Behavioural Traits in Bos taurus Cattle, Their Heritability, Potential Genetic Markers, and Associations with Production Traits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
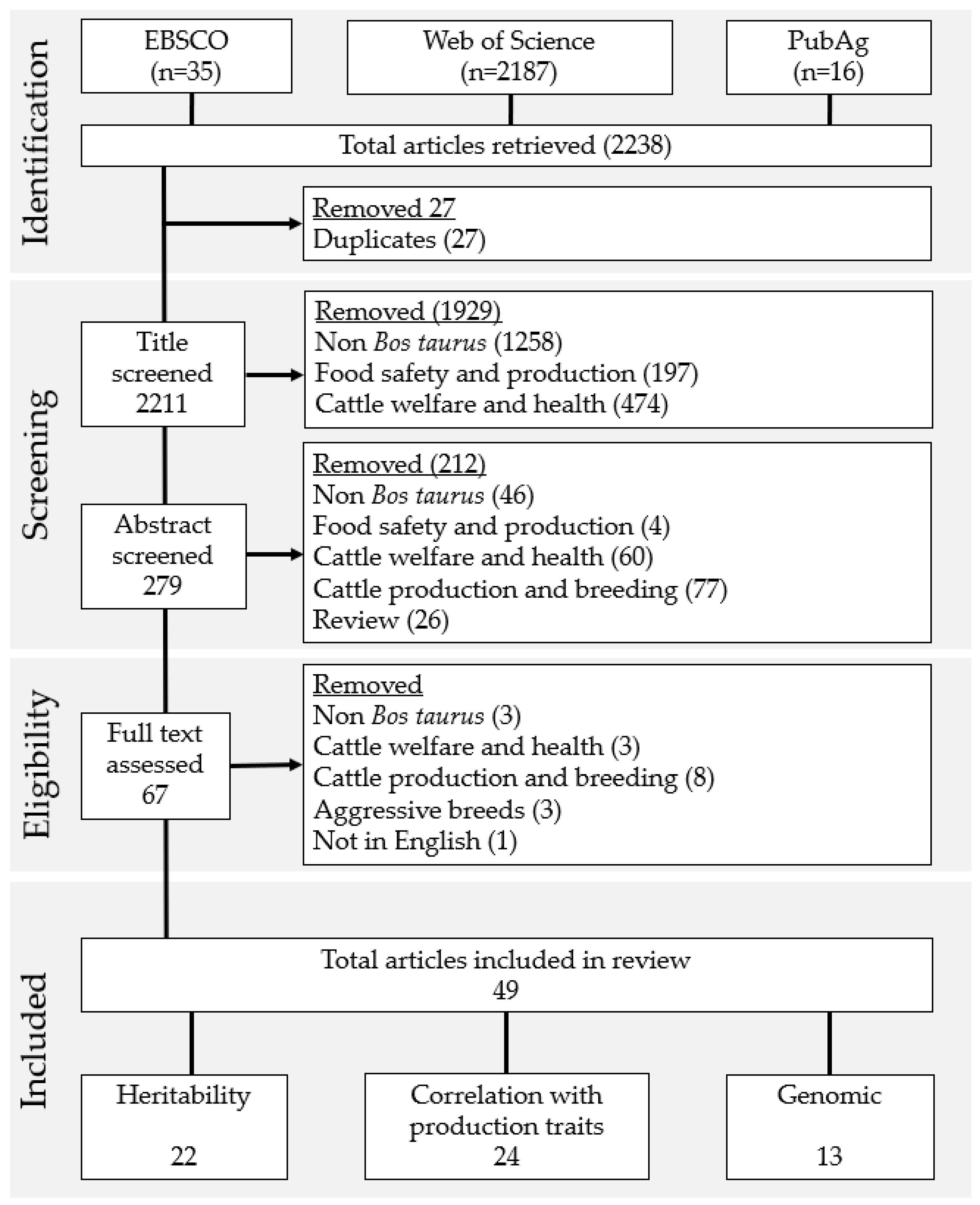
2.1. Search Strategy
2.2. Assessment and Selection of Papers Returned by the Search
2.3. Synthesis of Results
3. Results and Discussion
3.1. Study Selection
3.2. Behavioural Traits
3.2.1. Temperament, Disposition and/or Docility
3.2.2. Aggression
3.2.3. Chute Score (CS)
3.2.4. Flight Speed (FS)
3.2.5. Milking Temperament
3.2.6. Non-Restrained Behavioural Measures
3.2.7. Restrained Behavioural Measures
3.3. Heritability
3.3.1. Heritability of Temperament Disposition and Docility
3.3.2. Heritability of Aggression
3.3.3. Heritability of Chute Score
3.3.4. Heritability of Flight Speed
3.3.5. Heritability of Milking Temperament
3.3.6. Heritability of Non-Restrained Behavioural Measures
3.3.7. Heritability of Restrained Behavioural Measures
3.4. Genomics Background
3.5. Production Traits Related to Behaviour
3.5.1. Intake, Bodyweight and Growth
3.5.2. Carcass Traits
3.5.3. Fertility and Milk Production
3.6. Limitations of the Review and Search Protocol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheu, A.; Powell, A.; Bollongino, R.; Vigne, J.-D.; Tresset, A.; Çakırlar, C.; Benecke, N.; Burger, J. The genetic prehistory of domesticated cattle from their origin to the spread across Europe. BMC Genet. 2015, 16, 54. [Google Scholar] [CrossRef]
- Titterington, F.; Morrison, S.; Lively, F.; Wylie, A.; Gordon, A.; Browne, M. An analysis of Northern Ireland farmers’ experiences of using a target-driven beef heifer growth management plan and development of an empirical model leading to the launch of a decision support tool to promote first calving of beef heifers at 24 months. Agric. Syst. 2015, 132, 107–120. [Google Scholar] [CrossRef]
- Troy, D.J.; Kerry, J. Consumer perception and the role of science in the meat industry. Meat Sci. 2010, 86, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.; Hocquette, J.-F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, K.J.; Deboy, G.; Field, W.E.; Albright, J.L. Bull-related incidents: Their prevalence and nature. J. Agromed. 2009, 14, 357–369. [Google Scholar] [CrossRef]
- Fraser-Williams, A.P.; McIntyre, K.M.; Westgarth, C. Are cattle dangerous to walkers? A scoping review. Inj. Prev. 2016, 22, 437–441. [Google Scholar] [CrossRef]
- Titterington, F.M.; Knox, R.; Buijs, S.; Lowe, D.E.; Morrison, S.J.; Lively, F.O.; Shirali, M. Human–Animal Interactions with Bos taurus Cattle and Their Impacts on On-Farm Safety: A Systematic Review. Animals 2022, 12, 776. [Google Scholar] [CrossRef]
- Burrow, H.M. Measurements of temperament and their relationships with performance traits of beef cattle. Anim. Breed. Abstr. 1997, 65, 477–495. [Google Scholar]
- Adamczyk, K.; Pokorska, J.; Makulska, J.; Earley, B.; Mazurek, M. Genetic analysis and evaluation of behavioural traits in cattle. Livest. Sci. 2013, 154, 1–12. [Google Scholar] [CrossRef]
- Burrow, H.; Corbet, N. Genetic and environmental factors affecting temperament of zebu and zebu-derived beef cattle grazed at pasture in the tropics. Aust. J. Agric. Res. 2000, 51, 155–162. [Google Scholar] [CrossRef]
- Norris, D.; Ngambi, J.; Mabelebele, M.; Alabi, O.; Benyi, K. Genetic selection for docility: A review. J. Anim. Plant Sci. 2014, 24, 13–18. [Google Scholar]
- Haskell, M.J.; Simm, G.; Turner, S.P. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 2014, 5, 368. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Bettany-Saltikov, J. Learning how to undertake a systematic review: Part 2. Nurs. Stand. 2010, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.; Van Reenen, C. Animal behavior and well-being symposium: Interaction between coping style/personality, stress, and welfare: Relevance for domestic farm animals. J. Anim. Sci. 2016, 94, 2284–2296. [Google Scholar] [CrossRef]
- Rauw, W.M.; Johnson, A.K.; Gomez-Raya, L.; Dekkers, J. A hypothesis and review of the relationship between selection for improved production efficiency, coping behavior, and domestication. Front. Genet. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, A.B.; Oliveira, H.R.; Chen, S.-Y.; Miller, S.P.; Marchant-Forde, J.N.; Grigoletto, L.; Brito, L.F. A Systematic Review of Genomic Regions and Candidate Genes Underlying Behavioral Traits in Farmed Mammals and Their Link with Human Disorders. Animals 2021, 11, 715. [Google Scholar] [CrossRef]
- Fallahi, S. Behavioral genetics in Cattle-a review. J. Livest. Sci. 2019, 10, 102–108. [Google Scholar] [CrossRef]
- Lawlor, T. Genomic selection in the dairy industry: Excitement, challenges, and future directions. J. Anim. Sci. 2019, 97, 53–54. [Google Scholar] [CrossRef]
- Salvin, H.E.; Lees, A.M.; Cafe, L.M.; Colditz, I.G.; Lee, C. Welfare of beef cattle in Australian feedlots: A review of the risks and measures. Anim. Prod. Sci. 2020, 60, 1569–1590. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Interaction between stress hormones and phagocytic cells and its effect on the health status of dairy cows: A review. Vet. World 2020, 13, 1837. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Brito, L.F.; Alvarenga, A.B.; Wang, Y. Incorporating temperament traits in dairy cattle breeding programs: Challenges and opportunities in the phenomics era. Anim. Front. 2020, 10, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Brade, W.; Brade, E. Aspects of behavioural genetics in cattle 2nd communication. Ber. Über Landwirtsch. 2017, 95, 1. [Google Scholar]
- Dias Barbosa Silveira, I.; Fischer, V.; Dorneles Soares, G.J. Relation between genotype and temperament of grazing steers on meat quality. Rev. Bras. Zootec.-Braz. J. Anim. Sci. 2006, 35, 519–526. [Google Scholar]
- Eusebi, P.G.; Cortés, O.; Carleos, C.; Dunner, S.; Cañon, J. Detection of selection signatures for agonistic behaviour in cattle. J. Anim. Breed. Genet. 2018, 135, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, P.; Sevane, N.; Cortés, O.; Contreras, E.; Cañon, J.; Dunner, S. Aggressive behavior in cattle is associated with a polymorphism in the MAOA gene promoter. Anim. Genet. 2020, 51, 14–21. [Google Scholar] [CrossRef]
- Eusebi, P.G.; Sevane, N.; O’Rourke, T.; Pizarro, M.; Boeckx, C.; Dunner, S. Gene expression profiles underlying aggressive behavior in the prefrontal cortex of cattle. BMC Genom. 2021, 22, 245. [Google Scholar] [CrossRef] [PubMed]
- Ramey, H.R.; Decker, J.E.; McKay, S.D.; Rolf, M.M.; Schnabel, R.D.; Taylor, J.F. Detection of selective sweeps in cattle using genome-wide SNP data. BMC Genom. 2013, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Pelayo, R.; Solé, M.; Sánchez, M.; Molina, A.; Valera, M. Behavioural linear standardized scoring system of the Lidia cattle breed by testing in herd: Estimation of genetic parameters. J. Anim. Breed. Genet. 2016, 133, 414–421. [Google Scholar] [CrossRef]
- Menéndez-Buxadera, A.; Cortés, O.; Cañon, J. Genetic (co) variance and plasticity of behavioural traits in Lidia bovine breed. Ital. J. Anim. Sci. 2017, 16, 208–216. [Google Scholar] [CrossRef]
- Silva, B.; Gonzalo, A.; Cañón, J. Genetic parameters of aggressiveness, ferocity and mobility in the fighting bull breed. Anim. Res. 2006, 55, 65–70. [Google Scholar] [CrossRef]
- Parham, J.T.; Tanner, A.E.; Wahlberg, M.L.; Grandin, T.; Lewis, R.M. Subjective methods to quantify temperament in beef cattle are insensitive to the number and biases of observers. Appl. Anim. Behav. Sci. 2019, 212, 30–35. [Google Scholar] [CrossRef]
- Weaber, R.; Taxis, T.; Shafer, W.; Berger, L.; Faulkner, D.; Rolf, M.; Dow, D.; Taylor, J.; Lorenzen, C. Heritabilities, genetic and phenotypic correlations among Warner-Bratzler shear force and repeated objective measurements of temperament in fed cattle. J. Dairy Sci. 2010, 93, 744–745. [Google Scholar]
- Benhajali, H.; Boivin, X.; Sapa, J.; Pellegrini, P.; Boulesteix, P.; Lajudie, P.; Phocas, F. Assessment of different on-farm measures of beef cattle temperament for use in genetic evaluation. J. Anim. Sci. 2010, 88, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, S.; Stookey, J.; Winkelman-Sim, D.; Waltz, C.; Plante, Y.; Buchanan, F. A QTL study of cattle behavioral traits in embryo transfer families. J. Hered. 2001, 92, 290–292. [Google Scholar] [CrossRef]
- Garza-Brenner, E.; Sifuentes-Rincón, A.; Randel, R.; Paredes-Sánchez, F.; Parra-Bracamonte, G.; Arellano Vera, W.; Rodríguez Almeida, F.; Segura Cabrera, A. Association of SNPs in dopamine and serotonin pathway genes and their interacting genes with temperament traits in Charolais cows. J. Appl. Genet. 2017, 58, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gauly, M.; Mathiak, H.; Hoffmann, K.; Kraus, M.; Erhardt, G. Estimating genetic variability in temperamental traits in German Angus and Simmental cattle. Appl. Anim. Behav. Sci. 2001, 74, 109–119. [Google Scholar] [CrossRef]
- Takeda, K.; Uchida, H.; Inoue, K. Genetic relationships between temperament of calves at auction and carcass traits in Japanese Black cattle. Anim. Sci. J. 2017, 88, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Carlström, C.; Strandberg, E.; Pettersson, G.; Johansson, K.; Stålhammar, H.; Philipsson, J. Genetic associations of teat cup attachment failures, incomplete milkings, and handling time in automatic milking systems with milkability, temperament, and udder conformation. Acta Agric. Scand. Sect. A—Anim. Sci. 2016, 66, 75–83. [Google Scholar] [CrossRef]
- Hiendleder, S.; Thomsen, H.; Reinsch, N.; Bennewitz, J.; Leyhe-Horn, B.; Looft, C.; Xu, N.; Medjugorac, I.; Russ, I.; Kühn, C. Mapping of QTL for body conformation and behavior in cattle. J. Hered. 2003, 94, 496–506. [Google Scholar] [CrossRef]
- Varona, L.; Moreno, C.; Altarriba, J. Genetic correlation of longevity with growth, post-mortem, docility and some morphological traits in the Pirenaica beef cattle breed. Animal 2012, 6, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Erbe, M.; Bapst, B.; Bieber, A.; Simianer, H. Estimation of genetic parameters for novel functional traits in Brown Swiss cattle. J. Dairy Sci. 2013, 96, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Erbe, M.; Seefried, F.; Gredler, B.; Bapst, B.; Bieber, A.; Simianer, H. Accuracy of direct genomic values for functional traits in Brown Swiss cattle. J. Dairy Sci. 2014, 97, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Stephansen, R.; Fogh, A.; Norberg, E. Genetic parameters for handling and milking temperament in Danish first-parity Holstein cows. J. Dairy Sci. 2018, 101, 11033–11039. [Google Scholar] [CrossRef]
- Vallée, A.; Breider, I.; van Arendonk, J.; Bovenhuis, H. Genetic parameters for large-scale behavior traits and type traits in Charolais beef cows. J. Anim. Sci. 2015, 93, 4277–4284. [Google Scholar] [CrossRef]
- Le Neindre, P.; Trillat, G.; Sapa, J.; Ménissier, F.; Bonnet, J.; Chupin, J. Individual differences in docility in Limousin cattle. J. Anim. Sci. 1995, 73, 2249–2253. [Google Scholar] [CrossRef]
- Reinhardt, C.; Busby, W.; Corah, L. Relationship of various incoming cattle traits with feedlot performance and carcass traits. J. Anim. Sci. 2009, 87, 3030–3042. [Google Scholar] [CrossRef]
- Beckman, D.; Enns, R.; Speidel, S.; Brigham, B.; Garrick, D. Maternal effects on docility in Limousin cattle. J. Anim. Sci. 2007, 85, 650–657. [Google Scholar] [CrossRef][Green Version]
- Walkom, S.; Jeyaruban, M.G.; Tier, B.; Johnston, D. Genetic analysis of docility score of Australian Angus and Limousin cattle. Anim. Prod. Sci. 2016, 58, 213–223. [Google Scholar] [CrossRef]
- Phocas, F.; Boivin, X.; Sapa, J.; Trillat, G.; Boissy, A.; Le Neindre, P. Genetic correlations between temperament and breeding traits in Limousin heifers. Anim. Sci. 2006, 82, 805–811. [Google Scholar] [CrossRef]
- Vallée, A.; Daures, J.; Van Arendonk, J.; Bovenhuis, H. Genome-wide association study for behavior, type traits, and muscular development in Charolais beef cattle. J. Anim. Sci. 2016, 94, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vazquez, J.A.; Spangler, M.L. Genetic parameters for docility, weaning weight, yearling weight, and intramuscular fat percentage in Hereford cattle. J. Anim. Sci. 2016, 94, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, S.; Brandt, H.; König, S.; Erhardt, G.; Gauly, M. Temperament traits of beef calves measured under field conditions and their relationships to performance. J. Anim. Sci. 2010, 88, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Navajas, E.; Hyslop, J.; Ross, D.; Richardson, R.; Prieto, N.; Bell, M.; Jack, M.; Roehe, R. Associations between response to handling and growth and meat quality in frequently handled Bos taurus beef cattle. J. Anim. Sci. 2011, 89, 4239–4248. [Google Scholar] [CrossRef]
- Cafe, L.; Robinson, D.L.; Ferguson, D.; McIntyre, B.L.; Geesink, G.; Greenwood, P. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef]
- White, K.; Bormann, J.M.; Olson, K.; Jaeger, J.; Johnson, S.; Downey, B.; Grieger, D.M.; Waggoner, J.; Moser, D.; Weaber, R.L. Phenotypic relationships between docility and reproduction in Angus heifers. J. Anim. Sci. 2016, 94, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Bruno, K.; Vanzant, E.; Vanzant, K.; McLeod, K. Relationships of a novel objective chute score and exit velocity with growth performance of receiving cattle. J. Anim. Sci. 2016, 94, 4819–4831. [Google Scholar] [CrossRef] [PubMed]
- Glenske, K.; Prinzenberg, E.-M.; Brandt, H.; Gauly, M.; Erhardt, G. A chromosome-wide QTL study on BTA29 affecting temperament traits in German Angus beef cattle and mapping of DRD4. Animal 2011, 5, 195–197. [Google Scholar] [CrossRef]
- Lindholm-Perry, A.; Kuehn, L.; Freetly, H.; Snelling, W. Genetic markers that influence feed efficiency phenotypes also affect cattle temperament as measured by flight speed. Anim. Genet. 2015, 46, 60–64. [Google Scholar] [CrossRef]
- Rolfe, K.; Snelling, W.; Nielsen, M.; Freetly, H.; Ferrell, C.; Jenkins, T. Genetic and phenotypic parameter estimates for feed intake and other traits in growing beef cattle, and opportunities for selection. J. Anim. Sci. 2011, 89, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Jack, M.; Lawrence, A. Precalving temperament and maternal defensiveness are independent traits but precalving fear may impact calf growth. J. Anim. Sci. 2013, 91, 4417–4425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nkrumah, J.; Crews Jr, D.; Basarab, J.; Price, M.; Okine, E.; Wang, Z.; Li, C.; Moore, S.S. Genetic and phenotypic relationships of feeding behavior and temperament with performance, feed efficiency, ultrasound, and carcass merit of beef cattle. J. Anim. Sci. 2007, 85, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Sewalem, A.; Miglior, F.; Kistemaker, G.J. Genetic parameters of milking temperament and milking speed in Canadian Holsteins. J. Dairy Sci. 2011, 94, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Oliveira, H.R.; Schenkel, F.S.; Pedrosa, V.B.; Melka, M.G.; Brito, L.F. Using imputed whole-genome sequence variants to uncover candidate mutations and genes affecting milking speed and temperament in Holstein cattle. J. Dairy Sci. 2020, 103, 10383–10398. [Google Scholar] [CrossRef] [PubMed]
- Breuer, K.; Hemsworth, P.H.; Barnett, J.L.; Matthews, L.R.; Coleman, G.J. Behavioural response to humans and the productivity of commercial dairy cows. Appl. Anim. Behav. Sci. 2000, 66, 273–288. [Google Scholar] [CrossRef]
- Friedrich, J.; Brand, B.; Ponsuksili, S.; Graunke, K.L.; Langbein, J.; Knaust, J.; Kühn, C.; Schwerin, M. Detection of genetic variants affecting cattle behaviour and their impact on milk production: A genome-wide association study. Anim. Genet. 2016, 47, 12–18. [Google Scholar] [CrossRef]
- Aierqing, S.; Nakagawa, A.; Bungo, T. Association between temperament and polymorphisms of CRH and leptin in Japanese Black Cattle. J. Adv. Vet. Anim. Res. 2020, 7, 1. [Google Scholar] [CrossRef]
- Neave, H.W.; Costa, J.H.; Weary, D.M.; von Keyserlingk, M.A. Personality is associated with feeding behavior and performance in dairy calves. J. Dairy Sci. 2018, 101, 7437–7449. [Google Scholar] [CrossRef]
- Gutiérrez-Gil, B.; Ball, N.; Burton, D.; Haskell, M.; Williams, J.L.; Wiener, P. Identification of quantitative trait loci affecting cattle temperament. J. Hered. 2008, 99, 629–638. [Google Scholar] [CrossRef]
- Gauly, M.; Mathiak, H.; Erhardt, G. Genetic background of behavioural and plasma cortisol response to repeated short-term separation and tethering of beef calves. J. Anim. Breed. Genet. 2002, 119, 379–384. [Google Scholar] [CrossRef]
- Bacher, L.; Prieur, V.; Lardy, R.; Boivin, X. Does the avoidance distance test at the feed barrier have scientific validity for evaluating reactivity to humans in Limousin breeding bulls? Livest. Sci. 2021, 249, 104535. [Google Scholar] [CrossRef]
- Visscher, P.M.; Hill, W.G.; Wray, N.R. Heritability in the genomics era—Concepts and misconceptions. Nat. Rev. Genet. 2008, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Oltenacu, P.; Van Vleck, L.D.; Erb, H.; Smith, R. Heritabilities of and genetic correlations among six health problems in Holstein cows. J. Dairy Sci. 1989, 72, 180–186. [Google Scholar] [CrossRef]
- Wethal, K.; Svendsen, M.; Heringstad, B. Are farmer assessed temperament, milking speed, and leakage genetically the same traits in automatic milking systems and traditional milking systems? J. Dairy Sci. 2020, 103, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Recio, O.; Pryce, J.; Haile-Mariam, M.; Hayes, B. Incorporating heifer feed efficiency in the Australian selection index using genomic selection. J. Dairy Sci. 2014, 97, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Lühken, G.; Glenske, K.; Brandt, H.; Erhardt, G. Genetic variation in monoamine oxidase A and analysis of association with behaviour traits in beef cattle. J. Anim. Breed. Genet. 2010, 127, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kutzer, T.; Steilen, M.; Gygax, L.; Wechsler, B. Habituation of dairy heifers to milking routine—Effects on human avoidance distance, behavior, and cardiac activity during milking. J. Dairy Sci. 2015, 98, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- Elolimy, A.A.; Abdelmegeid, M.K.; McCann, J.C.; Shike, D.W.; Loor, J.J. Residual feed intake in beef cattle and its association with carcass traits, ruminal solid-fraction bacteria, and epithelium gene expression. J. Anim. Sci. Biotechnol. 2018, 9, 67. [Google Scholar] [CrossRef]
- Cziszter, L.T.; Gavojdian, D.; Neamt, R.; Neciu, F.; Kusza, S.; Ilie, D.-E. Effects of temperament on production and reproductive performances in Simmental dual-purpose cows. J. Vet. Behav. 2016, 15, 50–55. [Google Scholar] [CrossRef]
- Haile-Mariam, M.; Bowman, P.; Goddard, M. Genetic parameters of fertility traits and their correlation with production, type, workability, liveweight, survival index, and cell count. Aust. J. Agric. Res. 2004, 55, 77–87. [Google Scholar] [CrossRef]
- Zaborski, D.; Grzesiak, W.; Szatkowska, I.; Dybus, A.; Muszynska, M.; Jedrzejczak, M. Factors affecting dystocia in cattle. Reprod. Domest. Anim. 2009, 44, 540–551. [Google Scholar] [PubMed]
- Titterington, F.; Lively, F.; Dawson, S.; Gordon, A.; Morrison, S. The effects of breed, month of parturition and sex of progeny on beef cow fertility using calving interval as a measure. Adv. Anim. Biosci. 2017, 8, s67–s71. [Google Scholar] [CrossRef]
- Sawa, A.; Bogucki, M.; Neja, W.; Krężel-Czopek, S. Effect of temperament on performance of primiparous dairy cows. Ann. Anim. Sci. 2017, 17, 863–872. [Google Scholar] [CrossRef]
- Klaas, I.C.; Enevoldsen, C.; Ersbøll, A.K.; Tölle, U. Cow-related risk factors for milk leakage. J. Dairy Sci. 2005, 88, 128–136. [Google Scholar] [CrossRef]
| Population (Bovine) | Exposure (Genetics) | Outcome (Behavioural) | |
|---|---|---|---|
| Cattle, | QTL, | Temperament, | Docility, |
| Cow, | “Gene”, | Aggressi *, | Fear *, |
| “Steer”, | Genetic *, | “Chute Score”, | “Crush Score”, |
| Heifer, | Heritab *, | Flight, | “Exit Time”, |
| Bull, | Marker, | “Exit Speed”, | “Exit Score”, |
| Dairy, | SNP, | “Exit Velocity”, | Excit *, |
| Beef, | GWAS, | “Movement Measuring Device”, | |
| Herd, | Genomic * | “Strain Gauge”, | “Coping Style”, |
| “Bos taurus” | Boldness, | Personality, | |
| Proactive, | Reactive | ||
| Trait | Heritability ± SE | Breed | n | Age at Test | Reference |
|---|---|---|---|---|---|
| Temperament, disposition and/or docility | |||||
| Temperament (CMS) * | 0.09 (0.01) | Dairy breeds | 1,872,979 | First parity cows | [74] |
| Farmer assessed temperament | 0.10 (0.01) | Holstein | 126,614 | Mature cows | [44] |
| Temperament (AMS) † | 0.05 (0.01) | Dairy breeds | 72,683 | First parity cows | [74] |
| Handling temperament | 0.11 (0.02) | Holstein | 8108 | Mature cows | [44] |
| Temperament | 0.11–0.12 (≤0.05) | Japanese black | 7897 | 6–9 months | [38] |
| Temperament | 0.26 (0.01) | Dairy | 843 | Heifers | [75] |
| Docility | 0.19 (0.03) | Pirenaica | 2412 | Mature cows | [41] |
| Docility | 0.29–0.38 (≤0.03) | Limousin | 23,453 | Weaning | [48] |
| Docility | 0.21 (<0.01) | Angus | 50,935 | [49] | |
| Docility | 0.46 (<0.01) | Limousin | 50,930 | [49] | |
| Chute score | |||||
| Chute score | 0.29 (0.02) | Hereford | 25,037 | Weaning | [52] |
| Aggression | |||||
| During separation | 0 (0.05) | Simmental | 140 | Heifers | [37] |
| During gestation | 0.06 (0.02) | Charolais | 5954 | Mature cows | [45] |
| At parturition | 0.19 (0.05) | Charolais | 5881 | Mature cows | [45] |
| Aggressiveness | 0.08 (0.02) | Limousin | 2781 | Heifers | [50] |
| Milking temperament | |||||
| Milking temperament | 0.13 (0.01) | Dairy cows | 1,940,092 | First lactation | [63] |
| Milking temperament | 0.04 (0.04) | Brown Swiss | 2259 | Mature cows | [42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titterington, F.M.; Knox, R.; Morrison, S.J.; Shirali, M. Behavioural Traits in Bos taurus Cattle, Their Heritability, Potential Genetic Markers, and Associations with Production Traits. Animals 2022, 12, 2602. https://doi.org/10.3390/ani12192602
Titterington FM, Knox R, Morrison SJ, Shirali M. Behavioural Traits in Bos taurus Cattle, Their Heritability, Potential Genetic Markers, and Associations with Production Traits. Animals. 2022; 12(19):2602. https://doi.org/10.3390/ani12192602
Chicago/Turabian StyleTitterington, Frances Margaret, Rachel Knox, Steven James Morrison, and Masoud Shirali. 2022. "Behavioural Traits in Bos taurus Cattle, Their Heritability, Potential Genetic Markers, and Associations with Production Traits" Animals 12, no. 19: 2602. https://doi.org/10.3390/ani12192602
APA StyleTitterington, F. M., Knox, R., Morrison, S. J., & Shirali, M. (2022). Behavioural Traits in Bos taurus Cattle, Their Heritability, Potential Genetic Markers, and Associations with Production Traits. Animals, 12(19), 2602. https://doi.org/10.3390/ani12192602







