman, G. Roman Granaries and Store Buildings; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Cheung, C. Managing food storage in the Roman Empire. Quat. Int. 2021, 597, 63–75. [Google Scholar] [CrossRef]
- Lieber, E. Galen on contaminated cereals as a cause of epidemics. Bull. Hist. Med. 1970, 44, 332–345. [Google Scholar]
- Smith, D.; Kenward, H. ‘Well, Sextus what can we do with this?’ The disposal and use of insect-infested grain in Roman Britain. Environ. Archaeol. 2012, 17, 141–150. [Google Scholar] [CrossRef]
- Pals, J.P.; Hakbijl, T. Weed and insect infestation of a grain cargo in a ship at the Roman fort of Laurium in Woerden (Province of Zuid-Holland). Rev. Palaeobot. Palynol. 1992, 73, 287–300. [Google Scholar] [CrossRef]
- Ellis, S.J.R. The distribution of bars at Pompeii: Archaeological, spatial and viewshed analyses. J. Rom. Archaeol. 2004, 17, 371–384. [Google Scholar] [CrossRef]
- Ellis, S.J.R. The Pompeian Bar and the City: Defining Food and Drink Outlets and Identifying Their Place in an Urban Environment. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2005. [Google Scholar]
- Mac Mahon, A. The Taberna Counters of Pompeii and Herculaneum. In Roman Working Lives and Urban Living; Mac Mahon, A., Price, J., Eds.; Oxbow: Oxford, UK, 2005; pp. 70–87. [Google Scholar]
- Murphy, C.; Thompson, G.; Fuller, D.Q. Roman food refuse: Urban archaeobotany in Pompeii, Regio VI, Insula 1. Veg. Hist. Archaeobotany 2013, 22, 409–419. [Google Scholar] [CrossRef]
- Lloris, M.B. El pistrinum de la Colonia Victrix Iulia Lepida Celsa (Velilla de Ebro, Zaragoza). In Proceedings of the III Congreso de Arqueología y Patrimonio Aragonés CAPA, Zaragoza, Spain, 14–15 November 2019; pp. 195–206. [Google Scholar]
- Morgan, H. Bakers and the Baking Trade in the Roman Empire: Social and Political Responses from the Principate to Late Antiquity. Master’s Thesis, University of Cambridge, Cambridge, UK, 2015. [Google Scholar]
- Foss, P.W. Kitchens and Dining Rooms at Pompeii: The Spatial and Social Relationship of Cooking to Eating in the Roman Household. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 1994. [Google Scholar]
- Pagano, M. Commercio e Consumo del Grano ad Ercolano. In Le Ravitaillement en blé de Rome et des Centres Urbains des Débuts de la République Jusqu’au Haut Empire, Actes du Colloque International de Naples, 14–16 February 1991; École Française de Rome: Rome, Italy, 1994; pp. 141–147. [Google Scholar]
- Kent, R.G. The Edict of Diocletian fixing maximum prices. Univ. Pa. Law Rev. 1920, 69, 35–47. [Google Scholar] [CrossRef]
- Michell, H. The Edict of Diocletian: A study of price fixing in the Roman Empire. Can. J. Econ. Political Sci. 1947, 13, 1–12. [Google Scholar] [CrossRef]
- Paoli, U. Vita Romana: Usi, Costumi, Istituzioni, Tradizioni; Mondadori: Milano, Italy, 1990. [Google Scholar]
- Rowan, E. Bioarchaeological preservation and non-elite diet in the Bay of Naples: An analysis of the food remains from the Cardo V sewer at the Roman site of Herculaneum. Environ. Archaeol. 2016, 22, 318–336. [Google Scholar] [CrossRef]
- Ledger, M.L.; Rowan, E.; Marques, F.G.; Sigmier, J.H.; Sarkic, N.; Redzic, S.; Cahill, N.D.; Mitchell, P.D. Intestinal parasitic infection in the Eastern Roman Empire during the Imperial period and late antiquity. Am. J. Archaeol. 2020, 124, 631–657. [Google Scholar] [CrossRef]
- Jori, A. La Cultura Alimentare e L’arte Gastronomica dei Romani: Contributo alla Filosofia Dell’alimentazione e alla Storia Culturale del Mondo Mediterraneo; Accademia Nazionale Virgiliana di Scienze Lettere e Arti (Quaderni dell’Accademia 5): Mantova, Italy, 2016. [Google Scholar]
- Ellis, S.J.R. The rise and re-organization of the Pompeian salted fish industry. J. Rom. Archaeol. 2011, 85, 59–88. [Google Scholar]
- Curtis, R.I. Umami and the foods of classical antiquity. Am. J. Clin. Nutr. 2009, 90, 712–718. [Google Scholar] [CrossRef]
- Carannante, A. L’ultimo garum di Pompei. Analisi archeozoologiche sui resti di pesce dalla cosiddetta “Officina del garum”. Automata III–IV 2010, 1, 43–53. [Google Scholar] [CrossRef]
- Carannante, A. The last garum of Pompeii: Archaeozoological analyses on fish remains from the “Garum shop” and related ecological inferences. Int. J. Osteoarchaeol. 2019, 29, 377–386. [Google Scholar] [CrossRef]
- Rodríguez-Alcántara, A.; Roldán-Gómez, A.M.; Bernal-Casasola, D.; García-Vargas, E.; Palacios Macías, V.M. New technological contributions to roman garum elaboration from chemical analysis of archaeological fish remains from the ‘garum shop’ at Pompeii (I.12.8). Zephyrus 2018, 82, 149–163. [Google Scholar] [CrossRef]
- Romano, L.A.; Mejía, J. Infección por Streptococcus iniae: Una enfermedad emergente que afecta a peces de cultivo y a humanos. Rev. AquaTIC 2003, 18, 25–32. [Google Scholar]
- Boylan, S. Zoonoses associated with fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 427–438. [Google Scholar] [CrossRef]
- Gauthier, D.T. Bacterial zoonoses of fishes: A review and appraisal of evidence for linkages between fish and human infections. Vet. J. 2015, 203, 27–35. [Google Scholar] [CrossRef]
- Pappas, G.; Kiriaze, I.J.; Falagas, M.E. Insights into infectious disease in the era of Hippocrates. Int. J. Infect. Dis. 2008, 12, 347–350. [Google Scholar] [CrossRef]
- Capasso, L.; Di Tota, G. Tuberculosis in Herculaneum. In Tuberculosis: Past and Present; Palfi, G., Dutour, O., Deàks, I., Eds.; Golden Book Publishers and Tuberculosis Foundation: Budapest, Hungary; Szeged, Hungary, 1998; pp. 463–470. [Google Scholar]
- Alcock, J.P. Food in the Ancient World; Greenwood Press: Westport, CT, USA, 2006. [Google Scholar]
- Faas, P. Around the Roman Table: Food and Feasting in Ancient Rome; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Alcock, J.P. Milk and Its Products in Ancient Rome. In Milk: Beyond the Dairy, Proceedings of the Oxford Symposium on Food and Cookery, Oxford Symposium; Prospect Books: Devon, UK, 2000; pp. 31–38. [Google Scholar]
- Capasso, L. Brucellosis at Herculaneum. Int. J. Osteoarchaeol. 1999, 9, 277–288. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of egg contamination by Salmonella enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef]
- Bienert, B. A Roman ‘Toilet bowl’ from Speicher (Eifelkreis Bitburg-Prüm, Rhineland-Palatinate, Germany). In Latrinae: Roman Toilets in the Northwestern Provinces of the Roman Empire; Hoss, S., Ed.; Archaeopress: Oxford, UK, 2018; pp. 137–142. [Google Scholar]
- Peña, J.T. The Reuse of the Other Functional Categories of Pottery. In Roman Pottery in the Archaeological Record; Peña, J.T., Ed.; Cambridge University Press: New York, NY, USA, 2007; pp. 193–208. [Google Scholar]
- Havlíček, F.; Morcinek, M. Waste and pollution in the ancient Roman Empire. J. Landsc. Ecol. 2016, 9, 33–49. [Google Scholar] [CrossRef]
- Falcone, L.; Bloisi, F.; Califano, V.; Pagano, M.; Vicari, L. An old notice board at ancient Herculaneum studied using Near Infrared Reflectography. J. Archaeol. Sci. 2008, 35, 1708–1716. [Google Scholar] [CrossRef]
- Camardo, D.; Notomista, M.; Sirano, F. Herculaneum’s Roman toilets: The past and present of an ancient sewer system. Curr. World Archaeol. 2019, 96, 22–28. [Google Scholar]
- Ferrari, I. La Latrina Occidentale Delle Terme Centrali di Aquinum. In Atlante Tematico di Topografia Antica: Urbanistica e Monumenti, Strade, Insediamenti e Territorio; n. 29; Quilici, L., Quilici Gigli, S., Eds.; “L’Erma” di Bretschneider: Rome, Italy, 2019; pp. 113–132. [Google Scholar] [CrossRef]
- Jansen, G. Private Toilets at Pompeii: Appearance and Operation. In Sequence and Space in Pompeii; Bon, S.E., Jones, R., Eds.; Oxbow Books: Oxford, UK, 1997; pp. 121–134. [Google Scholar]
- Wiplinger, G. Der Gebrauch Des Xylospongiums: Eine Neue Theorie zu den hygienischen Verhältnissen in Römischen Latrinen. In Spa: Sanitas per Aquam, Proceedings of the International Frontinus-Symposium on the Technical and Cultural History of Ancient Baths, Aachen, Germany, 18–22 March 2009; Kreiner, R., Letzner, W., Eds.; Peeters: Leuven, Belgium, 2012; pp. 295–304. [Google Scholar]
- Cotruvo, J.A.; Dufour, A.; Rees, G.; Bartram, J.; Carr, R.; Cliver, D.O.; Craun, G.F.; Fayer, R.; Gannon, V.P.J. Waterborne Zoonoses: Identification, Causes, and Control; World Health Organization & IWA Publishing: London, UK, 2004. [Google Scholar]
- Heirbaut, E.; Jones, A.K.G.; Wheeler, K. Archaeometry: Methods and Analysis. In Roman Toilets: Their Archaeology and Cultural History; Jansen, G.C.M., Koloski-Ostrow, A.O., Moormann, E.M., Eds.; Peeters: Leuven, Belgium, 2011; pp. 7–20. [Google Scholar]
- Ledger, M.L.; Micarelli, I.; Ward, D.; Prowse, T.L.; Carroll, M.; Killgrove, K.; Rice, C.; Franconi, T.; Tafuri, M.A.; Manzi, G.; et al. Gastrointestinal infection in Italy during the Roman Imperial and Longobard periods: A paleoparasitological analysis of sediment from skeletal remains and sewer drains. Int. J. Paleopathol. 2021, 33, 61–71. [Google Scholar] [CrossRef]
- Ekroth, G. Animal Sacrifice in Antiquity. In The Oxford Handbook of Animals in Classical Thought and Life; Campbell, G.L., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 324–354. [Google Scholar]
- Ciaraldi, M.; Richardson, J. Food, ritual and rubbish in the making on Pompeii. Theor. Rom. Archaeol. J. 2000, 1999, 74–82. [Google Scholar] [CrossRef]
- Lepetz, S.; Van Andringa, W. Archéologie du Sacrifice Animal en Gaule Romaine: Rituels et Pratiques Alimentaires; Éditions Monique Mergoil: Montagnac, France, 2008. [Google Scholar]
- Robinson, M. Domestic burnt offerings and sacrifices at Roman and pre-Roman Pompeii, Italy. Veget. Hist. Archaeobot. 2002, 11, 93–99. [Google Scholar] [CrossRef]
- Van Andringa, W.; Lepetz, S. Le Ossa Animali nei Santuari: Per un’Archeologia delSacrificio. In Sanctuaires et Sources: Les Sources Documentaires et Leurs Limites Dans la Description des Lieux de Culte, (Collection du Centre Jean Bérard, 22); Cazanove, O., Scheid, J., Eds.; Centre Jean Bérard and Collège de France: Naples, Italy, 2003; pp. 85–96. [Google Scholar]
- Van Andringa, W. Du sanctuaire au macellum: Sacrifices, commerce et consommation de la viande à Pompéi. Food Hist. 2007, 5, 47–72. [Google Scholar] [CrossRef]
- Scheid, J. Le statut de la viande à Rome. Food Hist. 2007, 5, 19–28. [Google Scholar] [CrossRef]
- Belayche, N. Religion et consommation de la viande dans le monde romain: Des réalités voilées. Food Hist. 2007, 5, 29–43. [Google Scholar] [CrossRef]
- De Ruyt, C. Les produits vendus au macellum. Food Hist. 2007, 5, 135–150. [Google Scholar] [CrossRef]
- Ball, L.F.; Dobbins, J.J. Pompeii Forum Project: Current thinking on the Pompeii Forum. Am. J. Archaeol. 2013, 117, 461–492. [Google Scholar] [CrossRef]



| Site | Modern Country | Total Area (ha) | Population Density (People per ha) | Estimated Population |
|---|---|---|---|---|
| Roma | Italy | 1783 | 518 | 923,406 |
| Alexandria | Egypt | 972 | 422 | 410,535 |
| Antioch | Turkey | 399 | 313 | 124,936 |
| Carthage | Tunisia | 343 | 298 | 102,079 |
| Athens | Greece | 225 | 429 | 96,429 |
| Ephesus | Turkey | 263 | 272 | 71,587 |
| Lugdunum | France | 170 | 357 | 60,714 |
| Londinium | United Kingdom | 160 | 250 | 40,000 |
| Ostia | Italy | 154 | 227 | 35,017 |
| Neapolis | Italy | 82 | 275 | 22,550 |
| Verulamium | United Kingdom | 90 | 183 | 16,500 |
| Fregellae | Italy | 80 | 125 | 10,000 |
| Pompeii | Italy | 60 | 166 | 9938 |
| Emerita Augusta | Spain | 81 | 120 | 9720 |
| Volubilis | Morocco | 43 | 211 | 9058 |
| Calleva Atrebatum | United Kingdom | 45 | 80 | 3600 |
| Verona | Italy | 52 | 68 | 3525 |
| Augusta Praetoria | Italy | 41 | 83 | 3417 |
| Italica | Spain | 49 | 65 | 3178 |
| Iulia Valentia Banasa | Morocco | 15 | 183 | 2738 |
| Herculaneum | Italy | 20 | 115 | 2290 |
| Luna | Italy | 23 | 80 | 1840 |
| Conimbriga | Portugal | 23 | 66 | 1519 |
| Emporiae | Spain | 21 | 63 | 1313 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanga, C.; Remigio, M.; Viciano, J. Transmission of Zoonotic Diseases in the Daily Life of Ancient Pompeii and Herculaneum (79 CE, Italy): A Review of Animal–Human–Environment Interactions through Biological, Historical and Archaeological Sources. Animals 2022, 12, 213. https://doi.org/10.3390/ani12020213
Tanga C, Remigio M, Viciano J. Transmission of Zoonotic Diseases in the Daily Life of Ancient Pompeii and Herculaneum (79 CE, Italy): A Review of Animal–Human–Environment Interactions through Biological, Historical and Archaeological Sources. Animals. 2022; 12(2):213. https://doi.org/10.3390/ani12020213
Chicago/Turabian StyleTanga, Carmen, Marta Remigio, and Joan Viciano. 2022. "Transmission of Zoonotic Diseases in the Daily Life of Ancient Pompeii and Herculaneum (79 CE, Italy): A Review of Animal–Human–Environment Interactions through Biological, Historical and Archaeological Sources" Animals 12, no. 2: 213. https://doi.org/10.3390/ani12020213
APA StyleTanga, C., Remigio, M., & Viciano, J. (2022). Transmission of Zoonotic Diseases in the Daily Life of Ancient Pompeii and Herculaneum (79 CE, Italy): A Review of Animal–Human–Environment Interactions through Biological, Historical and Archaeological Sources. Animals, 12(2), 213. https://doi.org/10.3390/ani12020213






