Seasonal Variations in the Lipid Profile of the Ovarian Follicle in Italian Mediterranean Buffaloes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Collection of Follicular Fluid and Follicular Cells
2.3. Collection of Cumulus Cells and Oocytes
2.4. In Vitro Embryo Production
2.5. Extraction of the Apolar Fraction from Samples for 1H-NMR
2.6. 1H-NMR Metabolomic Analysis
2.7. Extraction of Apolar Samples for GC/MS
2.8. GC/MS Analysis
2.9. Statistical Analysis
3. Results
3.1. In Vitro Embryo Production
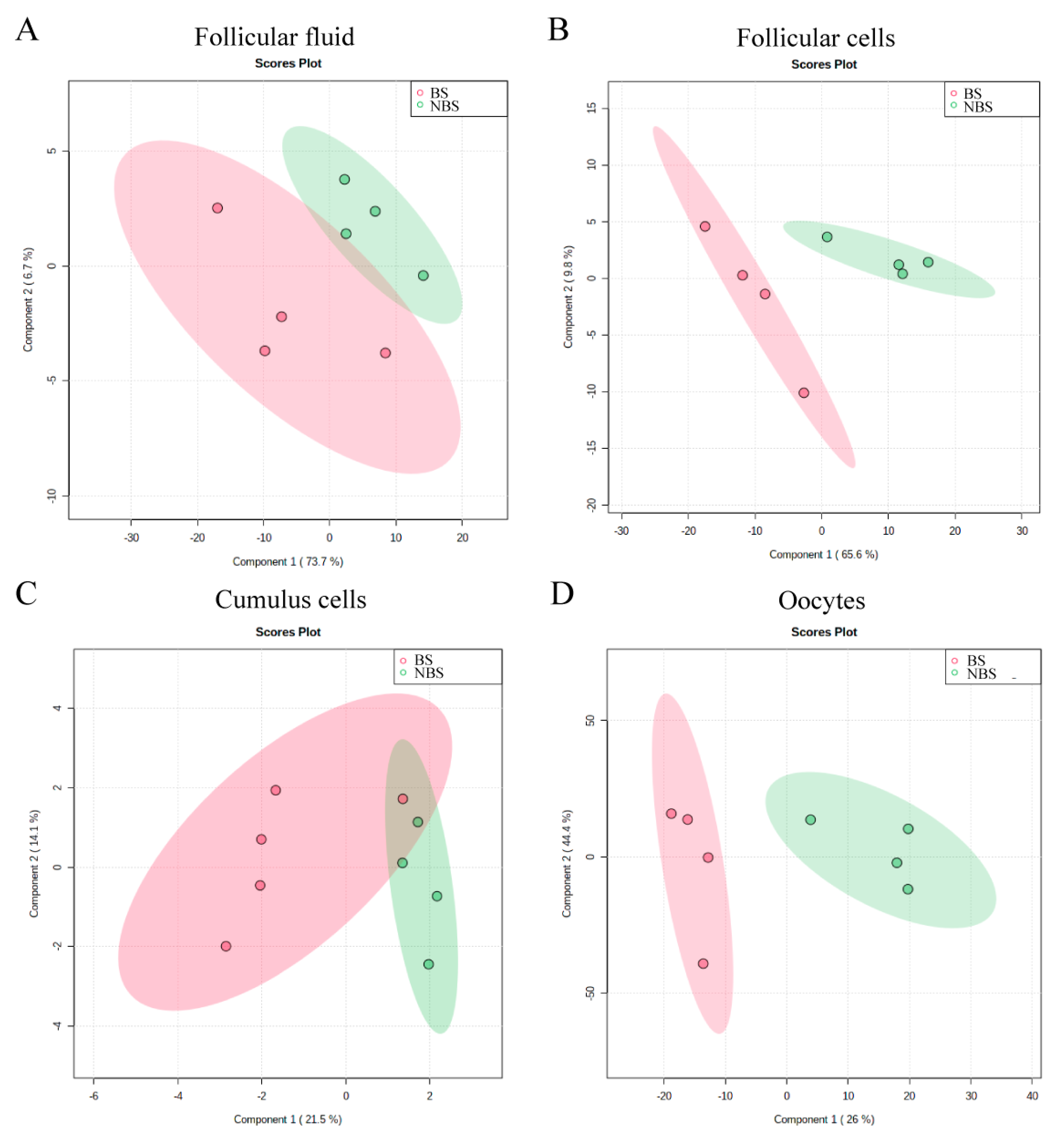
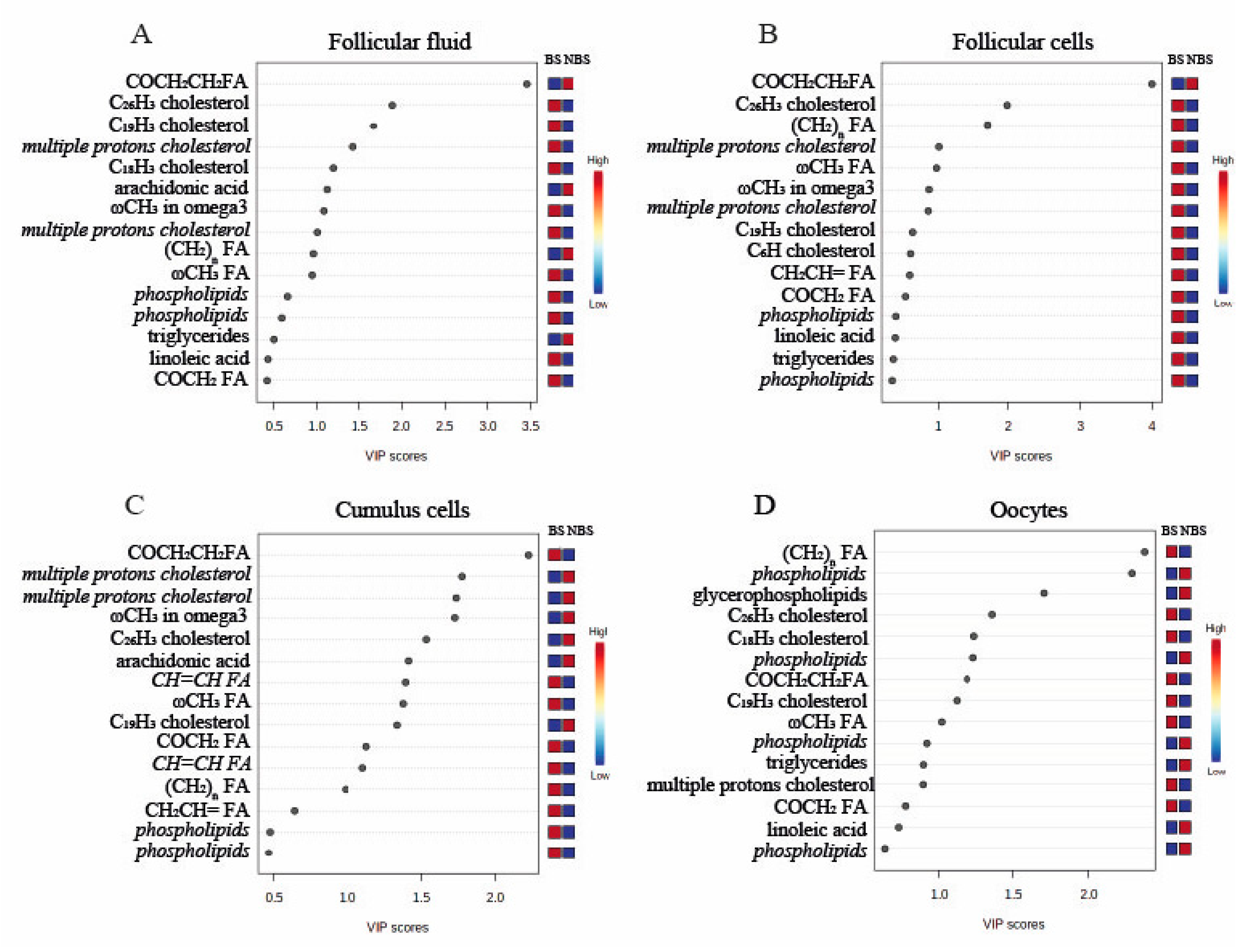
3.2. 1H-NMR Metabolomic Analysis of Follicular Fluid
3.3. 1H-NMR Metabolomic Analysis of Follicular Cells
3.4. 1H-NMR Metabolomic Analysis of Cumulus Cells
3.5. 1H-NMR Metabolomic Analysis of Oocytes
3.6. Analysis of Non-Esterified Fatty Acids in the Follicular Fluid by GC–MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Presicce, G.A. The Buffalo (Bubalus bubalis)-Production and Research; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014. [Google Scholar]
- Zicarelli, L. Can we consider buffalo a non precocious and hypofertile species? Ital. J. Anim. Sci. 2017, 6 (Suppl. 2), 143–154. [Google Scholar] [CrossRef]
- Campanile, G.; Neglia, G.; Gasparrini, B.; Galiero, G.; Prandi, A.; Di Palo, R.; Zicarelli, L. Embryonic mortality in buffaloes synchronized and mated by AI during the seasonal decline in reproductive function. Theriogenology 2005, 63, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Neglia, G.; Restucci, B.; Russo, M.; Vecchio, D.; Gasparrini, B.; Prandi, A.; Campanile, G. Early development and function of the corpus luteum and relationship to pregnancy in the buffalo. Theriogenology 2015, 83, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, S.; Novoa, M.V.S.; Vecchio, D.; Neglia, G.; Boccia, L.; Campanile, G.; Gasparrini, B. Ovum pick-up and in vitro embryo production (OPU-IVEP) in Mediterranean Italian buffalo performed in different seasons. Theriogenology 2012, 77, 148–154. [Google Scholar] [CrossRef]
- Salzano, A.; Gasparrini, B.; Vecchio, D.; Longobardi, V.; Baruselli, P.S.; Balestrieri, A.; Neglia, G. Effect of photoperiod on follicular IGF-1 and oocyte quality independently of metabolic status in buffalo heifers. Ital. J. Anim. Sci. 2019, 18, 949–956. [Google Scholar] [CrossRef]
- Capra, E.; Lazzari, B.; Russo, M.; Kosior, M.A.; Valle, G.; Longobardi, V.; Gasparrini, B. Seasonal effects on miRNA and transcriptomic profile of oocytes and follicular cells in buffalo (Bubalus bubalis). Sci. Rep. 2020, 10, 13557. [Google Scholar] [CrossRef]
- Prates, E.G.; Nunes, J.T.; Pereira, R.M. A role of lipid metabolism during cumulus-oocyte complex maturation: Impact of lipid modulators to improve embryo production. Mediat. Inflamm. 2014, 2014, 692067. [Google Scholar] [CrossRef]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta (BBA)—Mol. Cell. Biochem. Lipids 2017, 1862, 1260–1272. [Google Scholar] [CrossRef]
- Nuttinck, F.; Guienne, B.M.L.; Clément, L.; Reinaud, P.; Charpigny, G.; Grimard, B. Expression of genes involved in prostaglandin E2 and progesterone production in bovine cumulus-oocyte complexes during in vitro maturation and fertilization. Reproduction 2008, 135, 593–604. [Google Scholar] [CrossRef]
- Lew, B.J.; Meidan, R.; Wolfenson, D. Hormone concentration and follicular development in dairy cows under seasonal and acute hyperthermia. Arq. Bras. Med. Vet. Zootec. 2006, 58, 816–822. [Google Scholar] [CrossRef]
- Santos, J.E.P.; Bilby, T.R.; Thatcher, W.W.; Staples, C.R.; Silvestre, F.T. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod. Domest. Anim. 2008, 43, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.L.M.R.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; De Kruif, A.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Aardema, H.; Lolicato, F.; Van de Lest, C.H.; Brouwers, J.F.; Vaandrager, A.B.; Van Tol, H.T.; Gadella, B.M. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol. Reprod. 2013, 88, 164. [Google Scholar] [CrossRef]
- Aardema, H.; Gadella, B.M.; Van de Lest, C.H.A.; Brouwers, J.F.H.M.; Stout, T.A.E.; Roelen, B.A.J.; Vos, P.L.A.M. Free fatty acid levels in fluid of dominant follicles at the preferred insemination time in dairy cows are not affected by early postpartum fatty acid stress. J. Dairy Sci. 2015, 98, 2322–2336. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lazo, L.; Brisard, D.; Elis, S.; Maillard, V.; Uzbekov, R.; Labas, V.; Uzbekova, S. Fatty acid synthesis and oxidation in cumulus cells support oocyte maturation in bovine. Molec. Endoc. 2014, 28, 1502–1521. [Google Scholar] [CrossRef]
- de Andrade Melo-Sterza, F.; Poehland, R. Lipid metabolism in bovine oocytes and early embryos under in vivo, in vitro, and stress conditions. Int. J. Molec. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- Aardema, H.; van Tol, H.T.; Wubbolts, R.W.; Brouwers, J.F.; Gadella, B.M.; Roelen, B.A. Stearoyl-CoA desaturase activity in bovine cumulus cells protects the oocyte against saturated fatty acid stress. Biol. Reprod. 2017, 96, 982–992. [Google Scholar] [CrossRef]
- Lolicato, F.; Brouwers, J.F.; Van de Lest, C.H.A.; Wubbolts, R.; Aardema, H.; Priore, P.; Roelen, B.A.J.; Helms, J.B.; Gadella, B.M. The Cumulus Cell Layer Protects the Bovine Maturing Oocyte Against Fatty Acid-Induced Lipotoxicity. Biol. Reprod. 2015, 92, 16. [Google Scholar] [CrossRef]
- Di Francesco, S.; Boccia, L.; Campanile, G.; Di Palo, R.; Vecchio, D.; Neglia, G.; Gasparrini, B. The effect of season on oocyte quality and developmental competence in Italian Mediterranean buffaloes (Bubalus bubalis). Anim. Reprod. Sci. 2011, 123, 48–53. [Google Scholar] [CrossRef]
- Gasparrini, B.; Neglia, G.; Di Palo, R.; Campanile, G.; Zicarelli, L. Effect of cysteamine during in vitro maturation on buffalo embryo development. Theriogenology 2000, 54, 1537–1542. [Google Scholar] [CrossRef]
- Aardema, H.; Vos, P.L.; Lolicato, F.; Roelen, B.A.; Knijn, H.M.; Vaandrager, A.B.; Gadella, B.M. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol. Reprod. 2011, 85, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Coll, T.; Eyre, E.; Rodríguez-Calvo, R.; Palomer, X.; Sánchez, R.M.; Merlos, M.; Vázquez-Carrera, M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 2008, 283, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Dole, V.P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J. Clin. Investig. 1956, 35, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Hannaert, J.C.; Hoorens, A.; Eizirik, D.L.; Pipeleers, D.G. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 2001, 50, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Baddela, V.S.; Becker, F.; Dannenberger, D.; Viergutz, T.; Vanselow, J. Elevated free fatty acids affect bovine granulosa cell function: A molecular cue for compromised reproduction during negative energy balance. Endocr. Connect. 2019, 8, 493–505. [Google Scholar] [CrossRef]
- Seidel, G.E., Jr. Modifying oocytes and embryos to improve their cryopreservation. Theriogenology 2006, 65, 228–235. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kinoshita, M.; Ohnishi, M.; Fukui, Y. Lipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytes. Reproduction 2001, 122, 131–138. [Google Scholar] [CrossRef]
- Ferguson, E.M.; Leese, H.J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2006, 73, 1195–1201. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, B. In vitro embryo production in buffalo species: State of the art. Theriogenology 2002, 57, 237–256. [Google Scholar] [CrossRef]
- Mishra, R.; Simonson, M.S. Saturated free fatty acids and apoptosis in microvascular mesangial cells: Palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardio. Diabetol. 2005, 4, 2. [Google Scholar] [CrossRef]
- Mu, Y.M.; Yanase, T.; Nishi, Y.; Tanaka, A.; Saito, M.; Jin, C.H.; Nawata, H. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology 2001, 142, 3590–3597. [Google Scholar] [CrossRef]
- Calder, P. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Koga, F.; Kitagami, S.; Izumi, A.; Uemura, T.; Takayama, O.; Koga, T.; Mizoguchi, T. Relationship between nutrition and reproduction. Repro. Med. Bio. 2020, 19, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Luo, G.; Tang, X.; Ma, L.; Zheng, Y.; Jiang, Z. Arachidonic acid regulation of intracellular signaling pathways and target gene expression in bovine ovarian granulosa cells. Animals 2019, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shen, W.J.; Cortez, Y.; Tang, X.; Liu, L.F.; Kraemer, F.B.; Azhar, S. Hormonal regulation of microRNA expression in steroid producing cells of the ovary, testis and adrenal gland. PLoS ONE 2013, 8, e78040. [Google Scholar] [CrossRef]
- Yesilaltay, A.; Dokshin, G.A.; Busso, D.; Wang, L.; Galiani, D.; Chavarria, T.; Krieger, M. Excess cholesterol induces mouse egg activation and may cause female infertility. Proc. Natl. Acad. Sci. USA 2014, 111, E4972–E4980. [Google Scholar] [CrossRef]
- Buschiazzo, J.; Ialy-Radio, C.; Auer, J.; Wolf, J.P.; Serres, C.; Lefèvre, B.; Ziyyat, A. Cholesterol depletion disorganizes oocyte membrane rafts altering mouse fertilization. PLoS ONE 2013, 8, e62919. [Google Scholar] [CrossRef]
- Raffy, S.; Teissie, J. Control of lipid membrane stability by cholesterol content. Biophys. J. 1999, 76, 2072–2080. [Google Scholar] [CrossRef]
| FF | FC | CC | OO | |
|---|---|---|---|---|
| Triglycerides | ▲ | ▼ | ▲ | |
| Cholesterol | ▼ | ▼ | ▲ | ▼ |
| Phospholipids | ▼ | ▼ | ▼ | ▲ |
| Omega-3 FA | ▼ | ▼ | ▼ | ▼ |
| Arachidonic acid | ▲ | ▲ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosior, M.A.; Calabria, A.; Benitez Mora, M.P.; Russo, M.; Presicce, G.A.; Cocchia, N.; Monti, S.; Aardema, H.; Gasparrini, B. Seasonal Variations in the Lipid Profile of the Ovarian Follicle in Italian Mediterranean Buffaloes. Animals 2022, 12, 2108. https://doi.org/10.3390/ani12162108
Kosior MA, Calabria A, Benitez Mora MP, Russo M, Presicce GA, Cocchia N, Monti S, Aardema H, Gasparrini B. Seasonal Variations in the Lipid Profile of the Ovarian Follicle in Italian Mediterranean Buffaloes. Animals. 2022; 12(16):2108. https://doi.org/10.3390/ani12162108
Chicago/Turabian StyleKosior, Michal Andrzej, Alfonso Calabria, Maria Paz Benitez Mora, Marco Russo, Giorgio Antonio Presicce, Natascia Cocchia, Salvatore Monti, Hilde Aardema, and Bianca Gasparrini. 2022. "Seasonal Variations in the Lipid Profile of the Ovarian Follicle in Italian Mediterranean Buffaloes" Animals 12, no. 16: 2108. https://doi.org/10.3390/ani12162108
APA StyleKosior, M. A., Calabria, A., Benitez Mora, M. P., Russo, M., Presicce, G. A., Cocchia, N., Monti, S., Aardema, H., & Gasparrini, B. (2022). Seasonal Variations in the Lipid Profile of the Ovarian Follicle in Italian Mediterranean Buffaloes. Animals, 12(16), 2108. https://doi.org/10.3390/ani12162108






