Assessment of the Effects of Edible Microalgae in a Canine Gut Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Chemical Analyses
2.3. Microbial Analysis
2.4. Statistical Analyses
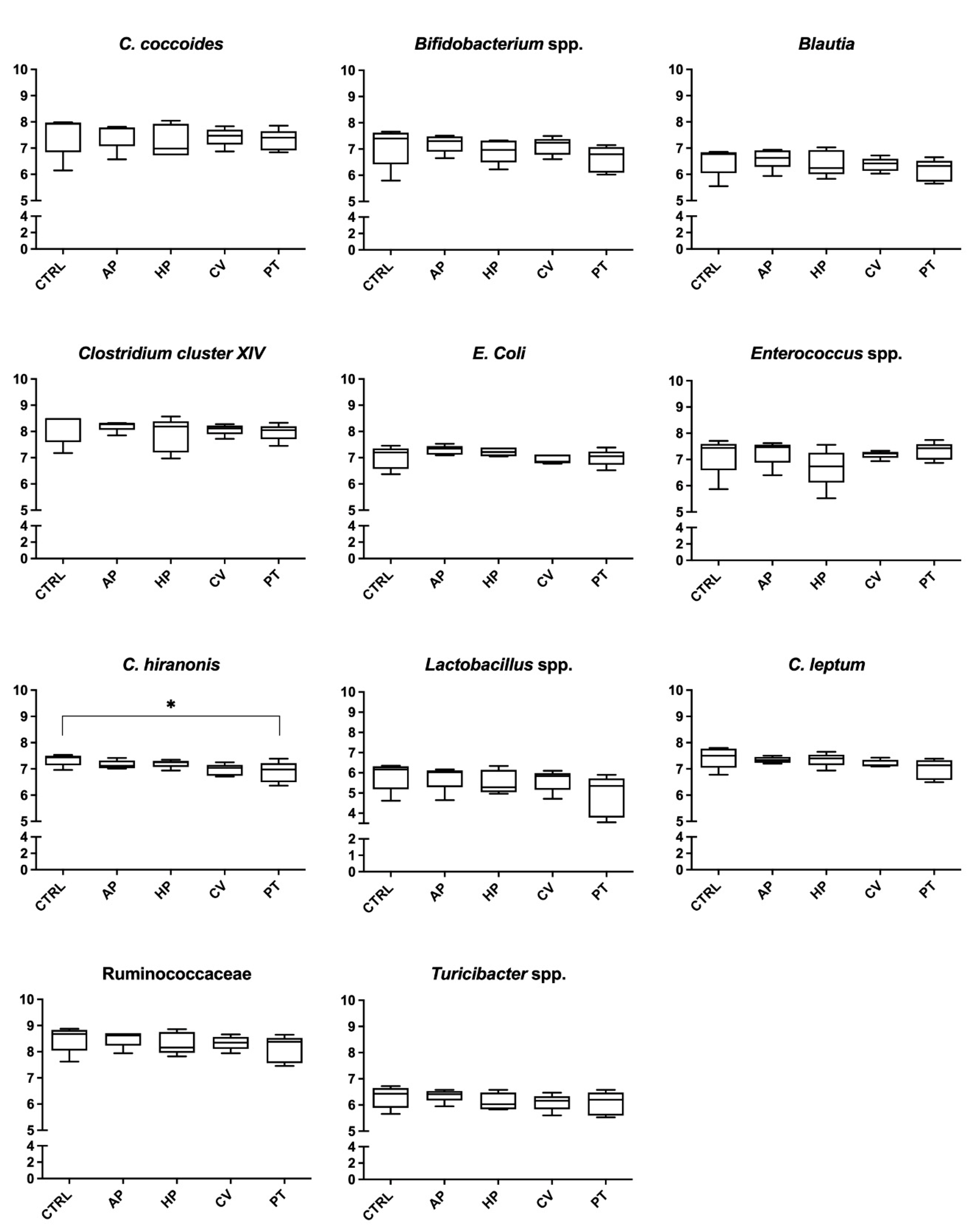
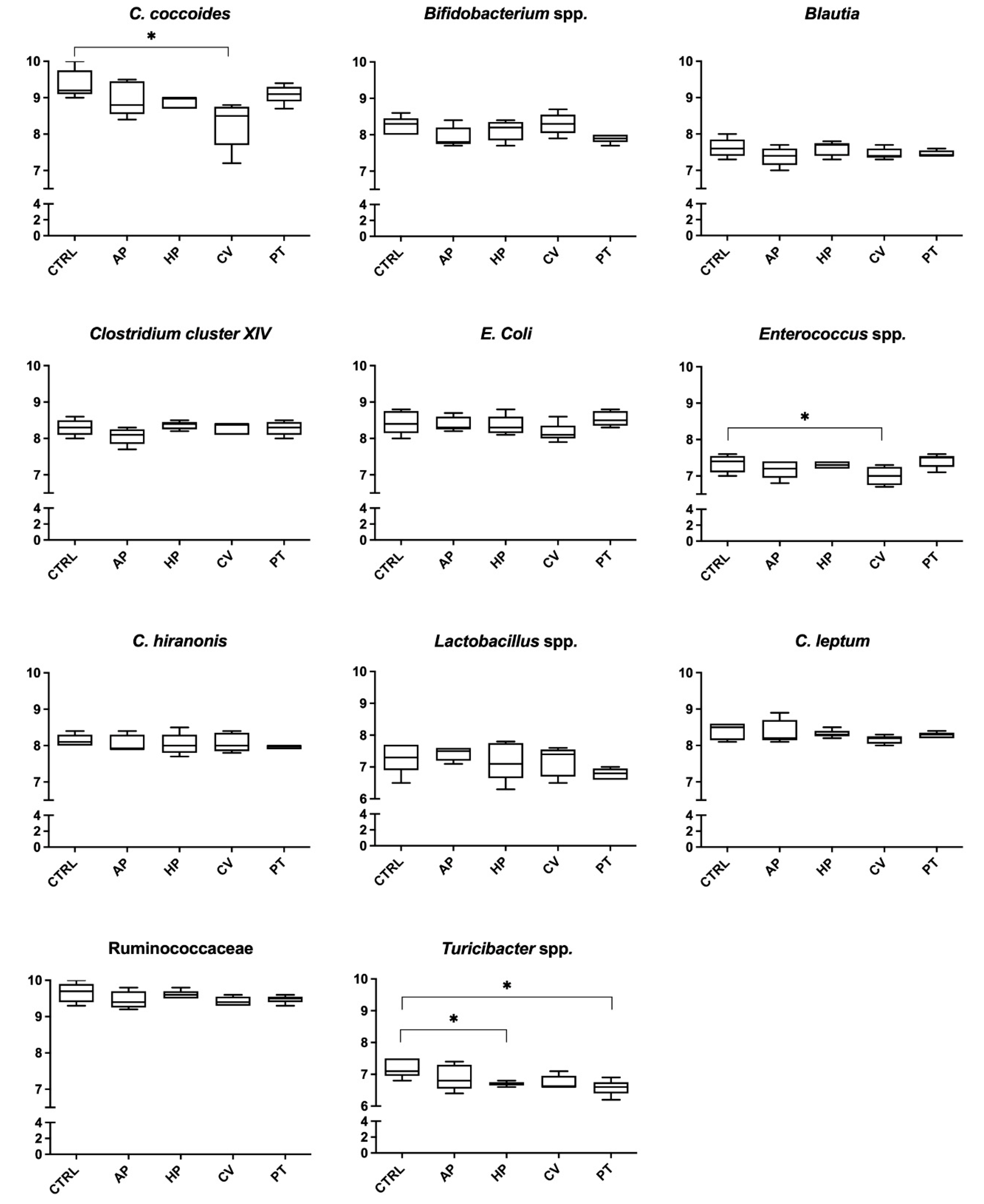
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuddenham, S.; Sears, C.L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 2015, 28, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2021, 50, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 2019, 9, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Ercolini, D.; Fogliano, V. Food Design to Feed the Human Gut Microbiota. J. Agric. Food Chem. 2018, 66, 3754–3758. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, Z.; Yang, R.; Bi, Y.; Xiong, X. The canine gastrointestinal microbiota: Early studies and research frontiers. Gut Microbes 2020, 11, 635–654. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Kay, R.A.; Barton, L.L. Microalgae as Food and Supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional high-value products from microalgae: A review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices (accessed on 27 June 2022).
- Joint Research Centre, Institute for Prospective Technological Studies; Barbosa, M.; Enzing, C.; Ploeg, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; Vigani, M., Parisi, C., Rodríguez Cerezo, E., Eds.; Publications Office: Luxembourg, 2016; pp. 19–37. [Google Scholar]
- Commission European Commission Regulation (EU) 2017/1017 of 15 June 2017 amending Regulation (EU) No 68/2013 on the Catalogue of feed materials. Off. J. Eur. Union 2017, 159, 48–119.
- Da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Silva Benavides, A.M.; Torzillo, G.; Kopecký, J.; Masojídek, J. Productivity and biochemical composition of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in tubular photobioreactors and open ponds. Biomass Bioenergy 2013, 54, 115–122. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21, 1–10. [Google Scholar] [CrossRef]
- De Medeiros, V.P.B.; Pimentel, T.C.; Sant’Ana, A.S.; Magnani, M. Microalgae in the meat processing chain: Feed for animal production or source of techno-functional ingredients. Curr. Opin. Food Sci. 2021, 37, 125–134. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Charoonnart, P.; Purton, S.; Saksmerprome, V. Applications of Microalgal Biotechnology for Disease Control in Aquaculture. Biology 2018, 7, 24. [Google Scholar] [CrossRef]
- Sagaram, U.S.; Gaikwad, M.S.; Nandru, R.; Dasgupta, S. Microalgae as feed ingredients: Recent developments on their role in immunomodulation and gut microbiota of aquaculture species. FEMS Microbiol. Lett. 2021, 368, fnab071. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; et al. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci. Rep. 2017, 7, 6286. [Google Scholar] [CrossRef] [PubMed]
- Satyaraj, E.; Reynolds, A.; Engler, R.; Labuda, J.; Sun, P. Supplementation of Diets With Spirulina Influences Immune and Gut Function in Dogs. Front. Nutr. 2021, 8, 667072. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gómez, O.; García-Rodríguez, R.V.; Quevedo-Corona, L.; Pérez-Pastén-Borja, R.; Rivero-Ramírez, N.L.; Ríos-Castro, E.; Pérez-Gutiérrez, S.; Pérez-Ramos, J.; Chamorro-Cevallos, G.A. Amelioration of ethanol-induced gastric ulcers in rats pretreated with phycobiliproteins of Arthrospira (Spirulina) maxima. Nutrients 2018, 10, 763. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-induced production of anti-inflammatory molecules in microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging prospects of macro- and microalgae as prebiotic. Microb. Cell Factories 2021, 20, 112. [Google Scholar] [CrossRef]
- De Medeiros, V.P.B.; de Souza, E.L.; de Albuquerque, T.M.R.; da Costa Sassi, C.F.; dos Santos Lima, M.; Sivieri, K.; Pimentel, T.C.; Magnani, M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Jin, J.B.; Cha, J.W.; Shin, I.S.; Jeon, J.Y.; Cha, K.H.; Pan, C.H. Supplementation with Chlorella vulgaris, Chlorella protothecoides, and Schizochytrium sp. increases propionate-producing bacteria in in vitro human gut fermentation. J. Sci. Food Agric. 2020, 100, 2938–2945. [Google Scholar] [CrossRef]
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Lanzilli, S.; Rubino, V.; Di Cerbo, A.; Centenaro, S.; Guidetti, G.; Canello, S.; Terrazzano, G. An immune-modulating diet increases the regulatory T cells and reduces T helper 1 inflammatory response in Leishmaniosis affected dogs treated with standard therapy. BMC Vet. Res. 2015, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Ohno, K.; Uchida, K.; Nakashima, K.; Fukushima, K.; Tsukamoto, A.; Nakajima, M.; Fujino, Y.; Tsujimoto, H. Decreased Immunoglobulin A Concentrations in Feces, Duodenum, and Peripheral Blood Mononuclear Cells of Dogs with Inflammatory Bowel Disease. J. Vet. Intern. Med. 2013, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Littler, R.M.; Batt, R.M.; Lloyd, D.H. Total and relative deficiency of gut mucosal IgA in German shepherd dogs demonstrated by faecal analysis. Vet. Rec. 2006, 158, 334–341. [Google Scholar] [CrossRef] [PubMed]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef]
- Biagi, G.; Cipollini, I.; Grandi, M.; Pinna, C.; Vecchiato, C.G.; Zaghini, G. A new in vitro method to evaluate digestibility of commercial diets for dogs. Ital. J. Anim. Sci. 2016, 15, 617–625. [Google Scholar] [CrossRef]
- Sunvold, G.D.; Hussein, H.S.; Fahey, G.C.; Merchen, N.R.; Reinhart, G.A. In vitro fermentation of cellulose, beet pulp, citrus pulp, and citrus pectin using fecal inoculum from cats, dogs, horses, humans, and pigs and ruminal fluid from cattle. J. Anim. Sci. 1995, 73, 3639–3648. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef]
- Pinna, C.; Giuditta Vecchiato, C.; Grandi, M.; Maria Eugenia Mammi, L.; Stefanelli, C.; Biagi, G. In vitro evaluation of the effects of tylosin on the composition and metabolism of canine fecal microbiota. Animals 2020, 10, 98. [Google Scholar] [CrossRef]
- Stefanelli, C.; Carati, D.; Rossoni, C. Separation of N1- and N8-acetylspermidine isomers by reversed-phase column liquid chromatography after derivatization with dansyl chloride. J. Chromatogr. B Biomed. Sci. Appl. 1986, 375, 49–55. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Suchodolski, J.S.; Jones, K.R.; Clark-Price, S.C.; Dowd, S.E.; Minamoto, Y.; Markel, M.; Steiner, J.M.; Dossin, O. Effect of the proton pump inhibitor omeprazole on the gastrointestinal bacterial microbiota of healthy dogs. FEMS Microbiol. Ecol. 2012, 80, 624–636. [Google Scholar] [CrossRef]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Malinen, E.; Rinttilä, T.; Kajander, K.; Mättö, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 2005, 100, 373–382. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.; Tanaka, R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 2004, 70, 7220–7228. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Miyamoto, Y.; Takada, T.; Matsumoto, K.; Oyaizu, H.; Tanaka, R. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 2002, 68, 5445–5451. [Google Scholar] [CrossRef]
- Alshawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, 136. [Google Scholar] [CrossRef]
- Malinen, E.; Kassinen, A.; Rinttilä, T.; Palva, A. Comparison of real-time PCR with SYBR Green I or 5′-nuclease assaysand dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantificationof selected faecal bacteria. Microbiology 2003, 149, 269–277. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Finegold, S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Marcus, A.K.; Kang, D.-W.; Rittmann, B.E.; Krajmalnik-Brown, R. pH-Mediated Microbial and Metabolic Interactions in Fecal Enrichment Cultures. mSphere 2017, 2, e00047-17. [Google Scholar] [CrossRef]
- Hamer, H.M.; de Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Cheng, N.; Nakata, P.A.; Zhao, L.; Hu, Q. Consumption of polysaccharides from Auricularia auricular modulates the intestinal microbiota in mice. Food Res. Int. 2019, 123, 383–392. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short chain fatty acids in the colon and peripheral tissues: A focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Wan, X.; Ai, C.; Chen, Y.; Gao, X.; Zhong, R.; Liu, B.; Chen, X.; Zhao, C. Physicochemical Characterization of a Polysaccharide from Green Microalga Chlorella pyrenoidosa and Its Hypolipidemic Activity via Gut Microbiota Regulation in Rats. J. Agric. Food Chem. 2019, 68, 1186–1197. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Jamar, G.; Santamarina, A.B.; Dias, G.C.; Masquio, D.C.L.; de Rosso, V.V.; Pisani, L.P. Relationship between fatty acids intake and Clostridium coccoides in obese individuals with metabolic syndrome. Food Res. Int. 2018, 113, 86–92. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Menezes, F.N.D.D.; da Cruz Almeida, É.T.; da Silva Vieira, A.R.; de Souza Aquino, J.; dos Santos Lima, M.; Magnani, M.; de Souza, E.L. Impact of Cashew (Anacardium occidentale L.) by-Product on Composition and Metabolic Activity of Human Colonic Microbiota In Vitro Indicates Prebiotic Properties. Curr. Microbiol. 2021, 78, 2264–2274. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal derivatives as potential nutraceutical and food supplements for human health: A focus on cancer prevention and interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Nami, Y.; Bakhshayesh, R.V.; Jalaly, H.M.; Lotfi, H.; Eslami, S.; Hejazi, M.A. Probiotic properties of enterococcus isolated from artisanal dairy products. Front. Microbiol. 2019, 10, 300. [Google Scholar] [CrossRef]
- Matos, Â.P. Microalgae as a Potential Source of Proteins. In Proteins: Sustainable Source, Processing and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 63–96. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amin. Acids 2006, 33, 547–562. [Google Scholar] [CrossRef]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.Y. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef]
- Musch, M.W.; Bookstein, C.; Xie, Y.; Sellin, J.H.; Chang, E.B. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G687–G693. [Google Scholar] [CrossRef]
- Jaskiewicz, J.; Zhao, Y.; Hawes, J.W.; Shimomura, Y.; Crabb, D.W.; Harris, R.A. Catabolism of isobutyrate by colonocytes. Arch. Biochem. Biophys. 1996, 327, 265–270. [Google Scholar] [CrossRef]
- Heby, O. Role of Polyamines in the Control of Cell Proliferation and Differentiation. Differentiation 1981, 19, 1–20. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Studies on amine production in the human colon: Enumeration of amine forming bacteria and physiological effects of carbohydrate and pH. Anaerobe 1996, 2, 285–297. [Google Scholar] [CrossRef]
| Item | Crude Protein | Crude Ash | Crude Fibre | Total Digestibility |
|---|---|---|---|---|
| Microalgae | ||||
| Arthrospira platensis | 70.9 | 5.03 | 0.70 | 86.2 |
| Haematococcus pluvialis | 10.4 | 4.03 | 15.67 | 7.87 |
| Phaeodactylum tricornutum | 39.6 | 22.4 | 0.40 | 67.5 |
| Chlorella vulgaris | 31.1 | 9.97 | 11.8 | 55.3 |
| Microalgae, undigested fraction | ||||
| Arthrospira platensis | 55.7 | 4.04 | 13.7 | |
| Haematococcus pluvialis | 10.2 | 1.53 | 9.84 | |
| Phaeodactylum tricornutum | 18.6 | 27.0 | 0.56 | |
| Chlorella vulgaris | 18.0 | 10.7 | 16.8 | |
| Treatment | Commercial Dry Food, Undigested Fraction (mg) | Algae, Undigested Fraction (mg) |
|---|---|---|
| Control (CTRL) | 210 | - |
| Arthrospira platensis (AP) | 210 | 11.6 |
| Haematococcus pluvialis (HP) | 210 | 77.4 |
| Phaeodactylum tricornutum (PT) | 210 | 37.5 |
| Chlorella vulgaris (CV) | 210 | 27.3 |
| Target | Primer | Sequence (5′→3′) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| Blautia spp. | Blautia_F | TCTGATGTGAAAGGCTGGGGCTTA | 62.0 | [44] |
| Blautia_R | GGCTTAGCCACCCGACACCTA | |||
| Turicibacter spp. | Turicibacter_F | CAGACGGGGACAACGATTGGA | 59.3 | [44] |
| Turicibacter_R | TACGCATCGTCGCCTTGGTA | |||
| Ruminococcaceae | Ruminococcaceae_F | ACTGAGAGGTTGAACGGCCA | 64.2 | [45] |
| Ruminococcaceae_R | CCTTTACACCCAGTAAWTCCGGA | |||
| Bifidobacterium spp. | Bif_F | TCGCGTCYGGTGTGAAAG | 62.0 | [46] |
| Bif_R | CCACATCCAGCRTCCAC | |||
| Lactobacillus spp. | Lac_F | AGCAGTAGGGAATCTTCCA | 64.2 | [47] |
| Lac_R | CACCGCTACACATGGAG | |||
| Clostridium leptum | sg-Clept-F | GCACAAGCAGTGGAGT | 59.3 | [48] |
| sg-Clept-R | CTTCCTCCGTTTTGTCAA | |||
| Clostridium coccoides | g-Ccoc-F | AAATGACGGTACCTGACTAA | 64.2 | [49] |
| g-Ccoc-R | CTTTGAGTTTCATTCTTGCGAA | |||
| Clostridium hiranonis | C.hiranonis_F | AGTAAGCTCCTGATACTGTCT | 65.4 | [50] |
| C.hiranonis_R | AGGGAAAGAGGAGATTAGTCC | |||
| Escherichia coli | Coli_F | GTTAATACCTTTGCTCATTGA | 62.0 | [51] |
| Coli_R | ACCAGGGTATCTAATCCTGTT | |||
| Enterococcus spp. | Ent_F | CCCTTATTGTTAGTTGCCATCATT | 59.3 | [46] |
| Ent_R | ACTCGTTGTACTTCCCATTGT | |||
| Clostridium cluster XIV | CloXIV-F | GAWGAAGTATYTCGGTATGT | 57.2 | [52] |
| CloXIV-R | CTACGCWCCCTTTACAC |
| Item | CTRL | AP | HP | PT | CV | Pooled SEM | Anova p-Value |
|---|---|---|---|---|---|---|---|
| pH | 6.71 | 6.63 | 6.58 * | 6.63 * | 6.56 * | 0.03 | 0.005 |
| Ammonia, mmol/L | 30.2 | 32.2 | 31.4 | 29.6 | 31.9 | 1.62 | 0.586 |
| Straight-chain SCFA, mmol/L | |||||||
| Acetate | 8.62 | 8.66 | 8.97 | 8.85 | 8.57 | 0.42 | 0.954 |
| Propionate | 4.54 | 4.92 | 5.13 | 6.19 * | 5.14 | 0.23 | 0.001 |
| Butyrate | 2.55 | 2.58 | 2.62 | 3.16 * | 2.69 | 0.12 | 0.013 |
| Total SCFA | 15.7 | 16.2 | 16.7 | 18.2 | 16.4 | 0.78 | 0.232 |
| BCFA, mmol/L | |||||||
| Isobutyrate | 0.27 | 0.15 | 0.15 | 0.13 * | 0.13 | 0.03 | 0.022 |
| Isovalerate | 0.46 | 0.26 * | 0.30 | 0.26 * | 0.26 * | 0.03 | 0.009 |
| Total BCFA | 0.73 | 0.41 | 0.45 | 0.39 * | 0.39 * | 0.08 | 0.006 |
| Individual SCFA proportions, % | |||||||
| Acetate | 51.5 | 52.2 | 52.1 | 47.6 * | 51.0 | 0.44 | <0.001 |
| Propionate | 27.1 | 29.7 * | 29.9 * | 33.3 * | 30.6 * | 0.23 | <0.001 |
| Butyrate | 15.2 | 15.5 | 15.2 | 17.0 * | 16.0 * | 0.15 | <0.001 |
| Isobutyrate | 1.54 | 0.88 | 0.90 | 0.71 * | 0.80 * | 0.13 | 0.001 |
| Isovalerate | 2.70 | 1.59 | 1.74 | 1.42 * | 1.56 * | 0.13 | <0.001 |
| Item | CTRL | AP | HP | PT | CV | Pooled SEM | Anova p-Value |
|---|---|---|---|---|---|---|---|
| pH | 5.84 | 5.84 | 5.81 | 5.95 | 5.81 | 0.01 | 0.004 |
| Ammonia, mmol/L | 39.6 | 39.9 | 36.0 | 38.0 | 35.7 | 1.29 | 0.065 |
| Straight-chain SCFA, mmol/L | |||||||
| Acetate | 16.7 | 16.9 | 16.6 | 16.5 | 16.5 | 0.48 | 0.960 |
| Propionate | 9.68 | 10.5 | 10.3 | 11.7 * | 10.7 | 0.28 | 0.001 |
| Butyrate | 5.43 | 5.73 | 5.31 | 5.65 | 5.61 | 0.14 | 0.271 |
| Total SCFA | 31.8 | 33.1 | 32.2 | 33.8 | 32.8 | 0.89 | 0.536 |
| BCFA, mmol/L | |||||||
| Isobutyrate | 0.60 | 0.64 | 0.60 | 0.64 | 0.62 | 0.02 | 0.289 |
| Isovalerate | 0.92 | 0.95 | 0.90 | 1.01 * | 0.94 | 0.02 | 0.041 |
| Total BCFA | 1.52 | 1.59 | 1.50 | 1.65 | 1.56 | 0.04 | 0.086 |
| Individual SCFA proportions, % | |||||||
| Acetate | 48.7 | 47.3 * | 47.9 * | 45.2 * | 46.7 * | 0.20 | <0.001 |
| Propionate | 28.3 | 29.4 * | 29.7 * | 32.2 * | 30.2 * | 0.09 | <0.001 |
| Butyrate | 15.9 | 16.0 | 15.4 * | 15.6 | 15.9 | 0.10 | 0.001 |
| Isobutyrate | 1.76 | 1.80 | 1.74 | 1.76 | 1.75 | 0.01 | 0.041 |
| Isovalerate | 2.68 | 2.66 | 2.59 | 2.78 | 2.65 | 0.05 | 0.254 |
| Item | CTRL | AP | HP | PT | CV | Pooled SEM | Anova p-Value |
|---|---|---|---|---|---|---|---|
| 6 h | |||||||
| Putrescine | 177.4 | 186.6 | 175.6 | 169.2 | 179.0 | 4.87 | 0.241 |
| Cadaverine | 101.0 | 124.6 | 132.4 | 87.4 | 96.4 | 15.1 | 0.371 |
| Spermidine | 24.4 | 68.8 | 36.4 | 21.6 | 23.6 | 9.55 | 0.043 |
| Spermine | 3.80 | 3.70 | 5.02 | 0.98 | 1.28 | 6.85 | 0.041 |
| 24 h | |||||||
| Putrescine | 166.4 | 174.0 | 111.4 | 140.4 | 107.8 | 10.2 | 0.007 |
| Cadaverine | 129.8 | 154.4 | 72.4 | 97.4 | 113.8 | 25.6 | 0.223 |
| Spermidine | 22.0 | 21.8 | 18.2 | 22.6 | 24.6 | 3.00 | 0.669 |
| Spermine | 1.32 | 1.16 | 1.04 | 0.58 | 2.12 | 0.53 | 0.414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delsante, C.; Pinna, C.; Sportelli, F.; Dalmonte, T.; Stefanelli, C.; Vecchiato, C.G.; Biagi, G. Assessment of the Effects of Edible Microalgae in a Canine Gut Model. Animals 2022, 12, 2100. https://doi.org/10.3390/ani12162100
Delsante C, Pinna C, Sportelli F, Dalmonte T, Stefanelli C, Vecchiato CG, Biagi G. Assessment of the Effects of Edible Microalgae in a Canine Gut Model. Animals. 2022; 12(16):2100. https://doi.org/10.3390/ani12162100
Chicago/Turabian StyleDelsante, Costanza, Carlo Pinna, Federica Sportelli, Thomas Dalmonte, Claudio Stefanelli, Carla G. Vecchiato, and Giacomo Biagi. 2022. "Assessment of the Effects of Edible Microalgae in a Canine Gut Model" Animals 12, no. 16: 2100. https://doi.org/10.3390/ani12162100
APA StyleDelsante, C., Pinna, C., Sportelli, F., Dalmonte, T., Stefanelli, C., Vecchiato, C. G., & Biagi, G. (2022). Assessment of the Effects of Edible Microalgae in a Canine Gut Model. Animals, 12(16), 2100. https://doi.org/10.3390/ani12162100







