1. Introduction
Restricted feeding is the approach to limit the calorie intake to attain particular digestive and physiological characteristics depending on the growth and development of an individual [
1]. Though ad libitum feeding is usually applied during rabbit rearing, it has been the major cause of feed waste and a high incidence of digestive diseases and mortality [
2]. Regardless of retaining the advantages such as reduced diarrhea and low mortality in young rabbits, the excessive nutritional restriction can also lead to reduced growth and low performance of rabbits [
3]. Congruently, one study showed that feed restriction in Hubbard broilers reduced average daily feed intake and had a considerably lower feed-to-weight ratio than the control group [
4]. Broiler chickens of 8 and 14 days of age, when subjected to compensation of energy restriction, showed similar levels of average daily gain(ADG), average daily feed intake(ADFI), and feed/gain(F/G) to that with the control group [
5]. Concomitantly, it is suggested that animals undergoing restriction can negate possible adverse effects on growth performance by providing nutrition.
The digestion and absorption of nutrients by the animals are mainly dependent on the digestive system and the level of nutrition, which further determine their growth and development [
6]. The use of 80–90% free-feeding restrictions throughout the production cycle of rabbits has been reported by Bovera, F et al. to produce rabbits with the same live weight at slaughter age [
7]. The effect of quantitatively and linearly reducing feed-intake levels (100-60%) on digestive health and growth in rabbits was reported by Gidenne, T. et al. During periods of restricted feeding, mortality and morbidity in rabbits were significantly reduced from 80% and 70% of the feeding level, respectively [
8]. Restriction on feed intake of 7–14-day-old broilers manifested a significant increase in several intestinal enzymes such as amylase, lipase, and trypsin but was unaffected after compensation [
9]. Skeletal muscle is the largest tissue in the body, weighing up to 40% of the body. After birth, the number of muscle fibers formed by the fusion of muscle satellite cells after activation is low, and the growth and development of skeletal muscle depend mainly on the increase in muscle fiber diameter [
10]. Studies have shown that there is a direct correlation between the cross-sectional area of muscle fibers and the net protein content of the muscle tissue. Muscle satellite cells are involved in muscle-fiber hypertrophy by increasing the nucleus of the muscle-fiber cell to maintain the balance between the nucleus and the cytoplasm of the myocyte [
11]. Studies have demonstrated an enhanced expression of IGFI in the exercised muscle, which further activated the PI3K/Akt pathway to promote muscle-fiber hypertrophy [
12] and was validated in transgenic mice overexpressing IGF1.
Therefore, this study conducted a detailed investigation on the effects of different levels of feeding restriction on the growth performance, intestinal immunity, and skeletal muscle development of meat rabbits. Additionally, regarding whether complete compensatory growth could be achieved post 2 weeks of restriction, we analyzed the effects of feeding restriction on growth performance, intestinal immunity, and skeletal muscle development of rabbits to provide some scientific basis for the selection of feeding restriction mode for rabbits.
2. Material and Methods
2.1. Animals and Experimental Design
The test animals were obtained from Xuzhou Meat Rabbit Ecological Science and Technology Park, Jiangsu Province. Of the 35-day-old weanling rabbits of similar weight and in good health, 198 were selected. The animals were randomly divided into three groups: the control (free range), the restricted-feeding group I (fed 85% of the control), and the restricted-feeding group Ⅱ (fed 70% of the control), with 66 animals in each group, half being male and half being female. A pre-test was carried out to calculate the amount of food intake by 12 ad libitum rabbits prior to initiation of the trial period. This provided the amount of food consumption given to the three groups during the restricted feeding period. The trial was divided into 21 days of a restricted feeding period and 14 days of a compensation period.
All the rabbit hutches and equipment were strictly cleaned and disinfected by high-temperature flame a day before the trial. All test rabbits were labeled and kept in single cages in the housing. The usual feeding management and immunization procedures were used during the test period, and the environmental conditions were kept consistent. The rabbits were fed twice a day at 08:00 and 18:00, with free access to water (the diet formula and nutritional levels shown in
Table 1). The food intake of growing rabbits was recorded daily. The average daily weight gain was calculated by weighing the rabbits before the start of the trial, at 56 days of age and before slaughter, and the disease and mortality of the rabbits during the trial were recorded. Six rabbits were randomly selected from each group at 56 and 70 days of age. Samples were collected from skeletal-muscle-tissue samples and three sections of small-intestine tissue.
2.2. Growth Performance Measurement and Health-Risk Assessment Indicators
The growth indicators such as average daily gain (ADG), average daily feed intake (ADFI), and feed-to-weight ratio (F/G) were measured as follow:
Average daily gain (ADG): All test rabbits were weighed empty stomach to calculate the average daily gain of meat rabbits from 36–56 days of age (restricted feeding period), 57–70 days of age (compensation period), and 36–70 days of age (full period).
Average daily feed intake (ADFI): The daily feed intake and the number of leftovers were recorded for the test rabbits for the restricted feeding period, the compensation period, and the whole period for the meat rabbits.
Feed-to weight ratio (F/G): The ratio of average feed intake to average daily weight gain is the average feed-to-weight ratio.
The morbidity, mortality, and health-risk indices were calculated for the restricted feeding period, the compensation period, and the full period. Mortality due to morbidity was counted as death only.
Morbidity rate = number of rabbits with disease/total number of rabbits × 100%
Mortality rate = number of rabbits killed/total number of rabbits × 100%
Health-risk index = morbidity + mortality
2.3. Measurement of Digestive Enzyme Activity and Immune and Antioxidant Indexes
In this test, the activity of jejunal amylase, lipase, chymotrypsin, and alkaline phosphatase (ALP/AKP) was determined using the α-AMS, lipase(LPS), chymotrypsin, and alkaline phosphatase kits (all from Nanjing Jiancheng, Nanjing, China), respectively. The levels of jejunal immunoglobulin and ileal inflammatory factors were measured using ELISA kits (Shanghai Enzyme Link, Shanghai, China). The jejunal malondialdehyde (MDA) content, total antioxidant capacity (T-AOC), and superoxide dismutase (T-SOD) activities were determined using kits (Nanjing Jiancheng Company).
2.4. Tissue Sample Collection
Six test rabbits were randomly selected from each of the control group, restricted-feeding group I, and restricted-feeding group II at 56 days of age. One part was rinsed with PBS and fixed in 4% paraformaldehyde solution for tissue sectioning. One part was washed with PBS several times to remove blood stains on the surface, then it was placed in lyophilization tubes and frozen in liquid nitrogen for extraction of total RNA.
2.5. HE Staining and Muscle Fibre Size Analysis
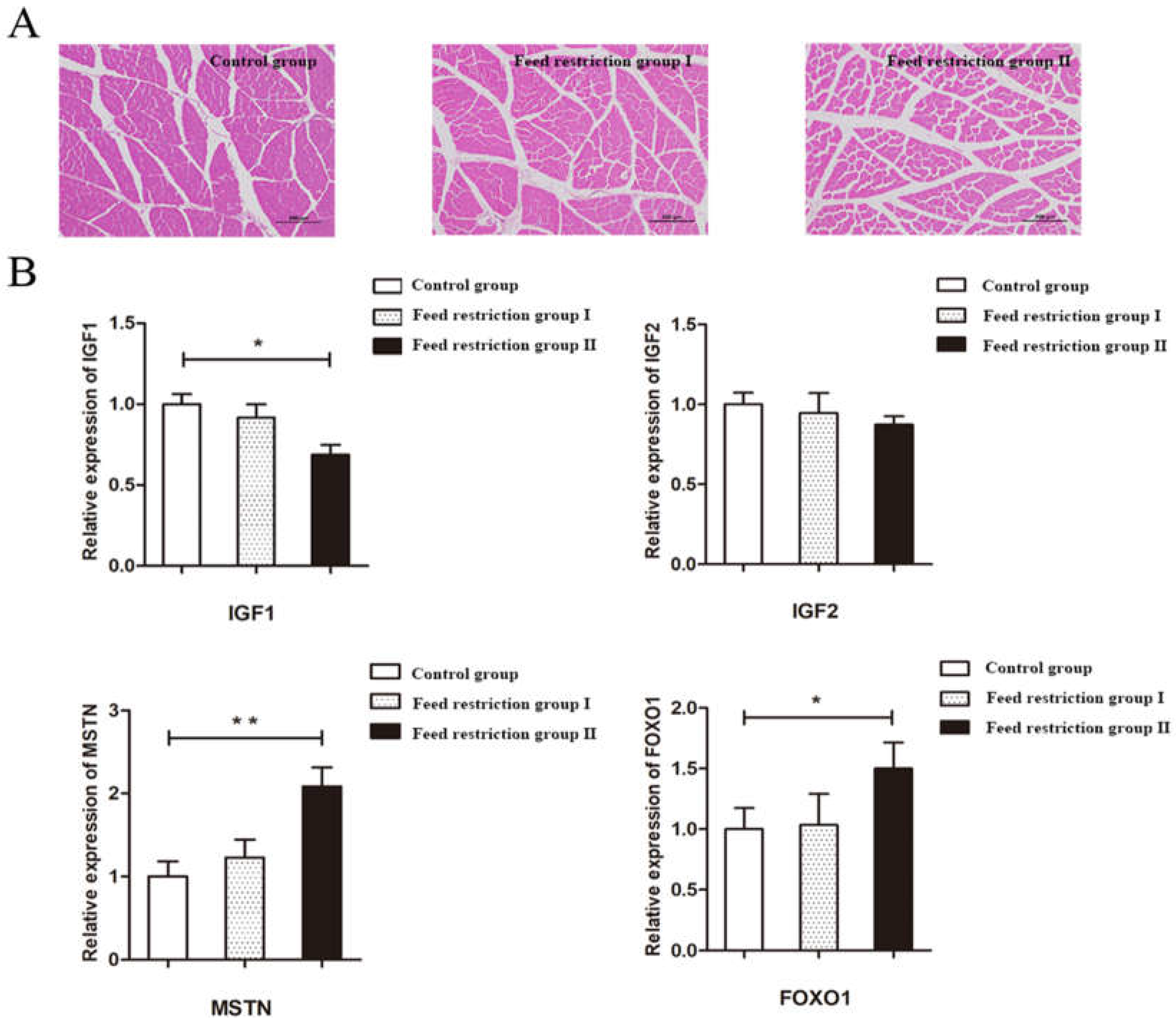
Tissues were fixed in 4% paraformaldehyde solution, trimmed to size, and then dehydrated in different concentrations of gradient ethanol and xylene before being waxed and embedded in an embedding machine and sliced using a microtome to a thickness of 7 µm. The sections were then dewaxed and rehydrated before being stained with hematoxylin-eosin (HE), sealed with neutral gum, and photographed with a magnification of 100×. The muscle cross-sectional area was analyzed using Image 6.0.
2.6. RNA Extraction and cDNA Synthesis
RNA was extracted from approximately 100 mg of skeletal muscle tissue using Trizol reagent (Invitrogen-Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. The concentration of extracted RNA was measured spectrophotometrically using a NanoDrop ND-1000 micro-UV-Vis spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA), and the purity of RNA was assessed by the A260/A280 ratio in the range of 1.8-2.0.
Total RNA (200 μg) was reverse transcribed into cDNA using HiScript® Ⅱ Q Select RT SuperMix for quantitative real-time PCR (qPCR) (Nanjing Novozymes Biotechnology Co., Ltd., Nanjing, China) according to the manufacturer’s protocol. Samples were stored at −20 °C for subsequent analysis.
2.7. Real-Time qPCR
Reaction system: 2 × ChamQ SYBR qPCR Master Mix 10 μL, 0.4 μL of each primer, 50×ROX Reference Dye2 0.4 μL, 1 μL of template DNA, ddH
2O to make up to 20 μL. Amplification procedure (three-step): 95 °C pre-denaturation for 30 s; 40 cycles of reaction: 95 °C for 10 S, 60 °C for 30 s; melting curve: 95 °C 15s, 60 °C 60s, 95 °C 15 S. Primer sequences are detailed in
Table 2.
2.8. Western Blot Analysis
The total protein was extracted by adding a certain percentage of protease inhibitor to Western and IP cell lysate and stored at −80 °C. The total protein content in skeletal muscle cells was measured using the BCA protein-concentration assay kit (Beyonce Biotechnology, Shanghai, China). The expression levels of Akt, p-Akt, p-70, and p-p70 proteins were measured using the Wes system Protein simple technique. The protein samples, the diluted primary anti-target protein antibody, GAPDH as an internal reference antibody (Proteintech, Wuhan, China), and other relevant test reagents were prepared followed by samples loading according to the Wes user procedure. Unlike traditional Western Blot, fully automated Wes gum preparation and running, membrane transfer, manual incubation or washing, press development, and subjective data processing are all done automatically in the capillary.
2.9. Statistical Analysis
The data were collated and counted using Excel software, and the relative gene expression was calculated using the 2−ΔΔct method. Data were analyzed using SPSS 22.0 statistical software and were analyzed by one-way ANOVA and paired t-test according to different trials. The results of the gender-difference analysis showed no significant gender effect, and the results of the trials were expressed as mean ± standard deviation; p < 0.05 indicated a significant difference; and p < 0.01 indicated an extremely significant difference.