Spawning Performance and Sex Steroid Levels in Female Pikeperch Sander lucioperca Treated with Poly(lactic-co-glycolic acid) Microparticles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Groups and Design
- 0.9% NaCl: saline solution only (Braun Melsungen AG, Melsungen, Germany), single injection of 0.9% NaCl at 1 mL/kg body weight (BW).
- mGnRHa: single injection of Supergestran® (Nordic Pharma, Jesenice, Czech Republic) at 20 µg/kg BW [mGnRHa (D-Tle6, Pro9, NEt-mGnRHa)].
- PLGA 5: 5-day release microparticles with encapsulated GnRHa ([D-Ala6, des-Gly10]GnRH-ethylamide) (APExBIO, Houston, TX, USA) at 5 µg/kg BW = GnRHa at 1 µg/kg BW per day.
- PLGA 20: 5-day release microparticles with encapsulated GnRHa ([D-Ala6, des-Gly10]GnRH-ethylamide) (APExBIO, Houston, TX, USA) at 20 µg/kg BW GnRHa = 4 µg/kg BW per day.
2.2. Microparticle Formation
2.3. Stripping of Broodfish
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Data Analysis
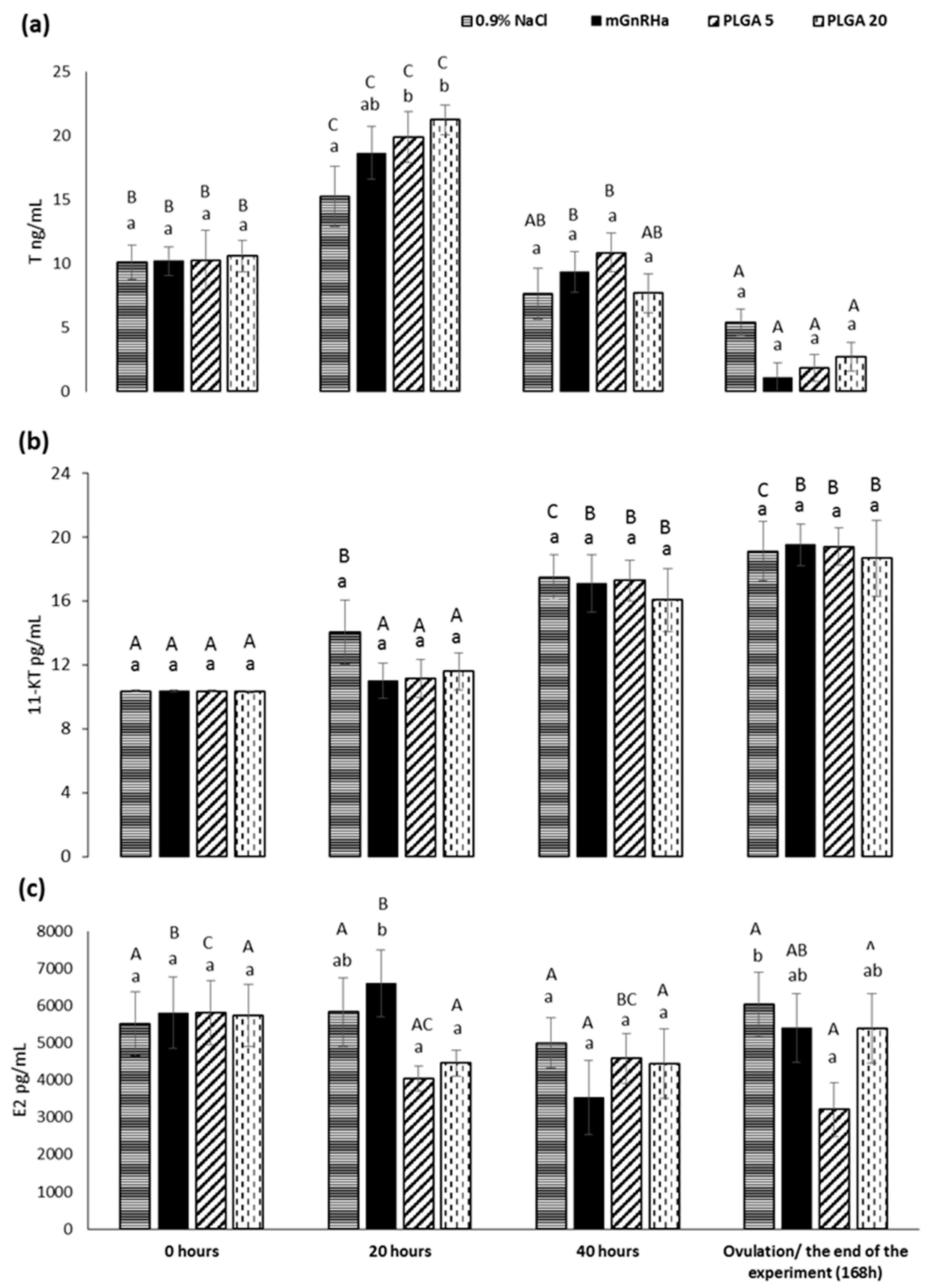
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Philipsen, A.; Van der Kraak, G. Excellence fish: Production of pikeperch in recirculating system. In Percid Fish Culture, from Research to Production, 1st ed.; Fontaine, P., Kestemont, P., Teletchea, F., Wang, N., Eds.; Presses Universitaires de Namur: Namur, Belgium, 2008; p. 67. [Google Scholar]
- Fontaine, P.; Wang, N.; Hermelink, B. Broodstock management and control of the reproductive cycle. In Biology and culture of percid fishes, 1st ed; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: New York, NY, USA, 2015; pp. 103–122. [Google Scholar]
- Sarameh, S.P.; Falahatkar, B.; Takami, G.A.; Efatpanah, I. Physiological changes in male and female pikeperch Sander lucioperca (Linnaeus, 1758) subjected to different photoperiods and handling stress during the reproductive season. Fish Physiol. Biochem. 2013, 39, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, S. Pikeperch (Lucioperca sandra Cuv.). Przegl ad Ryback 1928, 6, 175–188. [Google Scholar]
- Steffens, W.; Geldhauser, F.; Gerstner, P.; Hilge, V. German experiences in the propagation and rearing of fingerling pikeperch (Stizostedion lucioperca). Ann. Zool. Fennici. 1996, 33, 627–634. [Google Scholar]
- Malinovskyi, O.; Veselý, L.; Blecha, M.; Křišťan, J.; Policar, T. The substrate selection and spawning behaviour of pikeperch Sander lucioperca L. broodstock under pond conditions. Aquac. Res. 2018, 49, 3541–3547. [Google Scholar] [CrossRef]
- Blecha, M.; Samarin, A.; Křišťan, J.; Policar, T. Benefits of hormone treatment of both sexes in semi-artificial reproduction of pikeperch (Sander lucioperca L.). Czech J. Anim. Sci. 2016, 61, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Żarski, D.; Horváth, A.; Held, J.; Kucharczyk, D. Artificial reproduction of percid fishes. In Biology and culture of percid fishes, 1st ed; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer-Verlag GmbH: Heidelberg, Germany, 2015; pp. 123–161. [Google Scholar]
- Zakęś, Z.; Demska-Zakęś, K. Controlled reproduction of pikeperch Sander lucioperca (L.): A review. Arch. Pol. Fish. 2009, 17, 153–170. [Google Scholar] [CrossRef]
- Dadras, H.; Blecha, M.; Malinovskyi, O.; Flajšhans, M.; Lebeda, I.; Křišťan, J.; Policar, T. Triploidization in pikeperch (Sander lucioperca) induced by cold shock. Aquaculture 2021, 533, 736236. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Targońska, K.; Palińska, K.; Kupren, K.; Fontaine, P.; Kestemont, P. A new classification of pre-ovulatory oocyte maturation stages in pikeperch, Sander lucioperca (L.), and its application during artificial reproduction. Aquac. Res. 2012, 43, 713–721. [Google Scholar] [CrossRef]
- Zohar, Y.; Mylonas, C.C. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture 2001, 197, 99–136. [Google Scholar] [CrossRef]
- Falahatkar, B.; Poursaeid, S. Effects of hormonal manipulation on stress responses in male and female broodstocks of pikeperch Sander lucioperca. Aquac. Int. 2014, 22, 235–244. [Google Scholar] [CrossRef]
- Żarski, D.; Fontaine, P.; Roche, J.; Alix, M.; Blecha, M.; Broquard, C.; Król, J.; Milla, S. Time of response to hormonal treatment but not the type of a spawning agent affects the reproductive effectiveness in domesticated pikeperch, Sander lucioperca. Aquaculture 2019, 503, 527–536. [Google Scholar] [CrossRef]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 2001, 197, 3–24. [Google Scholar] [CrossRef]
- Matejkova, J.; Podhorec, P. Sustained drug delivery system in fish and the potential for use of PLGA microparticles: A review. Vet. Med. 2019, 64, 287–293. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Zohar, Y. Use of GnRHa-delivery systems for the control of reproduction in fish. Rev. Fish Biol. Fish. 2000, 10, 463–491. [Google Scholar] [CrossRef]
- Carolsfeld, J.; Sherwood, N.M.; Kreiberg, H.; Sower, S.A. Induced sexual maturation of herring using GnRH ‘quick-release’cholesterol pellets. Aquaculture 1988, 70, 169–181. [Google Scholar] [CrossRef]
- Arabacı, M.; Diler, I.; Sarı, M. Induction and synchronisation of ovulation in rainbow trout, Oncorhynchus mykiss, by administration of emulsified buserelin (GnRHa) and its effects on egg quality. Aquaculture 2004, 237, 475–484. [Google Scholar] [CrossRef]
- Mylonas, C.; Tabata, Y.; Langer, R.; Zohar, Y. Preparation and evaluation of polyanhydride microspheres containing gonadotropin-releasing hormone (GnRH), for inducing ovulation and spermiation in fish. J. Control. Release 1995, 35, 23–34. [Google Scholar] [CrossRef]
- Rather, M.A.; Sharma, R.; Gupta, S.; Ferosekhan, S.; Ramya, V.L.; Jadhao, S.B. Chitosan-Nanoconjugated Hormone Nanoparticles for Sustained Surge of Gonadotropins and Enhanced Reproductive Output in Female Fish. PLoS ONE 2013, 8, e57094. [Google Scholar] [CrossRef] [Green Version]
- Ruan, G.; Feng, S.-S. Preparation and characterization of poly(lactic acid)–poly(ethylene glycol)–poly(lactic acid) (PLA–PEG–PLA) microspheres for controlled release of paclitaxel. Biomaterials 2003, 24, 5037–5044. [Google Scholar] [CrossRef]
- Lewis, D.H. Controlled release of bioactive agents from lactide/glycolide polymers. J. Pharm. Sci. 1990, 45, 1–41. [Google Scholar]
- Rauta, P.R.; Nayak, B. Parenteral immunization of PLA/PLGA nanoparticle encapsulating outer membrane protein (Omp) from Aeromonas hydrophila: Evaluation of immunostimulatory action in Labeo rohita (rohu). Fish Shellfish. Immunol. 2015, 44, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.; St-Hilaire, S.; Zheng, Y.; Eley, J.; Marcum, R.; Sealey, W.; Donahower, B.C.; LaPatra, S.; Sheridan, P.P. Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA [Poly(D,L-Lactic-Co-Glycolic Acid)] nanoparticles. J. Fish Dis. 2012, 35, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Poly d,l-lactide-co-glycolic acid (PLGA)-encapsulated vaccine on immune system in Epinephelus bruneus against Uronema marinum. Exp. Parasitol. 2012, 131, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Křišt’an, J.; Alavi, S.M.H.; Stejskal, V.; Policar, T. Hormonal induction of ovulation in pikeperch (Sander lucioperca L.) using human chorionic gonadotropin (hCG) and mammalian GnRH analogue. Aquac. Int. 2013, 21, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, O.; Proteau, J.-P. Reproduction of pike-perch (Stizostedion lucioperca) in captivity. J. Appl. Ichthyol. 1996, 12, 149–152. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Microparticles used as drug delivery systems. Smart Colloid. Mater. 2006, 133, 15–21. [Google Scholar]
- Gothilf, Y.; Zohar, Y. Clearance of different forms of GnRH from the circulation of the gilthead seabream, Sparus aurata, in relation to their degradation and bioactivities. In Proceedings of the Fourth International Symposium on the Reproductive Physiology of Fish, Sheffield, UK, 7–12 July 1991; Scott, A.P., Kime, D.E., Rolfe, M.S., Eds.; pp. 35–37. [Google Scholar]
- Contreras-García, M.D.J.; Contreras-Sánchez, W.M.; Hernández-Vidal, U.; Mcdonal-Vera, A. Induced spawning of the common snook (Centropomus undecimalis) in captivity using GnRH-a implants. Ecosistemas y Recur. Agropecu. 2015, 2, 357–362. [Google Scholar]
- Ibarra-Castro, L.; Duncan, N.J. GnRHa-induced spawning of wild-caught spotted rose snapper Lutjanus guttatus. Aquaculture 2007, 272, 737–746. [Google Scholar] [CrossRef]
- Fakriadis, I.; Lisi, F.; Sigelaki, I.; Papadaki, M.; Mylonas, C.C. Spawning kinetics and egg/larval quality of greater amberjack (Seriola dumerili) in response to multiple GnRHa injections or implants. Gen. Comp. Endocrinol. 2019, 279, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.F.; Fordham, S.E.; Symonds, J.E.; Trippel, E.A.; Berlinsky, D.L. Hormonal induction of ovulation and spermiation in Atlantic cod (Gadus morhua). Aquaculture 2009, 296, 179–183. [Google Scholar] [CrossRef]
- Moon, S.; Lim, H.; Kwon, J.; Lee, J.; Chang, Y. Increased plasma 17-hydroxyprogesterone and milt production in response to gonadotropin-releasing hormone agonist in captive male starry flounder, Platichthys stellatus. Aquaculture 2003, 218, 703–716. [Google Scholar] [CrossRef]
- Schaerlinger, B.; Żarski, D. Evaluation and improvements of egg and larval quality in percid fishes. In Biology and Culture of Percid Fishes, 1st ed.; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, Netherlands, 2015; pp. 193–223. [Google Scholar]
- Rónyai, A. Induced out-of-season and seasonal tank spawning and stripping of pike perch (Sander lucioperca L.). Aquac. Res. 2007, 38, 1144–1151. [Google Scholar] [CrossRef]
- Zakęś, Z.; Demska-Zakęś, K. Artificial spawning of pikeperch (Sander lucioperca (L.)) stimulated with human chorionic gonadotropin (hCG) and mammalian GnRH analogue with a dopamine inhibitor. Fish. Aquat. Life 2005, 13, 63–75. [Google Scholar]
- Mylonas, C.C.; Hinshaw, J.M.; Sullivan, C.V. GnRHa-induced ovulation of brown trout (Salmo trutta) and its effects on egg quality. Aquaculture 1992, 106, 379–392. [Google Scholar] [CrossRef]
- Francescon, A.; Barbaro, A.; Bozzato, G.; Chiereghin, S.; Colombo, L.; Belvedere, P. Induction of multiple spawning in the gilthead seabream, Sparus aurata L., by LH-RH analogue treatments and their influence on egg quality. Riv. Ital. Acquacol. 1994, 29, 109–120. [Google Scholar]
- Poortenaar, C.W.; Pankhurst, N.W. Effect of Luteinising Hormone-Releasing Hormone Analogue and Human Chorionic Gonadotropin on Ovulation, Plasma and Ovarian Levels of Gonadal Steroids in Greenback Flounder Rhombosolea tapirina. J. World Aquac. Soc. 2000, 31, 175–185. [Google Scholar] [CrossRef]
- Glamuzina, B.; Skaramuca, B.; Glavić, N.; Kožul, V. Preliminary studies on reproduction and early life stages in rearing trials with dusky grouper, Epinephelus marginatus (Lowe, 1834). Aquac. Res. 1998, 29, 769–771. [Google Scholar] [CrossRef]
- Müller-Belecke, A.; Zienert, S. Out-of-season spawning of pike perch (Sander lucioperca L.) without the need for hormonal treatments. Aquac. Res. 2008, 39, 1279–1285. [Google Scholar] [CrossRef]
- Zohar, Y. Controlled release of gonadotropin releasing hormones for the manipulation of spawning in farmed fish. Control. Rel. Bioact. Mater. 1990, 17, 51–52. [Google Scholar]
- Mylonas, C.; Swanson, P.; Woods III, L.; Jonsson, E.; Jonasson, J.; Stefansson, S.; Zohar, Y. GnRHa-induced ovulation and sperm production in striped bass, Atlantic and Pacific salmon using controlled release devices. In Proceedings of the World Aquaculture Congress, Torremolinos, Spain, 3–7 June 1993; pp. 3–7. [Google Scholar]
- Kokokiris, L.; Canario, A.V.; Mylonas, C.C.; Pavlidis, M.; Kentouri, M.; Divanach, P. Induction of ovulation and spawning in the Mediterranean red porgy, Pagrus pagrus, by controlled delivery and acute injection of GnRHa. Isr. J. Aquac. 2005, 57, 223–230. [Google Scholar]
- Mugnier, C.; Guennoc, M.; Lebegue, E.; Fostier, A.; Breton, B. Induction and synchronisation of spawning in cultivated turbot (Scophthalmus maximus L.) broodstock by implantation of a sustained-release GnRH-a pellet. Aquaculture 2000, 181, 241–255. [Google Scholar] [CrossRef]
- Larsson, D.; Mylonas, C.; Zohar, Y.; Crim, L. Gonadotropin releasing hormone-analogue (GnRH-A) advances ovulation and improves the reproductive performance of a cold-water batch-spawning teleost, the yellowtail flounder (Pleuronectes ferrugineus). Can. J. Aquat. Fish. Sci. 1997, 54, 1957–1964. [Google Scholar] [CrossRef]
- Khendek, A.; Chakraborty, A.; Roche, J.; Ledoré, Y.; Personne, A.; Policar, T.; Żarski, D.; Mandiki, R.; Kestemont, P.; Milla, S.; et al. Rearing conditions and life history influence the progress of gametogenesis and reproduction performances in pikeperch males and females. Animal 2018, 12, 2335–2346. [Google Scholar] [CrossRef]
- Ishikawa, T.; Glidewell-Kenney, C.; Jameson, J.L. Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5α-reductase inhibitor. J. Steroid Biochem. Mol. Biol. 2006, 98, 133–138. [Google Scholar] [CrossRef]
- Sarameh, S.P.; Falahatkar, B.; Takami, G.A.; Efatpanah, I. Effects of different photoperiods and handling stress on spawning and reproductive performance of pikeperch Sander lucioperca. Anim. Reprod. Sci. 2012, 132, 213–222. [Google Scholar] [CrossRef]
- Linard, B.; Anglade, I.; Bennani, S.; Salbert, G.; Navas, J.M.; Bailhache, T.; Pakde, F.; Jego, P.; Valotaire, Y.; Saligaut, C.; et al. Some insights into sex steroid feedback mechanisms in the trout. In Reproductive Physiology of Fish; Goetz, F.W., Thomas, P., Eds.; Fish Symposium 95: Austin, TX, USA, 1995. [Google Scholar]
- Hermelink, B.; Wuertz, S.; Trubiroha, A.; Rennert, B.; Kloas, W.; Schulz, C. Influence of temperature on puberty and maturation of pikeperch, Sander lucioperca. Gen. Comp. Endocrinol. 2011, 172, 282–292. [Google Scholar] [CrossRef]
- Houchin, M.L.; Topp, E.M. Physical properties of PLGA films during polymer degradation. J. Appl. Polym. Sci. 2009, 114, 2848–2854. [Google Scholar] [CrossRef]


| Group | Injected/Ovulated | Latency Period (h) | Absolute Fecundity (Eggs/Female) | Relative Fecundity (eggs/kg BW) | Hatching Rate (%) |
|---|---|---|---|---|---|
| 0.9% NaCl | 10/0 a | ||||
| mGnRHa | 10/4 a | 84.5 ± 0.65 | 85,094 ± 39,731 | 83,943 ± 36,100 | 57.7 ± 7.9 |
| PLGA 5 | 10/10 b | 96.8 ± 1.03 | 109,781 ± 24,872 | 128,184 ± 27,811 | 68.0 ± 4.4 |
| PLGA 20 | 10/4 a | 90.5 ± 1.95 | 61,784 ± 41,289 | 52,784 ± 31,868 | 53.4 ± 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knowles, J.; Vysloužil, J.; Policar, T.; Milla, S.; Holická, M.; Podhorec, P. Spawning Performance and Sex Steroid Levels in Female Pikeperch Sander lucioperca Treated with Poly(lactic-co-glycolic acid) Microparticles. Animals 2022, 12, 208. https://doi.org/10.3390/ani12020208
Knowles J, Vysloužil J, Policar T, Milla S, Holická M, Podhorec P. Spawning Performance and Sex Steroid Levels in Female Pikeperch Sander lucioperca Treated with Poly(lactic-co-glycolic acid) Microparticles. Animals. 2022; 12(2):208. https://doi.org/10.3390/ani12020208
Chicago/Turabian StyleKnowles, Jindřiška, Jakub Vysloužil, Tomáš Policar, Sylvain Milla, Martina Holická, and Peter Podhorec. 2022. "Spawning Performance and Sex Steroid Levels in Female Pikeperch Sander lucioperca Treated with Poly(lactic-co-glycolic acid) Microparticles" Animals 12, no. 2: 208. https://doi.org/10.3390/ani12020208
APA StyleKnowles, J., Vysloužil, J., Policar, T., Milla, S., Holická, M., & Podhorec, P. (2022). Spawning Performance and Sex Steroid Levels in Female Pikeperch Sander lucioperca Treated with Poly(lactic-co-glycolic acid) Microparticles. Animals, 12(2), 208. https://doi.org/10.3390/ani12020208











