2. Materials and Methods
2.1. Animals and Procedures
The pigs used in the present experiment were cared for in accordance with the guidelines established in the Official Mexican Regulations on Animal Care [
9] and approved by the Ethical Committee of Institute of Agricultural Sciences at Universidad Autónoma de Baja California. The experiment was conducted during summer time of the year 2018 in northwestern Mexico, where ambient temperature usually fluctuates from 24 to 45 °C every day. Eighteen crossbred pigs (Landrace x Hampshire x Duroc) with an initial body weight of 36.0 ± 3.5 kg were used. Pigs were individually housed in metabolism pens (1.2 m wide, 1.2 m long, and 1.0 m high) with elevated iron-mesh floor, equipped with a stainless-steel self-feeder and a nipple water drinker. The AT and relative humidity (RH) inside the room was recorded during the study with the aid of a Higrothermograph (Thermotracker HIGRO; iButtonLink LLC, Whitewater, WI, USA) set to record those values every 15 min. The AT and RH data were used to calculate the heat index, according to the equation of Steadman [
10], modified by Rothfusz [
11]. All pigs were fed ad libitum a diet formulated with wheat, soybean meal, crystalline L-Lys HCl, L-Thr, DL-Met, and L-Val, as well as vitamins and minerals to meet current requirements [
12] for the 25 to 50 kg pigs (
Table 1). Additionally, purified water was provided ad libitum during the experiment.
The pigs were randomly assigned to one of three treatment groups (n = 6): (1) thermoneutral (TN), (2) 2 days exposure to HS (short, 2dHS), and (3) 7 days exposure to HS (long, 7dHS). The 2 days and 7 days exposure to HS were chosen because they represent the acute response and the start of the acclimating phase of the pigs to HS [
7,
8]. The experiment lasted 24 days, according to the following protocol. During the first 16 days, all pigs were housed inside an air-conditioned room with the thermostat set at 22 ± 2 °C. On day 16, the TN pigs were euthanized after spending 16 days under TN conditions, and this was defined as the TN treatment. Then, at 23.00 h on day 16, the cooling system was turned off; thus, the remaining 12 pigs were exposed to natural high AT conditions (natural HS) during a 2 day- (2dHS) or a 7 day- (7dHS) period. The 2dHS pigs were euthanized on day 19, and the 7dHS pigs were euthanized on day 24 after 2 or 7 days of natural exposure to HS, respectively. All pigs were weighed on days 1 and 16, and the remaining pigs at day 19 and day 24 of the trial to calculate weight gain for the TN, 2dHS, and 7dHS, periods, respectively. Feed intake and feed conversion were calculated for the same periods.
2.2. Collection of Samples
Two blood samples (approx. 6 mL each) were collected from each pig after overnight fasting (approx. 10 h) by venipuncture of the jugular vein into disposable Vacutainer® tubes containing ethylenediaminetetraacetic acid, EDTA (BD Vacutainer; Franklin Lakes, NJ, USA) right before they were euthanized. Pigs were euthanized by exsanguination after electrical stunning using a stunner (Best & Donovan, Cincinnati, OH, USA) with a voltage output of 620V during 2 to 3 s, immediately bled, and the carcasses were quickly eviscerated. Mucosal samples (approx. 500 mg) scratched from duodenum, middle jejunum, and ileum were collected into 2.0 mL micro tubes after flushing them with saline physiological solution. All samples were instantly frozen in liquid nitrogen and stored at −80 °C until mRNA expression analysis. Additionally, segments (approx. 5 cm long) from duodenum, middle jejunum, and ileum were rinsed in saline physiological solution and fixed in 10% formaldehyde solution buffered until histological analysis.
2.3. Blood Analyses
Hemogram tests were performed in one set of blood samples using a BC 2800 Vet auto hematology analyzer (Mindray Bio-Medical Electronics Co. Shenzen, China). The parameters analyzed were: count of white blood cells, neutrophils, lymphocytes, hematocrit, red blood cells, hemoglobin, mean corpuscular volume, and platelet count.
The other set of blood samples were centrifuged at 1500× g by 10 min at 4 °C, and blood (serum) chemistry was analyzed in an Elan ATAC 8000 Chemistry Analyzer (ELITech Clinical Data Inc. Elan Diagnostics, Smithfield, RI, USA). The parameters analyzed with this equipment were glucose, cholesterol, triglycerides, urea nitrogen, calcium, phosphorus, total protein, albumin, globulin, plasma proteins, aspartate NH2-transferase, alkaline phosphatase, γ-glutamyl transpeptidase, and amylase.
2.4. Intestinal Analyses
Intestinal histo-morphology, mucin content, and apoptosis were analyzed in segments of duodenum, middle jejunum, and ileum fixed in formaldehyde. Samples were embedded into paraffin blocks and sectioned at 3 μm. For intestinal histo-morphology, the sections were stained with hematoxylin–eosin [
13], and the mucosal structure was observed at 40x magnification under a light microscope (Zeiss AxioStar HBO50; Zeiss, Germany). Microphotographs were obtained by a photographic camera (Canon, Tokyo, Japan) at a resolution of 18 megapixels. Villus height and crypt depth of ten well-oriented villi per section of the intestine were measured in pixels (using the pixels measured in one mm as reference) and analyzed using the software Image J2 [
14]. The system was calibrated using a spatial calibration process performed by changing the pixels of the image to a known value in microns.
For mucin content analysis, slides prepared with tissues sections of 3 μm from each sample were processed for carbohydrate histochemistry detection by using the periodic acid-Schiff’s reaction (PAS) and Alcian blue staining [
15]. Microphotographs were acquired by using a digital camera (Cannon DS-L1 digital; Japan) adapted to a microscope Axiostar (Zeiss AxioStar HBO50) at 40x magnification. Ten well-oriented villi and crypts from each sample were considered to calculate the percentage of cells with positive staining as the number of purple, magenta-purple, and blue-purple cells per slide. The number of cells was enumerated from 20 fields using the software ImageJ2.
For the quantitation of apoptotic cells, 3 μm thick sections were adhered to poly-Lysine covered slides (Corning, Corning, NY, USA) and treated for dewaxing. The commercial kit Annexin V-FITC (Sigma Aldrich, St. Louis, MI, USA) was used to stain apoptotic cells according to the manufacturer’s specifications. Samples were observed under an epifluorescence microscope, Axioscop HB50 (Zeiss, Oberkochen, Germany), at wavelengths of 395–415 nm. Microphotographs from each intestinal segment were obtained by using a digital camera (Cannon DS-L1 digital; Japan) adapted to the microscope. An area of 0.01 mm2 was standardized for the quantitation of apoptotic cells at each sample by using the software ImageJ2.
2.5. RNA Extraction and Reverse Transcription
Samples from intestinal mucosae were treated for total RNA extraction by Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to Méndez et al. [
16]. RNA was eluted into RNase-free water and stored at −80 °C. The concentration of total RNA was determined spectrophotometrically at 260 nm (Helios β, Thermo Electron Co., Rochester, NY, USA); purity of RNA was assessed by using the A260/A280 ratio, which ranged from 1.8 to 2.0 [
17]; and integrity of RNA was evaluated by gel electrophoresis on 1% agarose gels. All RNA samples had good quality with a 28S:18S rRNA ratio around 2.0:1 [
17]. Approximately 2 µg of total RNA were treated with 1 U of DNase I (1 U µL
−1; Invitrogen), and reverse transcription was performed. Concentration of DNA samples was quantified and diluted into a final concentration of 50 ng∙µL
−1.
2.6. Real Time qPCR
Specific primers for Tight Junction Protein-1 (TJP-1), occludin, claudin-2 mRNA, and the 18S rRNA gene were designed according to their published sequences at the Genbank (
Table 2). A housekeeping 18S rRNA gene was used as an endogenous control to normalize variations in mRNA because its expression is very stable [
18]. Before starting, end point PCR was carried out to standardize the amplification conditions for each pair of primers, and in order to confirm the specificity of the PCR products related to its mRNA, a sample of each PCR product was purified and sequenced at the Genewiz Inc. (South Plainfield, NJ, USA). Sequencing results revealed that the products for TJP-1, Occludin, Claudin-2, and 18S rRNA showed 100% homology with their corresponding expected sequences reported in GenBank.
Expression of TJP-1, Occludin, and Claudin-2 was estimated by quantitative PCR (qPCR) assays using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, Inc., Carlsbad, CA, USA) into a CFX96-RealTime System (Bio-Rad, Herefordshire, England), and results were analyzed with the software CFX Manager 3.0 (Bio-Rad). Every qPCR reaction contained 50 ng of cDNA, 0.5 µM of each primer, 12.5 µL of 2x SYBR green/ROX qPCR Master Mix, and nuclease-free water to complete a final volume of 25 µL. Conditions for qPCR amplification and quantification were an initial denaturing stage (95 °C for 1 min), followed by 40 cycles of amplification (denaturing at 95 °C for 15 s; annealing at 56 °C for 15 s; and extension at 72 °C for 30 s), and a melting curve program (60 °C to 90 °C). Fluorescence was measured at the end of each cycle and every 0.5 °C during the melting program. Each sample was analyzed by duplicate, and 18S RNA was also amplified for each sample. The relative quantification of gene expression was analyzed by the 2
−ΔΔCt method [
19]; according to this method, the Ct values for each mRNA were corrected by the corresponding Ct value for its 18S RNA.
2.7. Statistical Analyses
Analyses of variance of the data were performed according to a Complete Randomized design; period was considered as a fixed effect, whereas pig and AT were considered as random effects. Performance, gene expression, intestinal histo-morphology, and hematological variables were analyzed. Three contrasts were constructed as follows: C1, TN vs. 2dHS; C2, TN vs. 7dHS; and C3, 2dHS vs. 7dHS. Daily variations in AT and RH data were also analyzed. Probability levels of p ≤ 0.05, and 0.05 < p ≤ 0.10 were defined as significant differences and tendencies, respectively.
4. Discussion
The effects of short- (2 d) and long-term (7 d) exposure of pigs to natural HS conditions on performance, hematologic parameters, and intestinal epithelium integrity of growing pigs, compared to pigs under TN conditions, were analyzed in the present study. The AT of pigs exposed to HS during the first 2 days (2dHS; 28.1 to 34.4 °C) and from d 3 to d 7 of HS (7dHS; 28.1 to 38.1 °C) was above the comfort zone of growing pigs [
20]. In contrast, the AT of pigs exposed to TN conditions (around 23 °C) and heat index (around 76) was within the comfort zone. Body temperature of pigs housed in the same room and exposed to AT similar to that of the 2dHS and 7dHS pigs ranged on average from 41.0 to 41.5 °C from 1300 to 2400 h every day compared to 39.1 °C of TN pigs. Hence, in agreement with previous reports [
3,
20,
21,
22], the AT recorded during both the 2dHS and 7dHS periods of the present experiment indicate that pigs were exposed to HS conditions.
Pigs exposed to HS reduce 50 to 80% of their voluntary feed intake during the first 24 h of exposure to HS [
5,
7], which causes a low or null body weight gain. As pigs become acclimated to HS, after 8 to 10 days of continuous exposure to high AT, their voluntary feed intake partially returns to levels before the onset of HS exposure [
3]. In the present experiment, pigs exposed to natural and fluctuating HS conditions reduced about 42% of the voluntary feed intake during the first two days of exposure in comparison with the TN pigs. This was associated with the 57% decrease in weight gain of 2dHS compared to TN pigs. However, the voluntary feed intake during the following 5 days of HS exposure was 35% lower than that of TN pigs, suggesting a partial feed intake recovery (11%) in the 7dHS pigs compared to the 2dHS pigs. Other authors [
1,
2,
3] reported similar results. Feed efficiency reduced 22% in 2dHS pigs compared to TN pigs, but it did not differ among 7dHS and TN. The depressed weight gain and feed efficiency during the first 2d of exposure to HS indicate that the animals could deviate some nutrients from growth to trigger the beginning of the acclimation response [
4]. In addition, it appears that 7dHS pigs used less of the ingested nutrients to fight HS than 2dHS pigs.
Feed intake and exposure to HS affect blood chemistry parameters. In the present experiment, although 2dHS pigs consumed 42% less feed, the serum concentration of glucose did not differ among 2dHS and TN pigs; in fact, serum glucose concentration relative to glucose intake was higher in 2dHS pigs than in TN pigs. This HS response appears to be related to alterations in absorption, metabolism, and endocrinology of pigs, as evidenced by the increased abundance of the glucose transporter SGLT1 and GLUT2 [
5,
23] and the increased glycogenolysis-related hepatic glucose production in HS pigs [
4]. On the other hand, Pearce et al. [
24] reported that both feed-restricted TN pigs and feed intake-depressed HS pigs reduced insulin serum concentration compared to ad libitum-fed TN pigs. Hypoinsulinemia is typical in animals with reduced feed intake [
4]. Hence, the relative high serum glucose concentration in 2dHS pigs may be explained by the higher intestinal absorption of glucose, increased hepatic glucose production, and reduced growth rate. In contrast, the lower glucose serum concentration in 7dHS pigs compared to TN pigs is likely explained by the prolonged reduced feed intake, but when compared to the 2dHS pigs, it may be explained by the higher growth rate (87%) of 7dHS pigs.
The 2-fold increment in the serum concentration of triglycerides in all HS pigs compared to TN pigs is in agreement with reports using pigs [
24] or chicks exposed to HS [
25]. The increment in post-absorptive serum concentration of triglycerides may reflect an increase in either the biosynthesis or the mobilization of adipose tissue (lipolysis), assuming triglycerides are exported by adipocytes and hepatocytes, which are the most relevant cells where biosynthesis occurs [
26]. Nonetheless, changes in lipid metabolism of HS pigs are highly complex and results are controversial. Pearce et al. [
24] reported that animals under energy intake restriction mobilize adipose tissue through a glucose-sparing mechanism to prioritize skeletal protein accretion. In the present experiment, HS pigs consumed 20 to 50% less energy than TN pigs. However, Schade [
27] found no difference in plasma triglycerides between pigs restricted to 65% or 90% of ad libitum feed intake. According to Baumgard and Rhoads [
28], hyperthermia in animals makes muscle to partially move from fatty acid oxidation to glycolysis to generate ATP. Hence, whether the increased serum triglyceride concentration in HS pigs was due to the reduced feed intake or to HS itself remains unclear; thus, further research is needed.
The serum concentration of urea N analyzed in the present experiment was 32% higher in 2dHS pigs than in TN pigs. Amino acid imbalance caused by either deficiency or excess of one or more dietary essential amino acids results in increased blood urea N, which is an indicator of protein utilization [
29]. We have shown that HS modifies the availability of some amino acids, such as Arg, Lys, Met, and Thr [
23,
30], by altering their absorptive and post-absorptive serum concentrations and their utilization, as indicated by the reduced myosin expression [
2] that might cause amino acid imbalance. Furthermore, it appears that HS affects the metabolism of protein in pigs by increasing the skeletal muscle catabolism [
4,
31], indicated by the increased serum concentration of 3-methyl-His [
23], a marker of muscle protein breakdown. Hence, post-absorptive amino acid imbalance, muscle protein synthesis, as well as the mobilization of amino acids from skeletal muscle seem to partially explain the increased concentration of serum urea N in pigs exposed to acute natural HS.
The higher serum content of total proteins and albumin in the 7dHS may reflect a partial dehydration, in comparison with the TN pigs, as both are within the normal values for pigs. The tendency of 7dHS to increase the serum albumin concentration in comparison with TN pigs is probably a component of the acclimation process of pigs to HS. The serum mineral unbalance is another effect of HS [
32]. In the present experiment, the reduced serum concentration of Ca (13%) and P (20%) in 7dHS pigs compared to TN pigs are mostly attributed to the combined effects of lower feed intake [
21], water loss or dehydration [
33], and reduced mineral retention [
34]. This response is consistent with the reduced hematocrit and hemoglobin in blood of 7dHS pigs, which combined with the reduced red blood cell count, may suggest a destruction of erythrocytes [
35] in chronic HS pigs.
Heat stress diverts blood from internal organs to peripheral tissues in an attempt to dissipate excess of body heat [
36,
37], but it results in decreased supply of nutrients and oxygen to intestinal cells, which provokes desquamation of mucosa and shortening of intestinal villi [
38]. In the present experiment, the jejunum villi height decreased 22% in the 2dHS pigs, but 7dHS pigs partially (11%) recovered it. In agreement, Yu et al. [
8] observed shorter villi height in jejunum of pigs exposed to 40 °C during 6 days, 5 h each day, compared with TN pigs, and those pigs also recovered pre-HS villi height after 10 days of HS exposure. Pearce et al. [
7] also reported that villi height decreased in jejunum of pigs after constant exposure to 35 °C during 24 h but remained without change during 7 days afterwards. This discrepancy may be explained by differences in the way pigs were exposed to HS; in our study, pigs were exposed to daily fluctuations in AT, as we exposed them to a natural environment. In duodenum and ileum, HS did not affect the villi height in the 2dHS pigs but it reduced them in the 7dHS pigs. This may be attributed to differences in the AT between these periods; the highest AT during the first 2 days (2dHS pigs) ranged from 32 to 34 °C for about 8 h every day, but it varied from 35 to 39 °C the following 5 days. These results confirm the negative impact of HS on intestinal epithelia but also showed a tendency to partially recover its normal height in jejunum after 7 days of HS exposure, as another component of the adaptation process [
3,
38].
The decreased supply of oxygen and nutrients to intestinal epithelia of HS pigs may also lead to diminished intestinal integrity and increased cell death [
24] and intestinal permeability [
39] to substances and pathogens [
7]. The immune system is the principal defense mechanism against ambient and biological stressors [
40]. Lymphocytes are immune cells that increase in circulating blood due to pathogen infection [
41] and, after stress, activate endocrine responses [
42]. In the present experiment, increased lymphocyte and white blood cell counts in 2dHS and 7dHS pigs, compared to TN pigs, could be attributed to an alert of pro-inflammatory state or increased intestinal permeability because we did not observe signs of infection in HS pigs. Likewise, the increments of platelet count (24%) and lymphocyte count in 2dHS pigs might suggest a pro-inflammatory state in these pigs [
43]. Hence, in agreement with other authors [
5,
44], HS seems to alter the inflammatory response of the small intestine of pigs.
Intestinal goblet cells produce mucin, which provides the frontline host defense against irritants and microbial attachment [
45]. Broilers exposed to HS during 21 days [
46] increased goblet cell count in duodenum and ileum. In the current experiment, the number of duodenum goblet cells of 7dHS pigs increased by 85% and 65% compared to TN and 2dHS pigs, respectively. This goblet cell hyperplasia coincided with that reported by Abuahamieh et al. [
47] in pigs after 7 days of HS treatment and Pearce et al. [
7] regarding mucin expression in pigs after 6 h of HS exposure. Our results suggest that an extended exposure of pigs to HS may increase mucin production to prevent the translocation of microorganisms or toxins through the duodenum intestinal barrier [
48] as a mechanism to compensate for the compromised intestinal epithelium. The reason why the number of goblet cells in jejunum and ileum was not affected remains unknown, although differences in digesta pH, vascularization, and epithelia histology between intestinal segments, among other factors, might explain it, but these hypotheses need to be tested.
The accumulation of oxidants in small intestine epithelial cells exposed to HS can induce apoptosis through activation of the lysosomal–mitochondrial apoptotic pathway [
49]. Pearce et al. [
7] reported increased death of the ileum epithelial cells after pigs were exposed to HS during 6 h, but no data were available beyond this time of exposure. In the current experiment, the number of apoptotic cells count in ileum increased 4.5- and 7.2-fold in 2dHS and 7dHS pigs, respectively, when compared to TN pigs. This increased apoptotic cell count and the decreased villi height observed in the small intestine of HS pigs in our experiment may indicate that the acute response reported by Pearce et al. [
7] may remain up to 7 days of HS exposure. Hence, these results suggest a chronic impact of HS on the integrity of the intestinal epithelia by increasing its permeability and becoming a “leaky gut” [
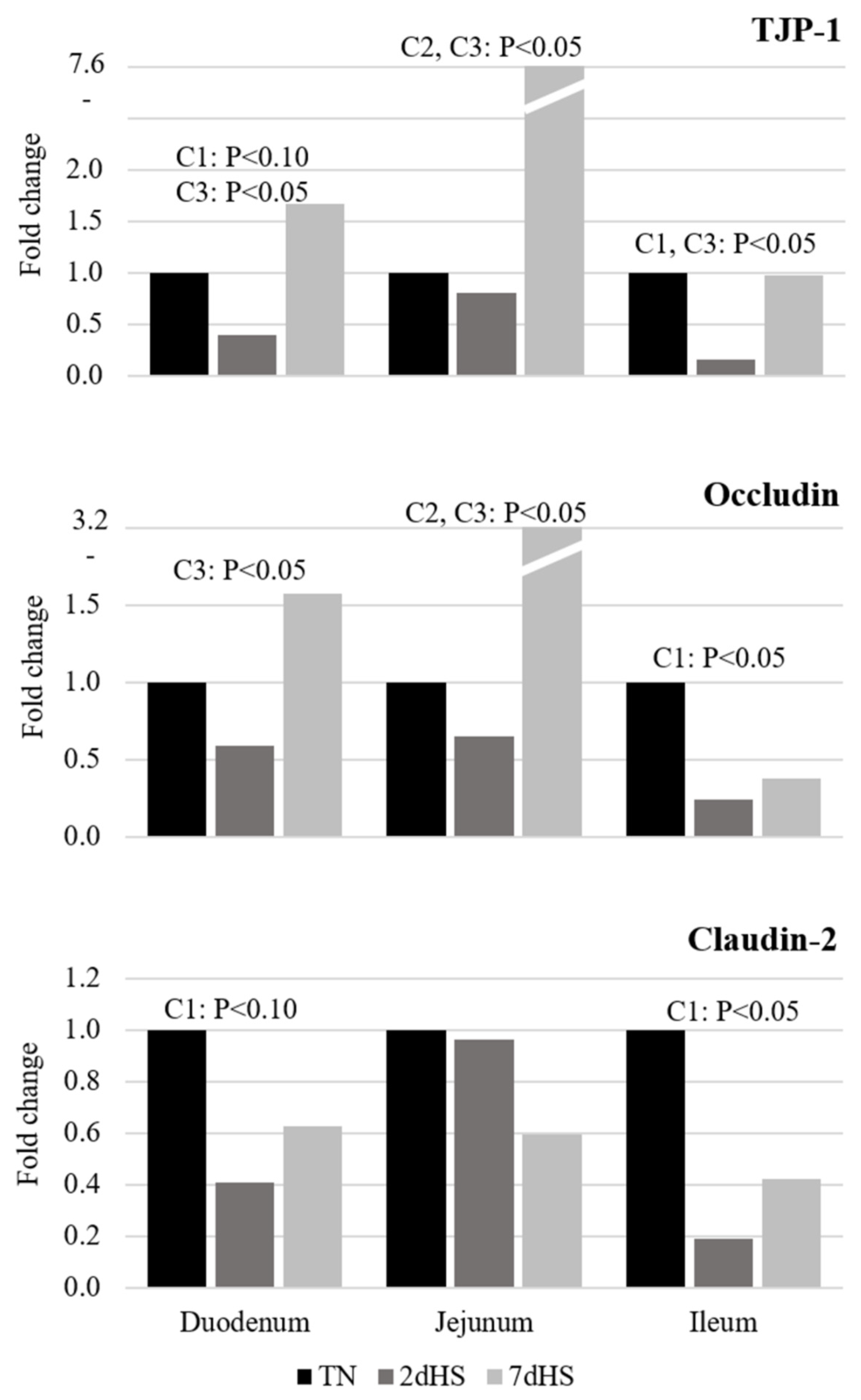
47]. Moreover, tight junction proteins (occludin, claudins, and TJP-1) in combination with mucin are essential components of the barrier that help to maintain a selective permeability of the intestinal epithelium and protect it against the entrance of pathogens and toxins [
50]. In the present experiment, the dramatic decrease in the expression of TJP-1 and claudin-2 in duodenum and ileum, as well as occludin in ileum of the 2dHS pigs, suggests an alteration in the integrity of these epithelia. However, the expression of TJP-1 and occludin extraordinarily increased in duodenum and jejunum of the 7dHS pigs. Pearce et al. [
51] reported similar increments in the expression of these proteins at day 7 of constant HS exposure (35 °C) of pigs. The latter response, therefore, might suggest a compensatory mechanism of the intestinal epithelia to overcome the acute negative impact of HS on the intestinal integrity as an additional component of the adaptation process of pigs to their exposure to high AT.