The Manifold Bioactivity and Immunoreactivity of Microbial Proteins of Cow and Human Mature Milk in Late Lactation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
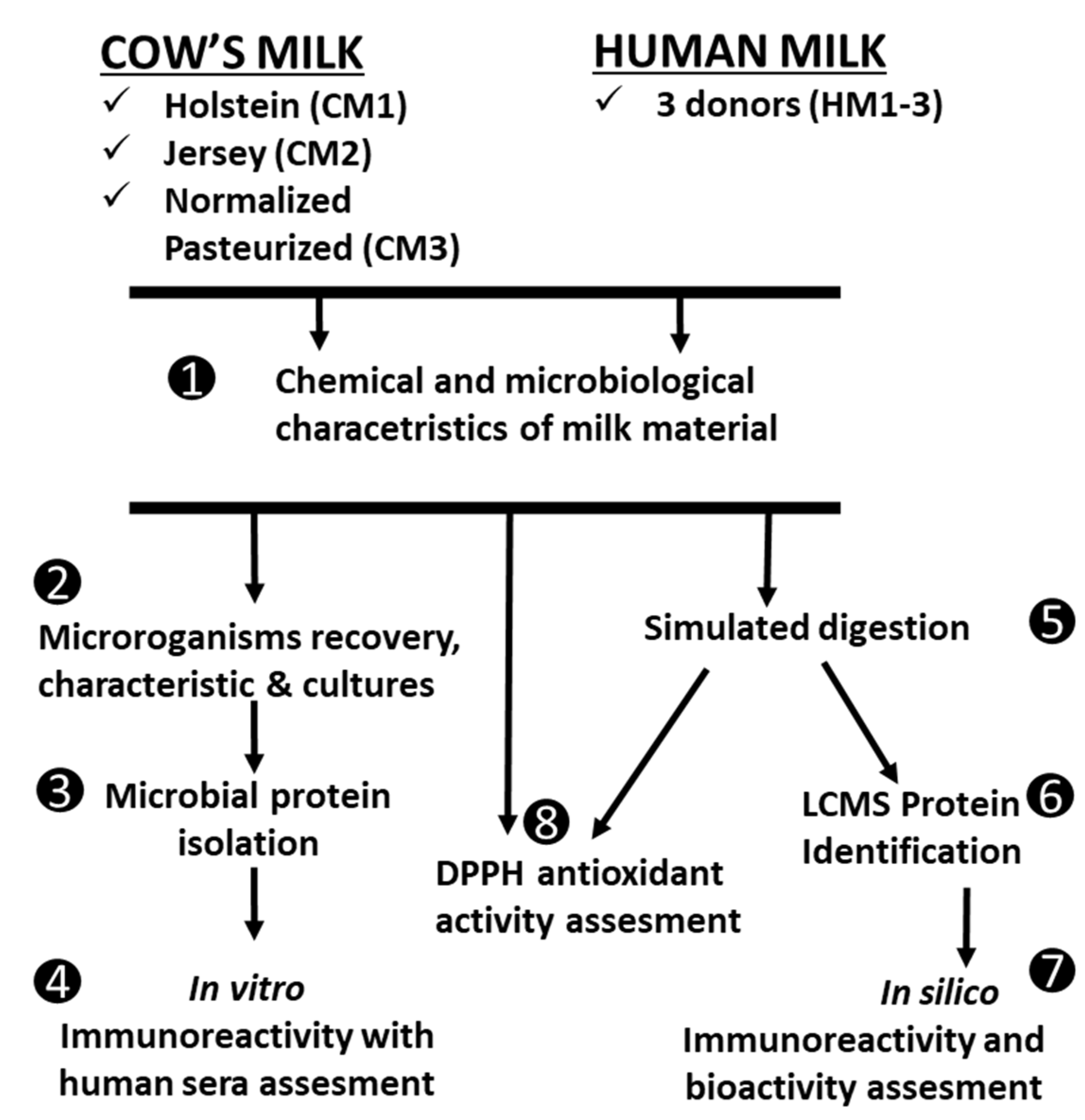
2.2. Experimental Design
2.3. Chemical Analysis of Milk Samples
2.4. Simulated Digestion of Milk Samples
2.5. Microbiological Analysis of Milk Samples
2.6. Direct Recovery of Microorganisms from Milk and Their Metabolic Activity Assessment
2.7. Microbial Cultures and Protein Isolation
2.8. Protein Visualization
2.9. In Vitro Assessment of Protein Immunoreactivity with Human Sera
2.10. Mass Spectral Analysis of Proteins
2.11. Antioxidant Activity with DPPH Radical-Scavenging Assay
2.12. In Silico Protein Immunoreactivity and Antioxidant Activity Analysis
2.13. Statistical Analysis
3. Results
3.1. Chemical Composition of Milk
3.2. Microbiological Analysis of Milk Samples
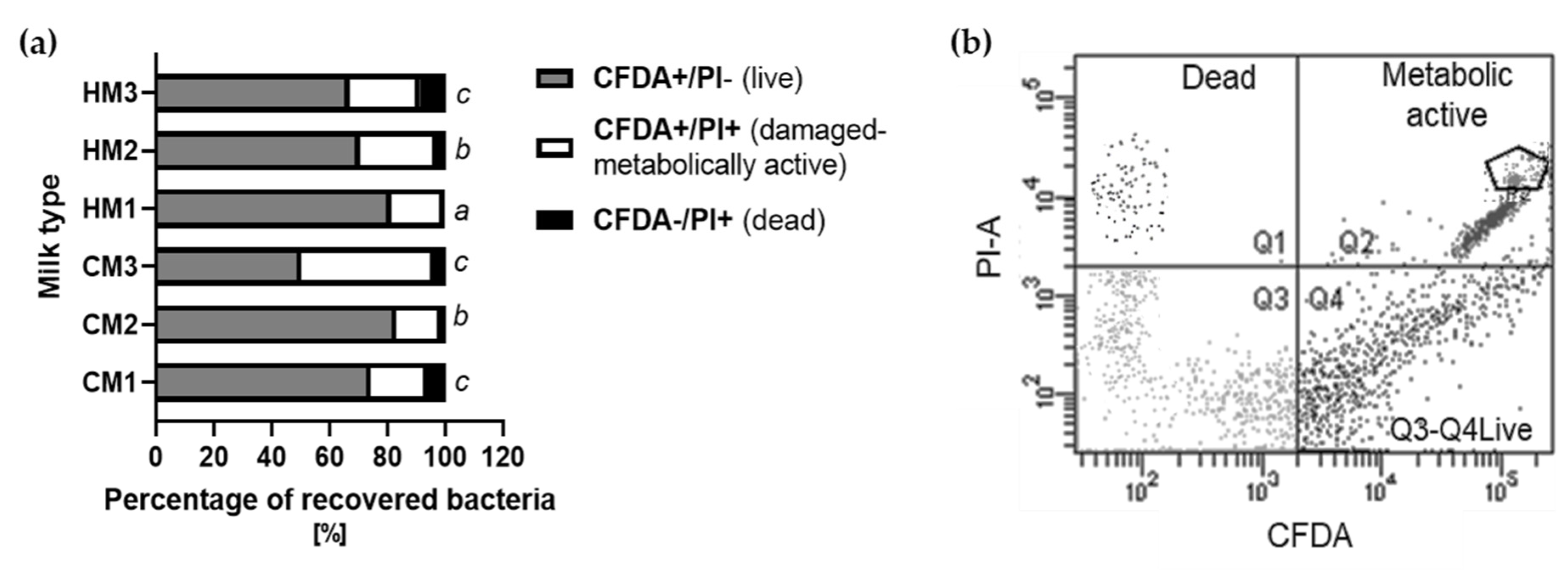
3.3. Microorganisms’ Activity after Recovery
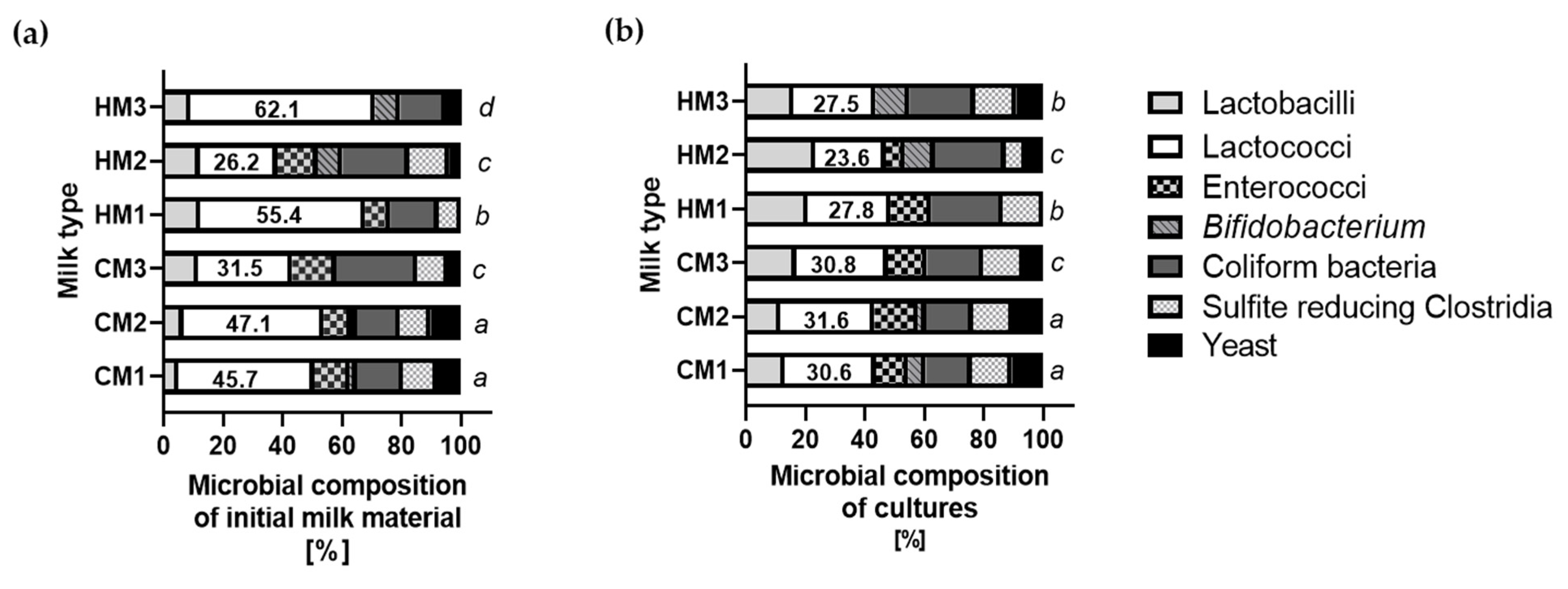
3.4. Microbial Composition of Cultures of Recovered Bacteria
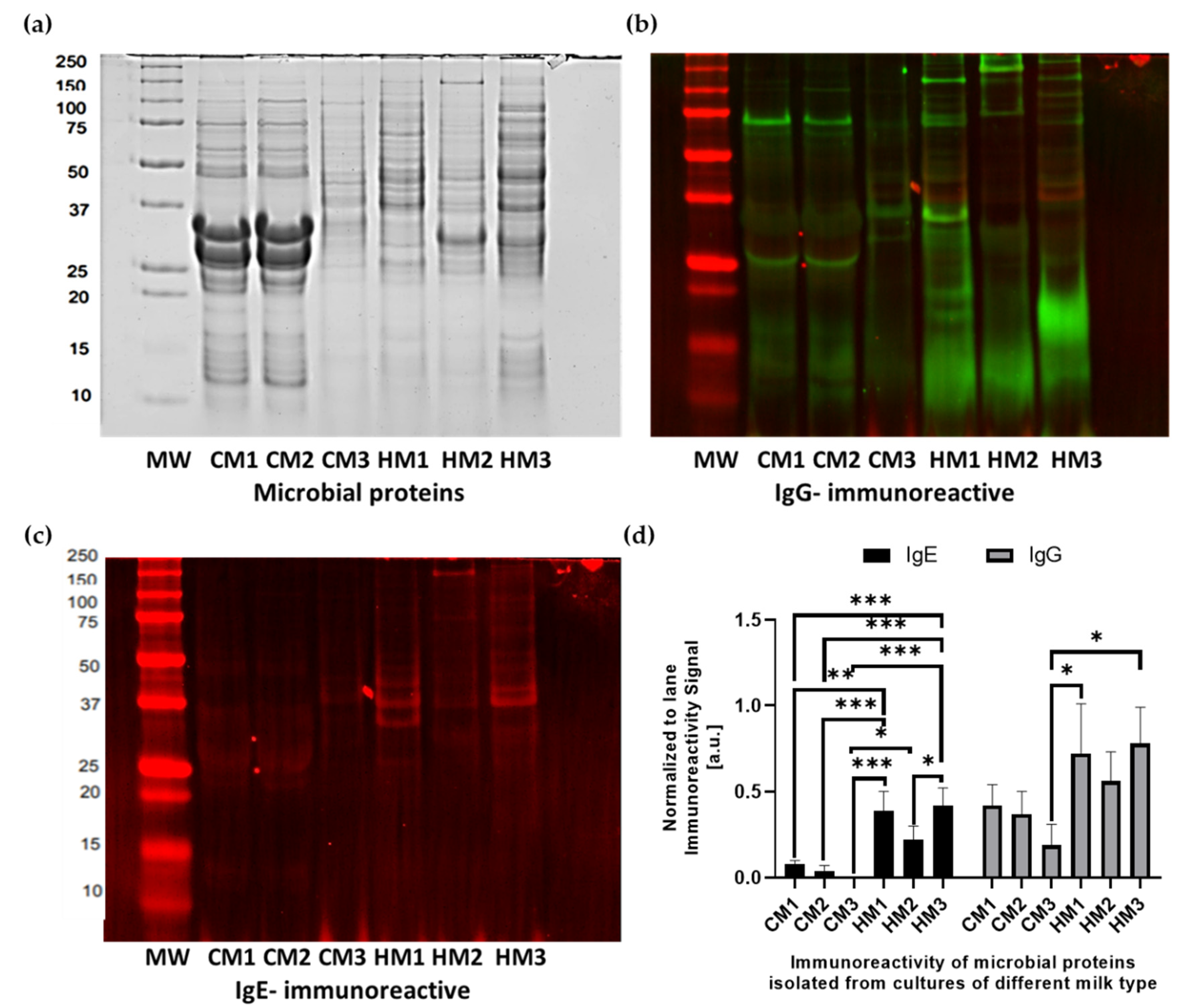
3.5. Characteristics of Protein Isolates
3.6. Immunoreactivity of Microbial Proteins with Human Sera
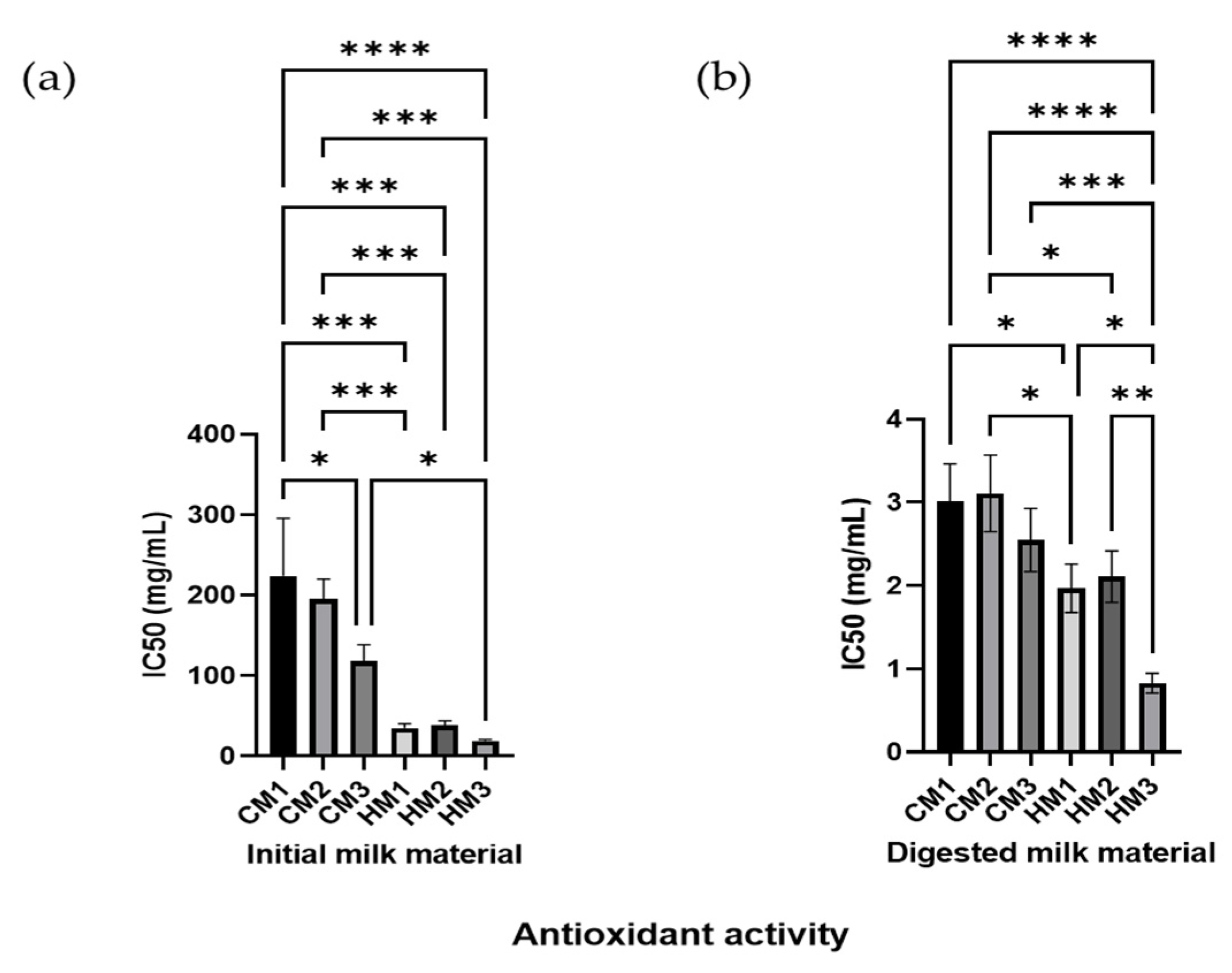
3.7. Milk and Digests Antioxidant Activity
3.8. In Silico Assessment of Bacterial Protein Immunoreactivity and Bioactive Properties
4. Discussion
4.1. Microbial Proteins Immunoeactivity in Milk
4.2. Study Design Limitations and Analytical Limits
4.3. Plans for Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Kong, C.; Wang, W.; Groeneveld, A.; Nauta, A.; Groves, M.R.; Kiewiet, M.B.G.; De Vos, P. The Human Milk Oligosaccharides 3-FL, Lacto-N-Neotetraose, and LDFT Attenuate Tumor Necrosis Factor-α Induced Inflammation in Fetal Intestinal Epithelial Cells In Vitro through Shedding or Interacting with Tumor Necrosis Factor Receptor 1. Mol. Nutr. Food Res. 2021, 65, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Davis, B.; Weishu, Z.; Nan, Z.; Di, M.; Allan, W. Short chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation. Am. J. Physiol. 2020, 320, G521–G530. [Google Scholar] [CrossRef] [PubMed]
- Burge, K.; Eckert, J.; Wilson, A.; Trammell, M.; Lueschow, S.R.; Mcelroy, S.J.; Dyer, D.; Chaaban, H. Formula Feeding Model of Necrotizing Enterocolitis. Nutrients 2022, 14, 1779. [Google Scholar] [CrossRef]
- Rasmussen, S.O.; Martin, L.; Østergaard, M.V.; Rudloff, S.; Roggenbuck, M.; Nguyen, D.N.; Sangild, P.T.; Bering, S.B. Human Milk Oligosaccharide Effects on Intestinal Function and Inflammation after Preterm Birth in Pigs; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 40, ISBN 2012152934001. [Google Scholar]
- Chatterton, D.E.W.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Thomas, S.; Gauglitz, J.M.; Tripathi, A.; Vargas, F.; Bertrand, K.; Kim, J.H.; Chambers, C.; Dorrestein, P.C.; Tsunoda, S. An untargeted metabolomics analysis of exogenous chemicals in human milk and transfer to the infant. bioRxiv 2022. [Google Scholar] [CrossRef]
- Poulsen, K.O.; Meng, F.; Lanfranchi, E.; Young, J.F.; Stanton, C.; Ryan, C.A.; Kelly, A.L.; Sundekilde, U.K. Dynamic Changes in the Human Milk Metabolome Over 25 Weeks of Lactation. Front. Nutr. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Walker, A. Breast Milk as the Gold Standard for Protective Nutrients. J. Pediatr. 2010, 156, S3–S7. [Google Scholar] [CrossRef]
- Lyons, K.E.; Shea, C.A.O.; Grimaud, G.; Ryan, C.A.; Dempsey, E.; Kelly, A.L.; Ross, R.P.; Stanton, C. The human milk microbiome aligns with lactation stage and not birth mode. Sci. Rep. 2022, 12, 5598. [Google Scholar] [CrossRef]
- Bravi, F.; Di Maso, M.; Eussen, S.R.B.M.; Agostoni, C.; Salvatori, G.; Profeti, C.; Tonetto, P.; Quitadamo, P.A.; Kazmierska, I.; Vacca, E.; et al. Dietary patterns of breastfeeding mothers and human milk composition: Data from the italian MEDIDIET study. Nutrients 2021, 13, 1722. [Google Scholar] [CrossRef]
- Ferrer-miralles, N.; Villaverde, A. Bacterial cell factories for recombinant protein production; expanding the catalogue. Microb. Cell Fact. 2013, 12, 113. [Google Scholar] [CrossRef]
- Stephen-victor, E.; Chatila, T.A. ScienceDirect Regulation of oral immune tolerance by the microbiome in food allergy. Curr. Opin. Immunol. 2019, 60, 141–147. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Stephen-Victor, E.; Gerber, G.K.; Noval, M.; Wang, S.; Harb, H.; Wang, L.; Li, N.; Crestani, E.; Secor, W.; et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat. Med. 2019, 25, 1164–1174. [Google Scholar] [CrossRef]
- Nalepa, B.; Olszewska, M.A.; Markiewicz, L.H. Seasonal variances in bacterial microbiota and volatile organic compounds in raw milk. Int. J. Food Microbiol. 2018, 267, 70–76. [Google Scholar] [CrossRef]
- Dudkiewicz, A.; Masmejean, L.; Arnaud, C.; Onarinde, B.A.; Sundara, R.; Pour-Taghi Anvarian, A.H.; Tucker, N. Approaches for improvement in digestive survival of probiotics, a comparative study. Polish J. Food Nutr. Sci. 2020, 70, 265–273. [Google Scholar] [CrossRef]
- European Commission Regulation (EC) N° 853/2004 of the European Parlamient and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L 139, 55.
- Skovgaard, N. Scientific Criteria to Ensure Safe Food; National Academies Press: Washington, DC, USA, 2004; Volume 94, ISBN 0309509203. [Google Scholar]
- Pollock, J.; Salter, S.J.; Nixon, R.; Hutchings, M.R. Milk microbiome in dairy cattle and the challenges of low microbial biomass and exogenous contamination. Anim. Microbiome 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Ma, T.; Shen, L.; Wen, Q.; Lv, R.; Hou, Q.; Kwok, L.Y.; Sun, Z.; Zhang, H. Pacbio sequencing revealed variation in the microbiota diversity, species richness and composition between milk collected from healthy and mastitis cows. Microbiology 2021, 167, 000968. [Google Scholar] [CrossRef]
- Metzger, S.A.; Hernandez, L.L.; Skarlupka, J.H.; Walker, T.M.; Suen, G.; Ruegg, P.L. A cohort study of the milk microbiota of healthy and inflamed bovine mammary glands from dryoff through 150 days in milk. Front. Vet. Sci. 2018, 5, 247. [Google Scholar] [CrossRef]
- Cremonesi, P.; Ceccarani, C.; Curone, G.; Severgnini, M.; Pollera, C.; Bronzo, V.; Riva, F.; Addis, M.F.; Filipe, J.; Amadori, M.; et al. Milk microbiome diversity and bacterial group prevalence in a comparison between healthy holstein friesian and rendena cows. PLoS ONE 2018, 13, e0205054. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, L.H.; Honke, J.; Haros, M.; Światecka, D.; Wróblewska, B. Diet shapes the ability of human intestinal microbiota to degrade phytate—In vitro studies. J. Appl. Microbiol. 2013, 115, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Brewster, J.D.; Paul, M. Short communication: Improved method for centrifugal recovery of bacteria from raw milk applied to sensitive real-time quantitative PCR detection of Salmonella spp. J. Dairy Sci. 2016, 99, 3375–3379. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Kocot, A.M.; Nynca, A.; Łaniewska-Trokenheim, Ł. Utilization of physiological and taxonomic fluorescent probes to study Lactobacilli cells and response to pH challenge. Microbiol. Res. 2016, 192, 239–246. [Google Scholar] [CrossRef]
- Klaassens, E.S.; De Vos, W.M.; Vaughan, E.E. Metaproteomics approach to study the functionality of the microbiota in the human infant gastrointestinal tract. Appl. Environ. Microbiol. 2007, 73, 1388–1392. [Google Scholar] [CrossRef]
- Wróblewska, B.; Markiewicz, L.H.; Szyc, A.M.; Dietrich, M.A.; Szymkiewicz, A.; Fotschki, J. Lactobacillus casei LcY decreases milk protein immunoreactivity of fermented buttermilk but also contains IgE-reactive proteins. Food Res. Int. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Schägger, H.; Von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Markiewicz, L.H.; Szymkiewicz, A.; Szyc, A.; Wróblewska, B. A simultaneous two-colour detection method of human IgG- and IgE-reactive proteins from lactic acid bacteria. J. Microbiol. Methods 2016, 126, 72–75. [Google Scholar] [CrossRef]
- Picot, L.; Ravallec, R.; Martine, F.P.; Vandanjon, L.; Jaouen, P.; Chaplain-Derouiniot, M.; Guérard, F.; Chabeaud, A.; Legal, Y.; Alvarez, O.M.; et al. Impact of ultrafiltration and nanofiltration of an industrial fish protein hydrolysate on its bioactive properties. J. Sci. Food Agric. 2010, 90, 1819–1826. [Google Scholar] [CrossRef]
- Espejo-Carpio, F.J.; García-Moreno, P.J.; Pérez-Gálvez, R.; Morales-Medina, R.; Guadix, A.; Guadix, E.M. Effect of digestive enzymes on the bioactive properties of goat milk protein hydrolysates. Int. Dairy J. 2016, 54, 21–28. [Google Scholar] [CrossRef]
- Ogrodowczyk, A.M.; Dimitrov, I.; Wróblewska, B. Two faces of milk proteins peptides with both allergenic and multidimensional health beneficial impact—Integrated in vitro/in silico approach. Foods 2021, 10, 163. [Google Scholar] [CrossRef]
- Dimitrov, I.; Garnev, P.; Flower, D.R.; Doytchinova, I. EpiTOP-a proteochemometric tool for MHC class II binding prediction. Bioinformatics 2010, 26, 2066–2068. [Google Scholar] [CrossRef]
- Nagpal, G.; Usmani, S.S.; Dhanda, S.K.; Kaur, H. Computer-aided designing of immunosuppressive peptides based on IL-10 inducing potential. Sci. Rep. 2017, 7, srep42851. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Górska, S.; Dylus, E.; Rudawska, A.; Brzozowska, E.; Srutkova, D. Immunoreactive Proteins of Bifidobacterium longum ssp. longum CCM 7952 and Bifidobacterium longum ssp. longum CCDM 372 Identified by Gnotobiotic Mono-Colonized Mice Sera, Immune Rabbit Sera and Non-immune Human Sera. Front. Microbiol. 2016, 7, 1537. [Google Scholar] [CrossRef]
- Fiedorowicz, E.; Markiewicz, L.H.; Sidor, K.; Świątecka, D.; Cieślińska, A.; Matysiewicz, M.; Piskorz-Ogórek, K.; Sienkiewicz-Szłapka, E.; Teodorowicz, M.; Świątecki, A.; et al. The influence of breast milk and infant formulae hydrolysates on bacterial adhesion and Caco-2 cells functioning. Food Res. Int. 2016, 89, 679–688. [Google Scholar] [CrossRef]
- Jeurink, P.V.; Van Bergenhenegouwen, J.; Jiménez, E.; Knippels, L.M.J.; Fernández, L.; Garssen, J.; Knol, J.; Rodríguez, J.M.; Martín, R. Human milk: A source of more life than we imagine. Benef. Microbes 2013, 4, 17–30. [Google Scholar] [CrossRef]
- Goodman, R.E. Allergen Online Vesion 21; University of Nebraska: Lincoln, NV, USA, 2021; ISBN 1018736824. [Google Scholar]
- Ho, B.; Baryshnikova, A.; Brown, G.W.; Ho, B.; Baryshnikova, A.; Brown, G.W. Unification of Protein Abundance Datasets Yields a Article Unification of Protein Abundance Datasets Yields a Quantitative Saccharomyces cerevisiae Proteome. Cell Syst. 2018, 6, 192–205.e3. [Google Scholar] [CrossRef]
- Zar, S.; Benson, M.J.; Kumar, D. Food-specific serum IgG4 and IgE titers to common food antigens in irritable bowel syndrome. Am. J. Gastroenterol. 2005, 100, 1550–1557. [Google Scholar] [CrossRef]
- Hussain, M.A.; Knight, M.I.; Britz, M.L. Proteomic analysis of lactose-starved Lactobacillus casei during stationary growth phase. J. Appl. Microbiol. 2009, 106, 764–773. [Google Scholar] [CrossRef]
- Koponen, J.; Laakso, K.; Koskenniemi, K.; Kankainen, M.; Savijoki, K.; Nyman, T.A.; De Vos, W.M.; Tynkkynen, S.; Kalkkinen, N.; Varmanen, P. Effect of acid stress on protein expression and phosphorylation in Lactobacillus rhamnosus GG. J. Proteom. 2011, 75, 1357–1374. [Google Scholar] [CrossRef]
- Abdelmoteleb, M.; Zhang, C.; Furey, B.; Kozubal, M.; Griffiths, H.; Champeaud, M.; Goodman, R.E. Evaluating potential risks of food allergy of novel food sources based on comparison of proteins predicted from genomes and compared to www.AllergenOnline.org. Food Chem. Toxicol. 2021, 147, 111888. [Google Scholar] [CrossRef]
- Kerro-Dego, O.; Prysliak, T.; Potter, A.A.; Perez-Casal, J. DNA-protein immunization against the GapB and GapC proteins of a mastitis isolate of Staphylococcus aureus. Vet. Immunol. Immunopathol. 2006, 113, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, W.; Lu, C. Identification of immunoreactive proteins of Streptococcus agalactiae isolated from cultured tilapia in China. Pathog. Dis. 2013, 69, 223–231. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Zhang, M.; Liang, S.; Xiao, J.; Wang, Q.; Liu, Q.; Zhang, Y. Secreted glyceraldehyde-3-phosphate dehydrogenase as a broad spectrum vaccine candidate against microbial infection in aquaculture. Lett. Appl. Microbiol. 2012, 54, 1–9. [Google Scholar] [CrossRef]
- Górska, S.; Buda, B.; Brzozowska, E.; Schwarzer, M.; Srutkova, D.; Kozakova, H.; Gamian, A. Identification of Lactobacillus proteins with different recognition patterns between immune rabbit sera and nonimmune mice or human sera. BMC Microbiol. 2016, 16, 1–11. [Google Scholar] [CrossRef][Green Version]
- Pancholi, V.; Fischetti, V.A. A major surface protein on group a streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 1992, 176, 415–426. [Google Scholar] [CrossRef]
- Henderson, B.; Martin, A. Bacterial virulence in the moonlight: Multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011, 79, 3476–3491. [Google Scholar] [CrossRef]
- Liu, H.; Jeffery, C.J. Cellular Metabolism Chapter. Molecules 2020, 25, 1–18. [Google Scholar]
- De Angelis, M.; Di Cagno, R.; Huet, C.; Crecchio, C.; Fox, P.F.; Gobbetti, M. Heat Shock Response in Lactobacillus plantarum. Appl. Environ. Microbiol. 2004, 70, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Tang, H.Y.; Chiang, M.L. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.X.; Yan, R.; Shi, H.Y.; Shi, D.; Fang, D.Q.; Jiang, H.Y.; Wu, W.R.; Guo, F.F.; Jiang, X.W.; Gu, S.L.; et al. Integrated transcriptomic and proteomic analysis of the bile stress response in probiotic Lactobacillus salivarius LI01. J. Proteom. 2017, 150, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Mbye, M.; Obaid, R.S.; Shah, N.P.; Baig, M.A.; Osaili, T.M.; Abuqamar, S.F.; Al-nabulsi, A.A.; El-tarabily, K.A.; Turner, M.S.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef]
- Pepper, S.J.; Britz, M.L. An acid up-regulated surface protein of lactobacillus paracasei strain GCRL 46 is phylogenetically related to the secreted glucan-(GpbB) and immunoglobulin-binding (sibA) protein of pathogenic streptococci. Int. J. Mol. Sci. 2019, 20, 1610. [Google Scholar] [CrossRef]
- Haq, I.U.; Brantl, S. Moonlighting in bacillus subtilis: The small proteins sr1p and sr7p regulate the moonlighting activity of glyceraldehyde 3-phosphate dehydrogenase a (gapa) and enolase in RNA degradation. Microorganisms 2021, 9, 1046. [Google Scholar] [CrossRef]
- Zarban, A.; Taheri, F.; Chahkandi, T.; Sharifzadeh, G.; Khorashadizadeh, M. Antioxidant and radical scavenging activity of human colostrum, transitional and mature milk. J. Clin. Biochem. Nutr. 2009, 45, 150–154. [Google Scholar] [CrossRef]
- Martysiak-Zurowska, D.; Wenta, W. A comparison of abts and dpph methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 11, 83–89. [Google Scholar]
- Zulueta, A.; Maurizi, A.; Frígola, A.; Esteve, M.J.; Coli, R.; Burini, G. Antioxidant capacity of cow milk, whey and deproteinized milk. Int. Dairy J. 2009, 19, 380–385. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Puta, M.; Barczak, N.; Dabrowska, J.; Malinowska-Pańczyk, E.; Kiełbratowska, B.; Kołodziejska, I. Effect of High Pressure and Sub-Zero Temperature on Total Antioxidant Capacity and the Content of Vitamin C, Fatty Acids and Secondary Products of Lipid Oxidation in Human Milk. Polish J. Food Nutr. Sci. 2017, 67, 117–122. [Google Scholar] [CrossRef]
- Pozzo, L.; Cirrincione, S.; Russo, R.; Karamać, M.; Amarowicz, R.; Coscia, A.; Antoniazzi, S.; Cavallarin, L.; Giribaldi, M. Comparison of oxidative status of human milk, human milk fortifiers and preterm infant formulas. Foods 2019, 8, 458. [Google Scholar] [CrossRef]
| Parameters/Samples | CM1 | CM2 | CM3 | HM1 | HM2 | HM3 | p Values CM vs. HM |
|---|---|---|---|---|---|---|---|
| Proteins [%] | 3.14 ± 0.31a | 3.35 ± 0.33 a | 3.09 ± 0.37 a | 1.12 ± 0.23 b | 1.25 ± 0.1 b | 1.42 ± 0.13 b | <0.0001 |
| Casein [g/100 mL] | 2.51 ± 0.21 b | 3.12 ± 0.13 a | 2.97 ± 0.05 a | 0.43 ± 0.08 c | 0.39 ± 0.08 c | 0.49 ± 0.12 c | 0.0048 |
| Total fat [%] | 3.58 ± 0.12 b | 4.32 ± 0.21a | 2.92 ± 0.11 c | 3.91 ± 0.45 a | 3.6 ± 0.14 b | 4.81 ± 0.28 a | 0.4101 |
| Dry skimmed mass [%] | 9.05 ± 0.28 b | 9.59 ± 0.20 a | 9.04 ± 0.16 b | 7.38 ± 0.48 c | 8.2 ± 0.41c | 8.21 ± 0.29 c | 0.0224 |
| Lactose [%] | 5.29 ± 0.21 b | 5.35 ± 0.29 b | 4.89 ± 0.06 b | 7.28 ± 0.32 a | 7.52 ± 0.39 a | 7.18 ± 0.4 a | 0.0005 |
| Urea [mg/L] * | 197 ± 16.5 a | 158 ± 13.5 b | 135 ± 10.0 d | 124 ± 18.2 e | 148 ± 10.0 c | 136 ± 11.5 d | 0.2671 |
| Functional parameters | |||||||
| Active acidity [°T] | 19.45 ± 0.1a | 18.71 ± 0.14 a | 17.63 ± 0.18 b | 9.81 ± 0.05 c | 8.75 ± 0.12 d | 9.92 ± 0.08 c | 0.0003 |
| Freezing point [°C] * | 0.536 ± 0.05 | 0.535 ± 0.07 | 0.496 ± 0.06 | 0.529 ± 0.06 | 0.526 ± 0.05 | 0.561 ± 0.06 | 0.3995 |
| Somatic cells [×103 cells/mL] | 122 ± 1.19 b | 141 ± 0.95 a | 98 ± 0.78 c | 3.65 ± 1.15 d | 5.17 ± 0.99 d | 4.3 ± 1.19 d | 0.0016 |
| Growth of Bacteria (log10 CFU/mL) | Milk Types | p Values CM vs. HM | |||||
|---|---|---|---|---|---|---|---|
| CM1 | CM2 | CM3 | HM1 | HM2 | HM3 | ||
| Aerobic mesophilic bacteria | 4.87 ± 0.24 a | 4.64 ± 0.45 a | 2.15 ± 1.02 b | 3.4 ± 0.7 a | 2.97 ± 0.27 b | 2.82 ± 0.75 b | 0.406 |
| Lactobacilli | 0.36 ± 0.09 | 0.48 ± 0.28 | 0.37 ± 0.18 | 0.54 ± 0.28 | 0.39 ± 0.16 | 0.3 ± 0.13 | 0.937 |
| Lactococci | 3.28 ± 0.76 a | 3.37 ± 0.25 a | 1 ± 0.35 c | 2.43 ± 0.42 b | 0.84 ± 0.6 c | 2.08 ± 0.08 b | 0.448 |
| Enterococci * | 0.85 ± 0.05 a | 0.67 ± 0.12 b | 0.47 ± 0.18 c | 0.37 ± 0.06 c | 0.43 ± 0.08 c | 0 | 0.084 |
| Bifidobacterium * | 0.17 ± 0.68 | 0.13 ± 0.58 | 0 | 0 | 0.27 ± 0.28 | 0.28 ± 0.18 | 0.472 |
| Coliform bacteria | 1.11 ± 0.31 a | 1.04 ± 0.07 a | 0.87 ± 0.07 a | 0.72 ± 0.2 a | 0.72 ± 0.3 a | 0.52 ± 0.2 b | 0.022 |
| Escherichia coli * | 0.16 ± 0.17 | 0.18 ± 0.03 | 0 | 0 | 0.13 ± 0.05 a | 0 | 0.383 |
| Sulfite reducing clostridia * | 0.84 ± 0.14 a | 0.74 ± 0.25 a | 0.33 ± 0.59 a | 0.33 ± 0.21 b | 0.43 ± 0.75 a | 0 | 0.132 |
| Yeast * | 0.56 ± 0.24 a | 0.72 ± 0.12 a | 0.13 ± 0.08 b | 0 | 0.13 ± 0.18 b | 0.17 ± 0.68 a | 0.114 |
| Protein Name | Accession a | Score b | Mass c | Matches d | emPAI e | Protein Sequence Coverage f | Host Organism |
|---|---|---|---|---|---|---|---|
| Microbial proteins from CM samples | |||||||
| Carboxylate-amine ligase | A0A0E2MAL2 | 278 | 51,305 | 40 | 0.09 | 9 | Lactobacillus casei |
| Lipid kinase | D8FRG9 | 262 | 37,442 | 15 | 0.36 | 15 | Lactobacillus rhamnosus |
| Transcriptional regulator | Q9CHX7 | 243 | 16,252 | 10 | 0.29 | 8 | Lactococcus lactis |
| 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | A0A0L0RK75 | 134 | 25,938 | 11 | 0.62 | 12 | Lactobacillus rhamnosus |
| Endo-1,4-beta-xylanase | Q9CIS3 | 107 | 41,689 | 10 | 0.25 | 13 | Lactococcus lactis |
| Pyruvate kinase | C2JX14 | 63 | 62,809 | 4 | 0.22 | 5 | Lactobacillus rhamnosus |
| Chaperone protein DnaK | B3WEQ7 | 57 | 67,523 | 5 | 0.21 | 4 | Lactobacillus casei |
| Putative Molybdenum transport system protein | L8JA30 | 57 | 30,581 | 7 | 0.15 | 6 | Photobacterium marinum |
| Cell division protein | S6AB11 | 57 | 33,270 | 4 | 0.23 | 12 | Sulfuricella denitrificans |
| ABC transporter | C2JYM1 | 56 | 66,162 | 4 | 0.29 | 5 | Lactobacillus rhamnosus |
| DNA damage-inducible protein | A0A0F8UZN5 | 52 | 47,252 | 2 | 0.09 | 5 | Aspergillus rambellii |
| Arginine exporter protein | K8X559 | 46 | 22,849 | 3 | 0.20 | 8 | Providencia burhodogranariea |
| Acetyl-coenzyme A | Q1GAF3 | 45 | 31,531 | 3 | 0.14 | 6 | Lactobacillus delbrueckii |
| Uncharacterized protein | F2NFM8 | 44 | 44,515 | 2 | 0.10 | 5 | Desulfobacca acetoxidans |
| Elongation factor Tu | A0A1X9TWP9 | 44 | 43,330 | 2 | 0.10 | 5 | Lactobacillus delbrueckii |
| Uncharacterized protein | Q9CFV8 | 44 | 12,459 | 2 | 0.39 | 9 | Lactococcus lactis |
| Enolase | D8FPZ1 | 44 | 46,282 | 2 | 0.20 | 4 | Lactobacillus delbrueckii |
| Microbial proteins from HM samples | |||||||
| ABC transporter ABC binding and permease protein | Q9CG37 | 155 | 68,327 | 11 | 0.11 | 6 | Lactococcus lactis |
| Beta-lactamase domain protein | B9XAB6 | 102 | 50,319 | 15 | 0.09 | 7 | Pedosphaera parvula |
| Uncharacterized protein | C4QZG1 | 78 | 7216 | 2 | 0.75 | 17 | Komagataella pastoris |
| Acyl_transf_3 domain-containing protein | R6MBA2 | 76 | 44,535 | 2 | 0.10 | 9 | Bacteroides clarus |
| Cyclopropane-fatty-acyl-phospholipid synthase | Q1G9E5 | 68 | 45,321 | 6 | 0.10 | 8 | Lactobacillus delbrueckii |
| Pyridine nucleotide-disulfide oxidoreductase class-II | A0A0D9MGW0 | 60 | 36,403 | 2 | 0.12 | 10 | Penicillium solitum |
| ABC transporter related | R7QYF5 | 57 | 29,417 | 4 | 0.15 | 13 | Roseburia sp. |
| Thymidylate kinase | Q9CIG4 | 55 | 23,984 | 2 | 0.38 | 8 | Lactococcus lactis |
| Glyceraldehyde 3-phosphate dehydrogenase | K2HVL5 | 52 | 37,976 | 6 | 0.18 | 11 | Bifidobacterium bifidum |
| ABC transporter protein | E3VVN9 | 50 | 80,255 | 5 | 0.11 | 2 | Lactobacillus casei |
| Elongation factor G | Q1GBM0 | 50 | 76,506 | 4 | 0.12 | 4 | Lactobacillus delbrueckii |
| Enolase | D8FPZ1 | 49 | 46,282 | 12 | 0.80 | 13 | Lactobacillus delbrueckii |
| Carboxylate-amine ligase | A0A0U3CLC8 | 47 | 48,369 | 2 | 0.19 | 3 | Lactobacillus delbrueckii |
| 60 kDa chaperonin | A0A0E3CQW5 | 46 | 57,357 | 3 | 0.16 | 3 | Lactobacillus rhamnosus |
| UPF0756 membrane protein | B3WE66 | 45 | 15,858 | 2 | 0.69 | 10 | Lactobacillus casei |
| 30S ribosomal protein S1 | Q1GAK0 | 44 | 44,211 | 3 | 0.21 | 5 | Lactobacillus delbrueckii |
| Lipoprotein | K0MV23 | 44 | 30,417 | 2 | 0.15 | 2 | Lactobacillus casei |
| A) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Name | Accession Number a | Allergenicity b | Proinflamatory Response c | Immunoglobulin Induction d | IL10 Inducers e | Bioactivity f | |||||||
| Anti-Inflammatory | Antioxidative | Antibacterial | Immuno- Modulating | Immuno- Stimulating | Regulating | Neuropeptides | |||||||
| Microbial proteins from CM samples | |||||||||||||
| Carboxylate-amine ligase | A0A0E2MAL2 | no matches | 214/1.52 | - | 1.36 | 0.0045 | 0.0839 | - | 0.0068 | - | 0.0091 | 0.0023 | |
| Lipid kinase | C2JW45 | 28.8% identity (63.0% similar) with allergen Lat c 1; E = 0.062 | 152/1.46 | IgG-1.365 | 1.28 | 0.0029 | 0.0698 | - | 0.0087 | - | - | 0.0116 | |
| Transcriptional regulator | Q9CHX7 | 29.0% identity with allergen Alt a 15.0101; E = 1.3 | 109/1.64 | IgG-1.059 | 1.73 | - | 0.1167 | - | - | - | 0.0333 | 0.0167 | |
| 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | C2JZH4 | 31% identity with Clo bo Toxin; E = 0.57 | 98/1.44 | IgG-0.944; IgE-1.167 | 1.28 | 0.0087 | 0.0873 | - | 0.0087 | - | - | 0.0087 | |
| Endo-1,4-beta-xylanase | Q9CIS3 | 26.4% identity (63.2% similar) with allergen Sus s Laminin; E = 0.68 | 168/1.44 | IgG-1.287; IgA-1.356 | 1.26 | - | 0.1167 | - | - | - | - | - | |
| Pyruvate kinase | C2JX14 | 41.9% identity (73.8% similar) with allergen Pan h 9; E < 1 ×10−7 | 266/2.11 | IgG-1.182; IgA-1.114 | 0.96 | - | 0.0391 | - | 0.0017 | 0.0017 | 0.0119 | 0.0068 | |
| Chaperone protein DnaK | B3WEQ7 | 53.0% identity (85% similar) with allergen Tri a 33.0101; E < 1 ×10−7 | 309/204 | IgG-1.359; IgA-0.998 | 0.72 | 0.0032 | 0.0689 | - | 0.0016 | 0.0016 | 0.0112 | 0.0032 | |
| Putative Molybdenum transport system protein | L8JA30 | no matches | 130/1.28 | IgG-1.180 | 1.03 | 0.0036 | 0.0427 | - | - | 0.0036 | 0.0071 | 0.0071 | |
| Cell division protein | S6AB11 | no matches | 171/1.68 | IgG-1.091; IgA-0.977 | 1.55 | - | 0.0596 | - | - | - | 0.0265 | - | |
| ABC transporter | C2JYM1 | no matches | 299/1.69 | IgG-1.458; IgA-0.946 | 1.40 | 0.0034 | 0.0604 | 0.0167 | 0.0017 | - | 0.0134 | 0.0168 | |
| NMD3 family protein | A0A0N8HZU5 | 25.7% identity (52.5% similar) with allergen Tyr p 2.0101; E = 0.17 | 58/1.13 | IgG-1.148; IgA-0.996 | 0.80 | - | 0.0568 | - | - | - | 0.0108 | 0.0081 | |
| DNA damage-inducible protein | A0A0F8UZN5 | 41.3% identity (60.9% similar) with allergen Alt a 7; E = 0.1 | 204/1.35 | IgG-1.291 | 1.05 | 0.0023 | 0.0508 | - | 0.0046 | 0.0046 | 0.0185 | 0.0023 | |
| Ragulator complex protein | A0A0P7YKP3 | no matches | 22/1.13 | IgG-1.599 | 1.38 | - | 0.0696 | - | - | - | 0.0087 | 0.0174 | |
| Arginine exporter protein | K8X559 | no matches | 72/1.11 | IgG-1.211 | 1.05 | - | 0.0878 | - | 0.0878 | 0.0049 | 0.0146 | 0.0195 | |
| Acetyl-coenzyme A | Q1GAF3 | 28.0% identity (60.0% similar) with allergen Pen m 2; E = 0.66 | 125/1.38 | IgG-1.345; | 1.23 | - | 0.0567 | - | 0.0106 | 0.0035 | 0.0106 | 0.0106 | |
| Uncharacterized protein | F2NFM8 | 44.7% identity (57.9% similar) with Art v 1-like protein; E = 0.4 | 113/1.28 | IgG-1.292; IgA-1.181 | 1.34 | 0.0050 | 0.0723 | - | - | - | 0.0249 | 0.015 | |
| Elongation factor Tu | A0A1X9TWP9 | 26.0% identity with allergen Der p 1; E = 2.3 | 139/1.43 | IgG-1.226; IgE-1.152; IgA- 1.111 | 1.21 | 0.0051 | 0.0530 | - | 0.0025 | - | 0.0202 | - | |
| Uncharacterized protein | Q9CFV8 | 26.7% identity (55.8% similar) with allergen Gly m 6.0501; E = 0.95 | 1/0.71 | IgG- 1.235 | 1.65 | - | 0.0500 | - | - | 0.0167 | 0.0167 | 0.0167 | |
| Enolase | D8FPZ1 | 50.2% identity (76.3% similar) with allergen Rho m 1; E < 1 ×10−7; score 0.75 | 155/1.21 | IgG-1.105; IgA-0.988 | 1.03 | - | - | - | - | - | - | - | |
| B) | |||||||||||||
| Protein Name | Accession Number a | Allergenicity b | Proinflamatory Response c | Immunoglobulin Induction d | IL10 Inducers e | Bioactivity f | |||||||
| Anti-Inflammatory | Antioxidative | Antibacterial | Immuno- Modulating | Immuno- Stimulating | Regulating | Neuropeptides | |||||||
| Microbial proteins from HM samples | |||||||||||||
| ABC transporter ABC binding and permease protein | Q9CG37 | no matches | 292/1.7 | IgG-1.314 | 1.5 | - | 0.1833 | - | 0.0167 | - | 0.0167 | - | |
| Beta-lactamase domain protein | B9XAB6 | 26.0% identity with allergen Eur m 4.0101; E = 1.3 | 186/1.25 | IgG-1.356; IgE-0.941; IgA-1.096 | 1.54 | 0.0022 | 0.0714 | - | - | 0.0022 | 0.0325 | 0.0087 | |
| Acyl_transf_3 domain-containing protein | R6MBA2 | 27.0% identity with allergen Can f 7; E = 0.91 | 210/1.36 | IgG-1.292 | 1.52 | 0.0104 | 0.1013 | - | 0.0026 | - | 0.0208 | 0.0208 | |
| Cyclopropane-fatty-acyl-phospholipid synthase | Q1G9E5 | no matches | 184/1.55 | IgG-1.286; IgA-1.262 | 1.25 | 0.0102 | 0.0738 | - | 0.0051 | - | 0.0153 | 0.0102 | |
| Pyridine nucleotide-disulfide oxidoreductase class-II | A0A0D9MGW0 | 38.0% identity with putative allergen Ory s 3; E = 1.3 | 108/1.22 | IgG-1.787 | 0.93 | - | 0.0950 | - | 0.0059 | - | 0.0148 | 0.0178 | |
| ABC transporter related | R7QYF5 | no matches | 104/1.48 | IgG-1.304 | 1.44 | - | 0.1541 | - | - | - | 0.0077 | 0.0154 | |
| Thymidylate kinase | Q9CIG4 | no matches | 58/1.23 | IgG-1.324; IgE-1.049 | 1.13 | - | 0.0333 | - | - | - | 0.0500 | - | |
| Glyceraldehyde 3-phosphate dehydrogenase | K2HVL5 | 41.4% identity (71.7% similar) with allergen Tri a 34.0101; E < 1 ×10−7 | 101/1.52 | IgG-1.252; IgA-1.257 | 0.83 | 0.0667 | - | - | - | - | 0.0333 | ||
| ABC transporter protein | E3VVN9 | no matches | 328/1.64 | IgG-1.197; IgE-0.951; IgA-1.054 | 1.27 | 0.0028 | 0.1723 | - | 0.0028 | 0.0014 | 0.0153 | 0.0111 | |
| Elongation factor G | Q1GBM0 | 24.4% identity (52.6% similar) with allergen Phl p 5; E = 0.67; score 0.21 | 234/1.87 | IgG-1.495; IgA-1.246 | 1.18 | 0.0029 | 0.0533 | - | 0.0029 | 0.0029 | 0.0130 | 0.0101 | |
| Enolase | D8FPZ1 | 50.2% identity (76.3% similar) with allergen Rho m 1; E < 1 ×10−7; score 0.75 | 155//1.2 | IgG-1.105; IgA-0.988 | 1.03 | - | - | - | - | - | - | - | |
| Carboxylate-amine ligase | A0A0U3CLC8 | 31.6% identity (55.7% similar) with alergen Fel d 3; E = 0.59; score: 0.87 | 200/1.86 | IgG- 1.281; IgE-1.296 | 1.26 | 0.0048 | 0.0692 | - | 0.0072 | - | 0.0167 | 0.0095 | |
| 60 kDa chaperonin (Chaperonin GroEL) | A0A0E3CQW5 | 26.5% identity (57.6% similar) with alergen Cra g 1.0102; E < 1 ×10−3; score: 0.88 | 212/1.73 | IgG-1.237; IgE-0.972; IgA-1.184 | 0.78 | 0.0074 | 0.0423 | 0.0167 | 0.0018 | - | 0.0037 | 0.0037 | |
| UPF0756 membrane protein | B3WE66 | 44.0% identity with allergen Bla g 1; E = 0.13 | 108/1.45 | IgA-1.048 | 1.48 | 0.0327 | - | - | - | 0.0196 | - | ||
| 30S ribosomal protein S1 | Q1GAK0 | no matches; score:0.77 to putative allergenes | 83/1.27 | IgG-1.265; IgE-0.906; IgA-0.958 | 1.08 | 0.0025 | 0.0524 | - | - | 0.0025 | 0.0175 | 0.0125 | |
| Lipoprotein | K0MV23 | no matchess; core: 0.71 to putative allergenes | 133/1.83 | IgG-1.045; IgA-1.096 | 1.09 | 0.1055 | - | - | - | 0.0145 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogrodowczyk, A.M.; Jeż, M.; Wróblewska, B. The Manifold Bioactivity and Immunoreactivity of Microbial Proteins of Cow and Human Mature Milk in Late Lactation. Animals 2022, 12, 2605. https://doi.org/10.3390/ani12192605
Ogrodowczyk AM, Jeż M, Wróblewska B. The Manifold Bioactivity and Immunoreactivity of Microbial Proteins of Cow and Human Mature Milk in Late Lactation. Animals. 2022; 12(19):2605. https://doi.org/10.3390/ani12192605
Chicago/Turabian StyleOgrodowczyk, Anna Maria, Maja Jeż, and Barbara Wróblewska. 2022. "The Manifold Bioactivity and Immunoreactivity of Microbial Proteins of Cow and Human Mature Milk in Late Lactation" Animals 12, no. 19: 2605. https://doi.org/10.3390/ani12192605
APA StyleOgrodowczyk, A. M., Jeż, M., & Wróblewska, B. (2022). The Manifold Bioactivity and Immunoreactivity of Microbial Proteins of Cow and Human Mature Milk in Late Lactation. Animals, 12(19), 2605. https://doi.org/10.3390/ani12192605







