The Complete Mitochondrial Genomes of Three Sphenomorphinae Species (Squamata: Scincidae) and the Selective Pressure Analysis on Mitochondrial Genomes of Limbless Isopachys gyldenstolpei
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Specimen Collection
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Mitochondrial Genome Annotation and Sequence Analyses
2.4. Phylogenetic Analyses
| Family | Subfamily | Species | Length | Accession No. | Reference |
|---|---|---|---|---|---|
| Scincidae | Mabuyinae | Eutropis multifasciata | 17,062 bp | MN938934 | [44] |
| Scincinae | Ateuchosaurus chinensis | 16,840 bp | MW327509 | [45] | |
| Plestiodon chinensis | 17,175 bp | KT279358 | [46] | ||
| Plestiodon elegans | 17,304 bp | KJ643142 | [47] | ||
| Plestiodon liui | 17,643 bp | MT662111 | [48] | ||
| Plestiodon tunganus | 17,263 bp | MK370739 | [49] | ||
| Sphenomorphinae | Ablepharus himalayanus | 17,304 bp | MN885892 | [59] | |
| Isopachys gyldenstolpei | 17,210 bp | MH020638 | This study | ||
| Scincella huanrenensis | 17,212 bp | KU507306 | [50] | ||
| Scincella modesta | 17,466 bp | MN702771 | [51] | ||
| Scincella modesta | 17,511 bp | MN786972 | [52] | ||
| Scincella reevesii | 15,424 bp | MN832615 | [53] | ||
| Scincella vandenburghi | 17,103 bp | KU646826 | [50] | ||
| Sphenomorphus indicus | 16,944 bp | OM117611 | This study | ||
| Sphenomorphus indicus | 17,027 bp | MK450438 | [55] | ||
| Sphenomorphus incognitus | 17,417 bp | MH329292 | [54] | ||
| Tropidophorus hainanus | 17,001 bp | OM117612 | This study | ||
| Tropidophorus hangnam | 16,777 bp | MN977920 | [56] | ||
| Cordyloidea | Cordylidae | Smaug warreni | 17,184 bp | NC005962 | [57] |
| Xantusiidae | Xantusiinae | Lepidophyma flavimaculatum | 16,158 bp | NC008775 | [58] |
2.5. Detecting Selective Pressure
3. Results
3.1. Organization and Characteristics of Three Skink Mitochondrial Genomes
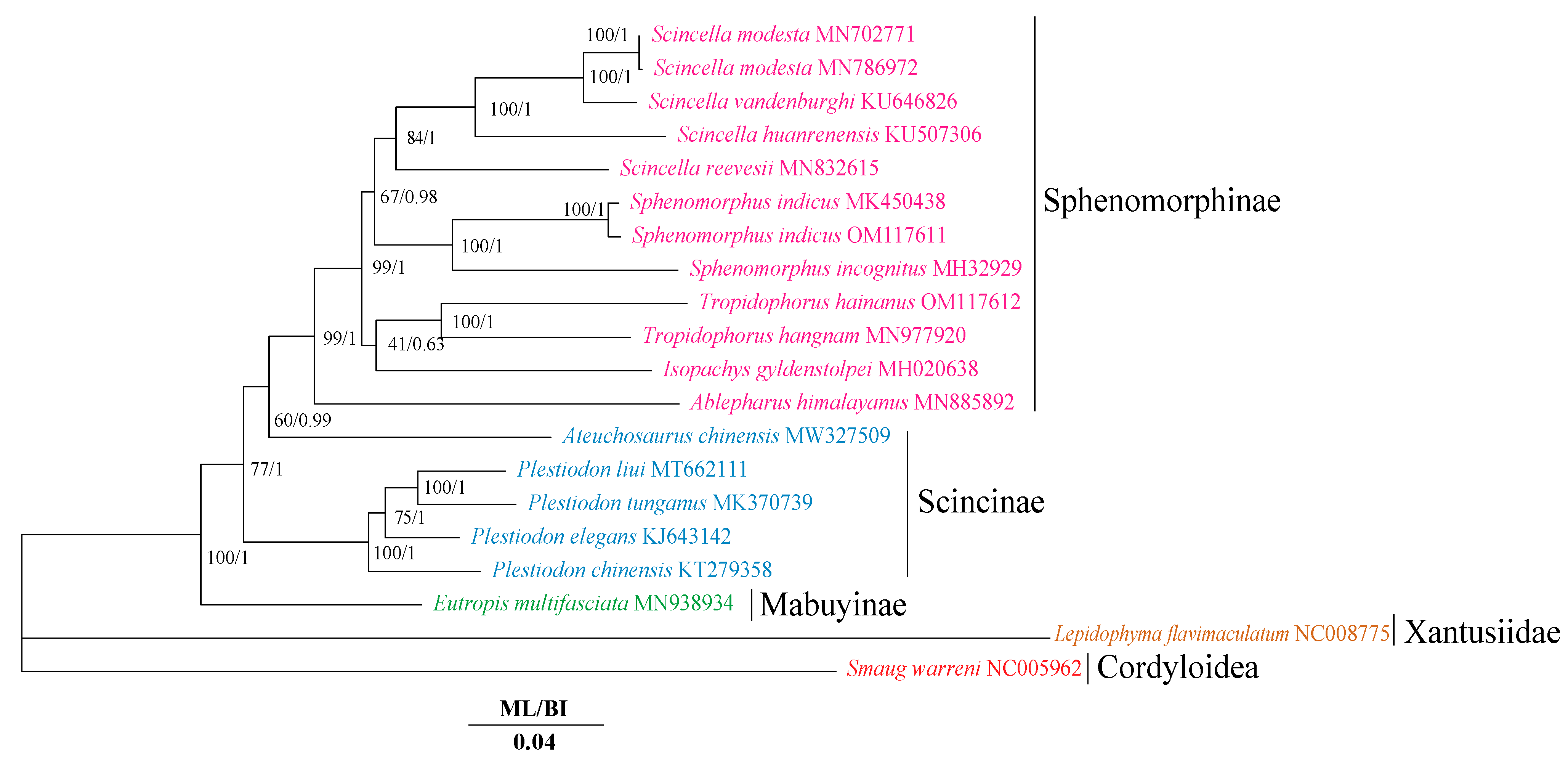
3.2. Phylogenetic Relationships
3.3. Positive Selection Analysis of 13 Protein-Coding Genes
4. Discussion
4.1. Phylogenetic Analyses
4.2. Positive Selection Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uetz, P.; Koo, M.S.; Aguilar, R.; Brings, E.; Catenazzi, A.; Chang, A.T.; Chaitanya, R.; Freed, P.; Gross, J.; Hammermann, M.; et al. A quarter century of reptile and amphibian databases. Herpetol. Rev. 2021, 52, 246–255. [Google Scholar]
- Grismer, L.L.; Wood, P.L.; Quah, E.S.; Anuar, S.; Poyarkov, N.A.; Thy, N.; Orlov, N.L.; Thammachoti, P.; Seiha, H. Integrative taxonomy of the Asian skinks Sphenomorphus stellatus (Boulenger, 1900) and S. praesignis (Boulenger, 1900) with the resurrection of S. annamiticus (Boettger, 1901) and the description of a new species from Cambodia. Zootaxa 2019, 4683, 381–411. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.E. Limb reduction in squamates: Identification of the lineages and discussion of the trends. J. Herpetol. 1991, 95, 166–173. [Google Scholar] [CrossRef]
- Greer, A.E. The Biology and Evolution of Scincid Lizards; Surrey Beatty & Sons: Chipping Norton, NSW, Australia, 2007. [Google Scholar]
- Greer, A.E.; Wilson, G.D. Comments on the scincid lizard genus Ophiomorus, with a cladistic analysis of the species. Hamadryad 2001, 26, 261–271. [Google Scholar]
- Poulakakis, N.; Pakaki, V.; Mylonas, M.; Lymberakis, P. Molecular phylogeny of the Greek legless skink Ophiomorus punctatissimus (Squamata: Scincidae): The impact of the Mid-Aegean trench in its phylogeography. Mol. Phylogenet. Evol. 2008, 47, 396–402. [Google Scholar] [CrossRef]
- Schmitz, A.; Brandley, M.C.; Mausfeld, P.; Vences, M.; Glaw, F.; Nussbaum, R.A.; Reeder, T.W. Opening the black box: Phylogenetics and morphological evolution of the Malagasy fossorial lizards of the subfamily “Scincinae”. Mol. Phylogenet. Evol. 2005, 34, 118–133. [Google Scholar] [CrossRef]
- Wassersug, R.J.; Roberts, L.; Gimian, J.; Hughes, E.; Saunders, R.; Devison, D.; Woodbury, J.; O’Reilly, J.C. The behavioral responses of amphibians and reptiles to microgravity on parabolic flights. Zoology 2005, 108, 107–120. [Google Scholar] [CrossRef]
- Kunya, K.; Kamolnorranath, S.; Chan-ard, T.; Cota, M.; Makchai, S. Additional locality of an endemic legless skink, Isopachys roulei (Angel, 1920) (Squamata, Scincidae) from northeastern Thailand. Thailand Nat. Hist. Mus. J. 2011, 5, 89–93. [Google Scholar]
- Grismer, L.L.; Quah, E.S. The rediscovery of Sphenomorphus malayanus Doria, 1888 (Squamata: Scincidae) from the Titiwangsa Mountain range of Peninsular Malaysia and its re-description as S. senja sp. nov. Zootaxa 2015, 3931, 63–70. [Google Scholar] [CrossRef]
- Grismer, L.L. Lizards of Peninsular Malaysia, Singapore, and their Adjacent Archipelagos: Their Description, Distribution, and Natural History; Edition Chimaira: Frankfurt am Main, Germany, 2011. [Google Scholar]
- Sumarli, A.; Grismer, L.L.; Wood, P.L.; Ahmad, A.B.; Rizal, S.; Ismail, L.H.; Izam, N.A.M.; Ahmad, N.; Linkem, C. The first riparian skink (Genus: Sphenomorphus Strauch, 1887) from Peninsular Malaysia and its relationship to other Indochinese and Sundaic species. Zootaxa 2016, 4173, 029–044. [Google Scholar] [CrossRef]
- Barbour, T. Aquatic skincs and arboreal monitors. Copeia 1921, 97, 42–44. [Google Scholar] [CrossRef]
- Bauer, A.M.; Jackman, T. Global Diversity of Lizards in Freshwater (Reptilia: Lacertilia). In Freshwater Animal Diversity Assess; Springer: Dordrecht, NL, USA, 2007; pp. 581–586. [Google Scholar]
- Guo, K.J.; Shu, F.; Wu, N.F.; Chen, S.D.; Hou, M.; Shi, S.C.; Deng, X.J. Neotype designation and redescription of Tropidophorus guangxiensis Wen, 1992 (Squamata: Sauria: Scincidae), with description of a new subspecies from central South China. Zool. Res. 2021, 42, 606–613. [Google Scholar] [CrossRef]
- Zheng, Y.; Wiens, J.J. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 2016, 94, 537–547. [Google Scholar] [CrossRef]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Hutter, C.R.; Mulcahy, D.G.; Noonan, B.P.; Townsend, T.M.; Sites Jr, J.W.; Reeder, T.W. Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol. Lett. 2012, 8, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.M.; Reeder, T.W.; Wiens, J.J. When do species-tree and concatenated estimates disagree? An empirical analysis with higher-level scincid lizard phylogeny. Mol. Phylogenet. Evol. 2015, 82, 146–155. [Google Scholar] [CrossRef]
- Avise, J.C.; Arnold, J.; Ball, R.M.; Bermingham, E.; Lamb, T.; Neigel, J.E.; Reeb, C.A.; Saunders, N.C. Intraspecific phylogeography: The mitochondrial DNA bridge between population genetics and systematics. Annu. Rev. Ecol. Syst. 1987, 18, 489–522. [Google Scholar] [CrossRef]
- Escalante, I.; Ellis, V.R.; Elias, D.O. Leg loss decreases endurance and increases oxygen consumption during locomotion in harvestmen. J. Comp. Physiol. A 2021, 207, 257–268. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Das, J. The role of mitochondrial respiration in physiological and evolutionary adaptation. BioEssays 2006, 28, 890–901. [Google Scholar] [CrossRef]
- Sun, Y.B.; Shen, Y.Y.; Irwin, D.M.; Zhang, Y.P. Evaluating the roles of energetic functional constraints on teleost mitochondrial-encoded protein evolution. Mol. Biol. Evol. 2011, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Shi, P.; Sun, Y.B.; Zhang, Y.P. Relaxation of selective constraints on avian mitochondrial DNA following the degeneration of flight ability. Genome Res. 2009, 19, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L. Mitochondrial genome organization and vertebrate phylogenetics. Genet. Mol. Biol. 2000, 23, 745–752. [Google Scholar] [CrossRef]
- Galtier, N.; Jobson, R.W.; Nabholz, B.; Glémin, S.; Blier, P.U. Mitochondrial whims: Metabolic rate, longevity and the rate of molecular evolution. Biol. Lett. 2009, 5, 413–416. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Liang, L.; Zhu, Z.H.; Zhou, W.P.; Irwin, D.M.; Zhang, Y.P. Adaptive evolution of energy metabolism genes and the origin of flight in bats. Proc. Natl. Acad. Sci. USA 2010, 107, 8666–8671. [Google Scholar] [CrossRef]
- Chong, R.A.; Mueller, R.L. Low metabolic rates in salamanders are correlated with weak selective constraints on mitochondrial genes. Evol. Int. J. Org. Evol. 2013, 67, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Castoe, T.A.; Jiang, Z.J.; Gu, W.; Wang, Z.O.; Pollock, D.D. Adaptive evolution and functional redesign of core metabolic proteins in snakes. PLoS ONE 2008, 3, e2201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Jiang, G.F.; Yan, L.Y.; Li, R.; Mu, Y.; Deng, W.A. Positive selection drove the adaptation of mitochondrial genes to the demands of flight and high-altitude environments in grasshoppers. Front. Genet. 2018, 9, 605. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, S.; Yang, Y.; Zhou, K.; Yang, G. Phylogenomic analyses and improved resolution of Cetartiodactyla. Mol. Phylogenet. Evol. 2011, 61, 255–264. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Ren, J.; Peng, C.; Jiang, D.; Li, J. Evolution of phenotype and mitochondrial genome reveals limbless and body-elongated Squamates may change their energy basis for locomotion. Asian Herpetol. Res. 2021, 12, 213–220L. [Google Scholar]
- Kumazawa, Y.; Endo, H. Mitochondrial genome of the Komodo dragon: Efficient sequencing method with reptile-oriented primers and novel gene rearrangements. DNA Res. 2004, 11, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, S. Primer Premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Zhang, L.P.; Yu, D.N.; Storey, K.B.; Cheng, H.Y.; Zhang, J.Y. Higher tRNA gene duplication in mitogenomes of praying mantises (Dictyoptera, Mantodea) and the phylogeny within Mantodea. Int. J. Biol. Macromol. 2018, 111, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Burland, T.G. DNASTAR’s lasergene sequence analysis software. Bioinf. Methods Protoc. 2000, 132, 71–91. [Google Scholar]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, W181–W184. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Golding, M. Real World Adobe Illustrator CS4; Peachpit Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Chen, L.; Lin, Y.; Xiao, Q.; Lin, Y.; Du, Y.; Lin, C.; Ward-Fear, G.; Hu, C.; Qu, Y.; Li, H. Characterization of the complete mitochondrial genome of the many-lined sun skink (Eutropis multifasciata) and comparison with other Scincomorpha species. Genomics 2021, 113, 2526–2536. [Google Scholar] [CrossRef]
- Zhong, J.J.; Wu, Q.Q.; Wang, Y.M.; Guo, K.J.; Ding, G.H.; Luo, S.T. The first complete mitochondrial DNA of the Chinese short-limbed skink (Ateuchosaurus chinensis Gray, 1845) determined by next-generation sequencing. Mitochondrial DNA Part B 2021, 6, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, X.; Chen, L.; Xiao, W.; Zhu, X.; Xia, Y.; Chen, J.; Wang, H.; Zhang, B. The complete mitochondrial genome of Eumeces chinensis (Squamata: Scincidae) and implications for Scincidae taxonomy. Mitochondrial DNA Part A 2016, 27, 4691–4692. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, C.; Huang, X.; Zhang, B. Complete mitochondrial genome of Eumeces elegans (Squamata: Scincidae). Mitochondrial DNA Part A 2016, 27, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Cai, B.; Chen, M.; Guo, X. Next-generation sequencing yields a nearly complete mitochondrial genome of Plestiodon liui (Reptilia, Squamata, Scincidae) endemic to China. Mitochondrial DNA Part B 2020, 5, 3637–3638. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Chen, D.; Guo, X. The complete mitochondrial genome of a blue-tailed skink (Plestiodon tunganus) endemic to Sichuan Basin. Mitochondrial DNA Part B 2019, 4, 1109–1110. [Google Scholar] [CrossRef]
- Park, J.; Koo, K.S.; Kim, I.H.; Park, D. Complete mitochondrial genomes of Scincella vandenburghi and S. huanrenensis (Squamata: Scincidae). Mitochondrial DNA Part B 2016, 1, 237–238. [Google Scholar] [CrossRef][Green Version]
- Zhong, J.; Ma, L.; Guo, K.; Du, Y. Complete mitochondrial genome of Scincella modesta (Squamata: Scincidae) and phylogenetic analysis. Mitochondrial DNA Part B 2020, 5, 373–374. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Y.; Lin, Y.; Hu, Y.; Xiao, Q.; Zhou, X.; Liu, Y.; Li, H. Complete mitochondrial genome of Scincella modesta (Squamata: Scincidae). Mitochondrial DNA Part B 2020, 5, 676–677. [Google Scholar] [CrossRef]
- Zhong, J.; Guo, K.; Ma, L. First record of mitochondrial genome of Scincella reevesii (Squamata: Scincidae) and phylogenetic analysis. Mitochondrial DNA Part B 2021, 6, 564–565. [Google Scholar] [CrossRef]
- Tang, X.S.; Yang, D.C.; Huang, S. The complete mitochondrial genome of Sphenomorphus incognitus (Reptilia: Scincidae). Mitochondrial DNA Part B 2019, 4, 307–308. [Google Scholar] [CrossRef]
- Tang, X.S.; Yang, D.C.; Lin, Y.J.; Dai, L.L. The complete mitochondrial genome of Sphenomorphus indicus (Reptilia: Scincidae). Mitochondrial DNA Part B 2019, 4, 2727–2728. [Google Scholar] [CrossRef] [PubMed]
- Duangjai, S.; Srisodsuk, S.; Chuaynkern, C.; Chuaynkern, Y. Complete mitochondrial genome of Tropidophorus hangnam (Squamata: Scincidae) with phylogenetic analysis. Mitochondrial DNA Part B 2020, 5, 3683–3684. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y. Mitochondrial DNA sequences of five squamates: Phylogenetic affiliation of snakes. DNA Res. 2004, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y. Mitochondrial genomes from major lizard families suggest their phylogenetic relationships and ancient radiations. Gene 2007, 388, 19–26. [Google Scholar] [CrossRef]
- Weng, S.Y.; Huang, S.; Yang, L.; Peng, L.F.; Wei, C.; Li, J.C. The complete mitochondrial genome of Asymblepharus himalayanus (Scincidae; Sauria) and its phylogenetic position within Scincidae. Mitochondrial DNA Part B 2021, 6, 3031–3032. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model apace. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Consortium, U. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2018, 46, D115–D119. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Chen, D.; Guo, X. Complete mitochondrial genome of a blue-tailed skink Plestiodon capito (Reptilia, Squamata, Scincidae) and comparison with other Scincidae lizards. Genetica 2020, 148, 229–241. [Google Scholar] [CrossRef]
- Brandley, M.C.; OTA, H.; Hikida, T.; Nieto Montes De Oca, A.; Feria-Ortiz, M.; Guo, X.; Wang, Y. The phylogenetic systematics of blue-tailed skinks (Plestiodon) and the family Scincidae. J. Linn. Soc. Lond. Zool. 2012, 165, 163–189. [Google Scholar] [CrossRef]
- Pyron, R.A. Novel approaches for phylogenetic inference from morphological data and total-evidence dating in squamate reptiles (lizards, snakes, and amphisbaenians). Syst. Biol. 2017, 66, 38–56. [Google Scholar] [CrossRef]
- Hedges, S.B. The high-level classification of skinks (Reptilia, Squamata, Scincomorpha). Zootaxa 2014, 3765, 317–338. [Google Scholar] [CrossRef]
- Honda, M.; Ota, H.; Murphy, R.W.; Hikida, T. Phylogeny and biogeography of water skinks of the genus Tropidophorus (Reptilia: Scincidae): A molecular approach. Zool. Scr. 2006, 35, 85–95. [Google Scholar] [CrossRef]
- Cho, S.; Zwick, A.; Regier, J.C.; Mitter, C.; Cummings, M.P.; Yao, J.; Du, Z.; Zhao, H.; Kawahara, A.Y.; Weller, S.; et al. Can deliberately incomplete gene sample augmentation improve a phylogeny estimate for the advanced moths and butterflies (Hexapoda: Lepidoptera)? Syst. Biol. 2011, 60, 782–796. [Google Scholar] [CrossRef]
- Wiens, J.J.; Fetzner Jr, J.W.; Parkinson, C.L.; Reeder, T.W. Hylid frog phylogeny and sampling strategies for speciose clades. Syst. Biol. 2005, 54, 778–807. [Google Scholar] [CrossRef]
- Zhang, J.; Nielsen, R.; Yang, Z. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 2005, 22, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Dotson, E.M.; Beard, C.B. Sequence and organization of the mitochondrial genome of the Chagas disease vector, Triatoma dimidiata. Insect Mol. Biol. 2001, 10, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Brandt, U. Energy converting NADH: Quinone oxidoreductase (complex I). Annu. Rev. Biochem. 2006, 75, 69–92. [Google Scholar] [CrossRef]
- Wirth, C.; Brandt, U.; Hunte, C.; Zickermann, V. Structure and function of mitochondrial complex I. Biochim. Biophys. Acta Biosyst. 2016, 1857, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Bridges, H.R.; Birrell, J.A.; Hirst, J. The mitochondrial-encoded subunits of respiratory complex I (NADH: Ubiquinone oxidoreductase): Identifying residues important in mechanism and disease. Biochem. Soc. Trans. 2011, 39, 799–860. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, C.; Hägerhäll, C. Transmembrane topology of the NuoL, M and N subunits of NADH: Quinone oxidoreductase and their homologues among membrane-bound hydrogenases and bona fide antiporters. Biochim. Biophys. Acta Biosyst. 2002, 1556, 121–132. [Google Scholar] [CrossRef][Green Version]
- Da Fonseca, R.R.; Johnson, W.E.; O’Brien, S.J.; Ramos, M.J.; Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genom. 2008, 9, 119. [Google Scholar] [CrossRef]
- Gross, M.R. Evolution of diadromy in fishes. Am. Fish. Soc. Symp. 1987, 1, 14–25. [Google Scholar]
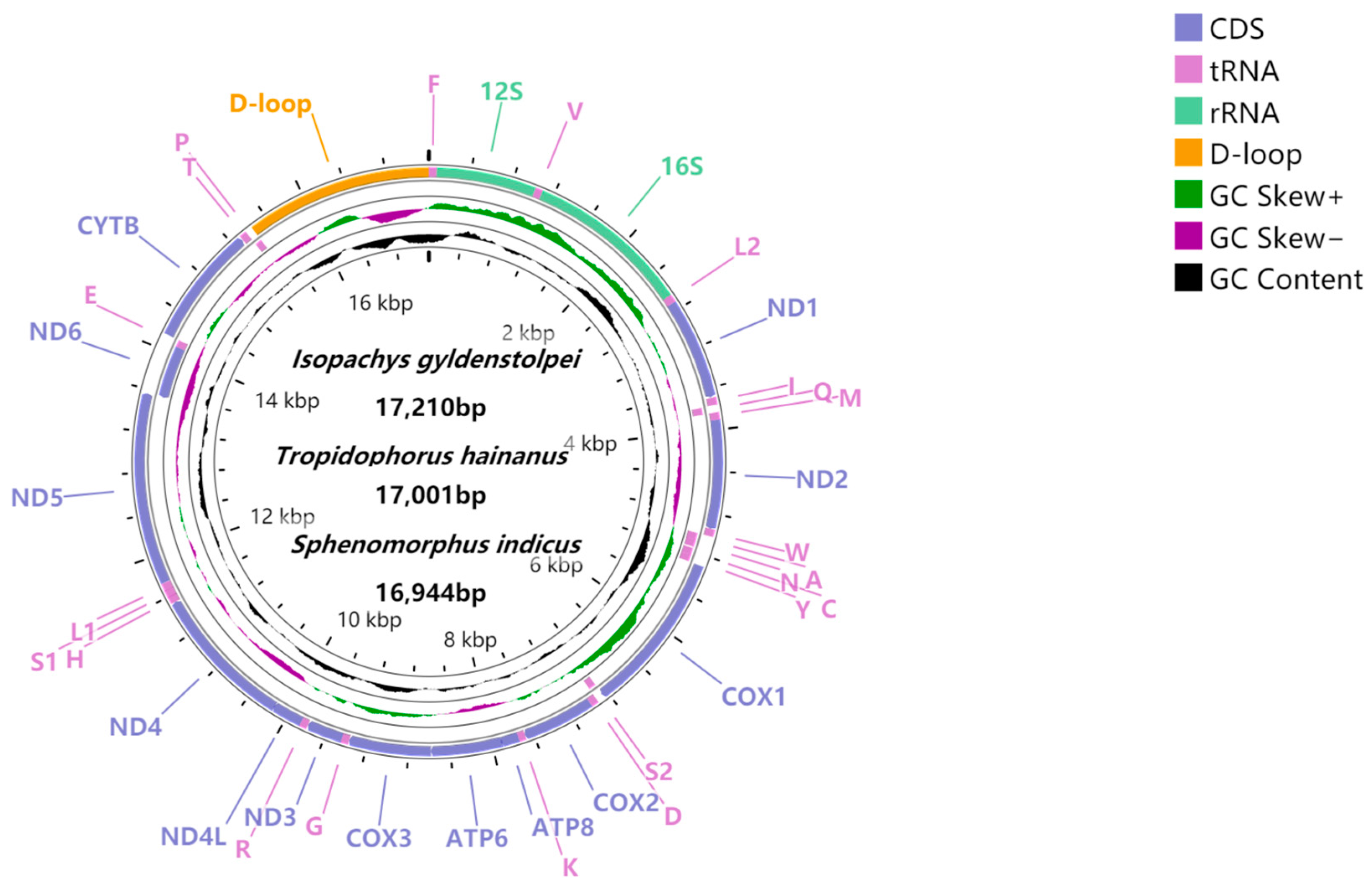
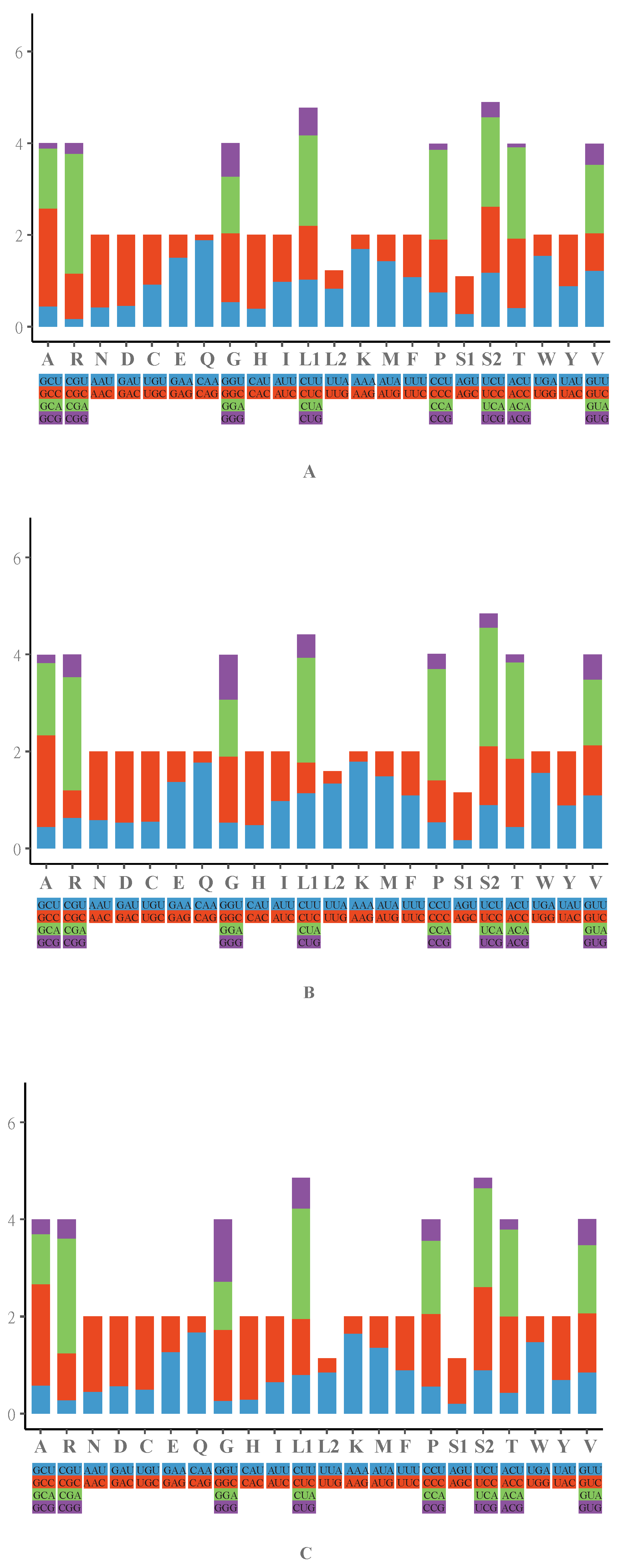
| Region | I. gyldenstolpei | S. indicus | T. hainanus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (bp) | A + T (%) | AT Skew | GC Skew | Length (bp) | A + T (%) | AT Skew | GC Skew | Length (bp) | A + T (%) | AT Skew | GC Skew | ||
| mito | 17,210 | 56 | 0.121 | −0.358 | 16,944 | 57.9 | 0.11 | −0.308 | 17,001 | 54.2 | 0.118 | −0.33 | |
| PCGs | J | 11,382 | 55.52 | 0.058 | −0.392 | 11,382 | 57.28 | 0.056 | −0.35 | 11,379 | 53.06 | 0.063 | −0.363 |
| N | −0.651 | 0.602 | −0.51 | 0.508 | −0.595 | 0.462 | |||||||
| rRNAs | 2507 | 54.2 | 0.313 | −0.207 | 2470 | 57.4 | 0.29 | −0.147 | 2485 | 53.7 | 0.296 | −0.191 | |
| A + T-rich region | 1774 | 61.5 | 0.086 | −0.46 | 1566 | 61.1 | 0.9 | −0.359 | 1615 | 60.4 | 0.07 | −0.404 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Tong, Y.; Ayivi, S.P.G.; Storey, K.B.; Zhang, J.-Y.; Yu, D.-N. The Complete Mitochondrial Genomes of Three Sphenomorphinae Species (Squamata: Scincidae) and the Selective Pressure Analysis on Mitochondrial Genomes of Limbless Isopachys gyldenstolpei. Animals 2022, 12, 2015. https://doi.org/10.3390/ani12162015
Wu L, Tong Y, Ayivi SPG, Storey KB, Zhang J-Y, Yu D-N. The Complete Mitochondrial Genomes of Three Sphenomorphinae Species (Squamata: Scincidae) and the Selective Pressure Analysis on Mitochondrial Genomes of Limbless Isopachys gyldenstolpei. Animals. 2022; 12(16):2015. https://doi.org/10.3390/ani12162015
Chicago/Turabian StyleWu, Lian, Yao Tong, Sam Pedro Galilee Ayivi, Kenneth B. Storey, Jia-Yong Zhang, and Dan-Na Yu. 2022. "The Complete Mitochondrial Genomes of Three Sphenomorphinae Species (Squamata: Scincidae) and the Selective Pressure Analysis on Mitochondrial Genomes of Limbless Isopachys gyldenstolpei" Animals 12, no. 16: 2015. https://doi.org/10.3390/ani12162015
APA StyleWu, L., Tong, Y., Ayivi, S. P. G., Storey, K. B., Zhang, J.-Y., & Yu, D.-N. (2022). The Complete Mitochondrial Genomes of Three Sphenomorphinae Species (Squamata: Scincidae) and the Selective Pressure Analysis on Mitochondrial Genomes of Limbless Isopachys gyldenstolpei. Animals, 12(16), 2015. https://doi.org/10.3390/ani12162015






