Snail Mucus Filtrate Reduces Inflammation in Canine Progenitor Epidermal Keratinocytes (CPEK)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Snail Secretion Filtrate
2.2. Cell Culture and Treatment
2.3. MTT Assay
2.4. Measurement of Cytokine Production
2.5. RT-PCR
2.6. Statistical Analysis
3. Results
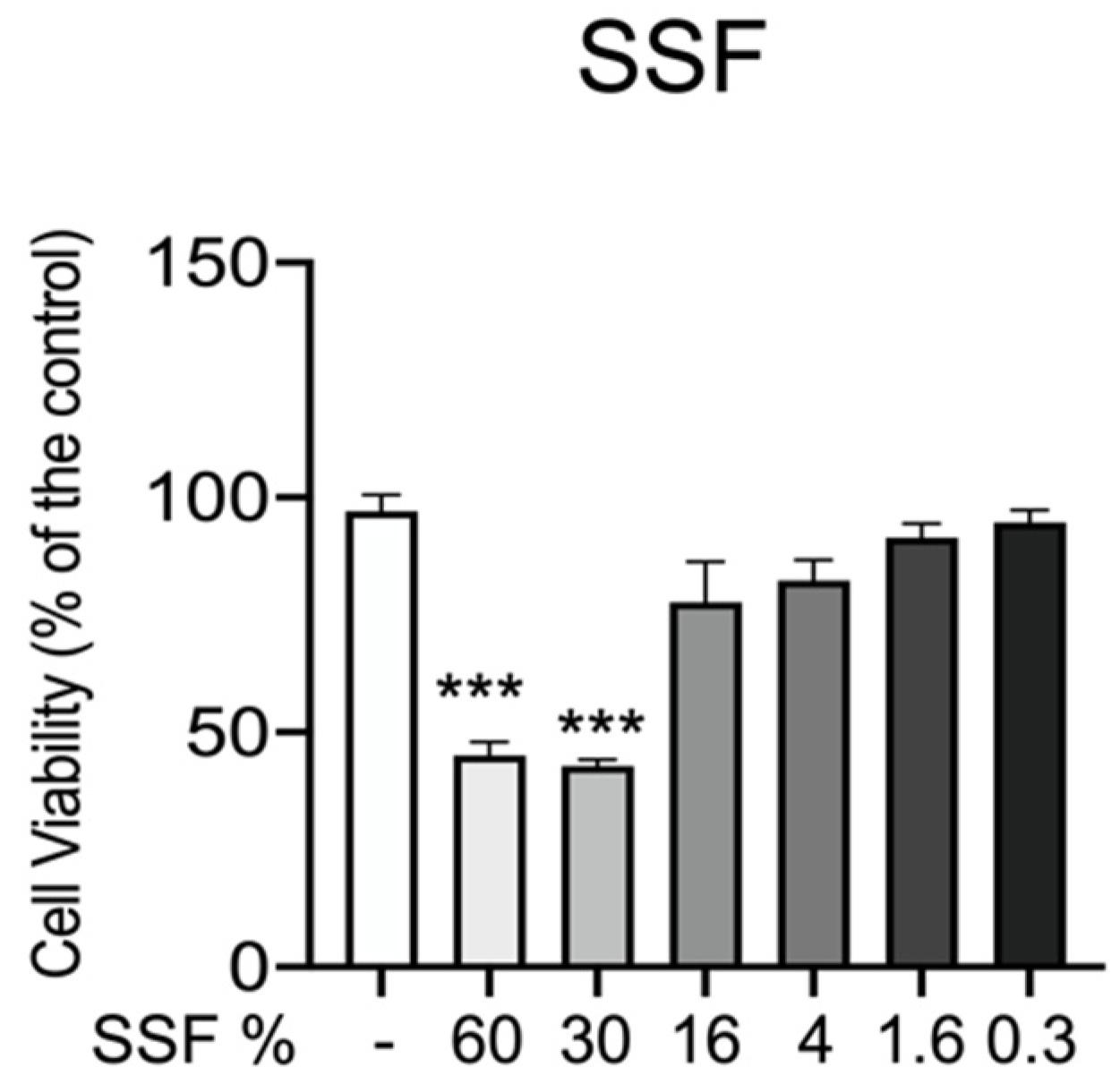
3.1. Effect of SSF on CPEK Cell Viability
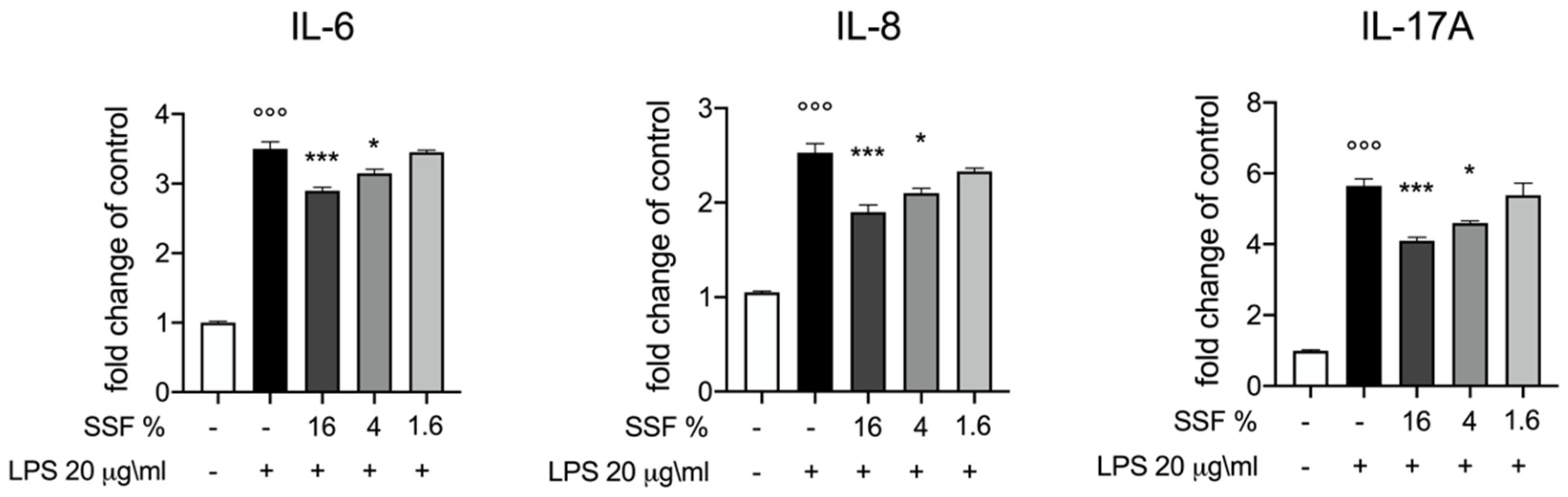
3.2. Effect of SSF on IL-6, IL-8 and IL-17A Release
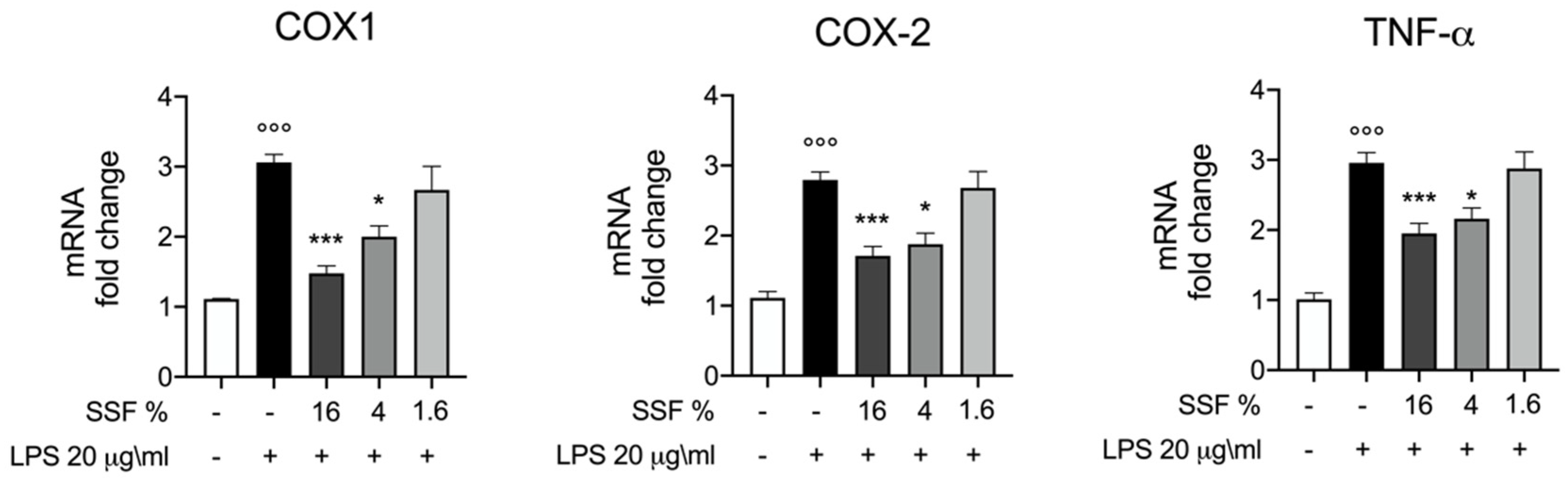
3.3. Effect of SSF on COX1, COX2 and TNF-α Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halliwell, R. Revised nomenclature for veterinary allergy. Vet. Immunol. Immunopathol. 2006, 114, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Brement, T.; Laly, M.J.; Combarros, D.; Guillemaille, D.; Bourdeau, P.J.; Bruet, V. Reliability of different sets of criteria in diagnosing canine atopic dermatitis applied to a population of 250 dogs seen in a veterinary teaching hospital. Vet. Dermatol. 2019, 30, 188-e59. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.; Gajewska, M.; Dembele, K.; Maciejewski, H.; Prostek, A.; Jank, M. Lymphocytic, cytokine and transcriptomic profiles in peripheral blood of dogs with atopic dermatitis. BMC Vet. Res. 2016, 12, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldenhoff, W.E.; Frank, G.R.; DeBoer, D.J. Comparison of the results of intradermal test reactivity and serum allergen-specific IgE measurement for Malassezia pachydermatis in atopic dogs. Vet. Dermatol. 2014, 25, 507–511, e84-5. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R. Atopic Dermatitis in Domestic Animals: What Our Current Understanding Is and How This Applies to Clinical Practice. Vet. Sci. 2021, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Martel, B.C.; Lovato, P.; Baumer, W.; Olivry, T. Translational Animal Models of Atopic Dermatitis for Preclinical Studies. Yale J. Biol. Med. 2017, 90, 389–402. [Google Scholar]
- Noli, C.; Colombo, S.; Cornegliani, L.; Ghibaudo, G.; Persico, P.; Vercelli, A.; Galzerano, M. Quality of life of dogs with skin disease and of their owners. Part 2: Administration of a questionnaire in various skin diseases and correlation to efficacy of therapy. Vet. Dermatol. 2011, 22, 344–351. [Google Scholar] [CrossRef]
- Noli, C.; Minafo, G.; Galzerano, M. Quality of life of dogs with skin diseases and their owners. Part 1: Development and validation of a questionnaire. Vet. Dermatol. 2011, 22, 335–343. [Google Scholar] [CrossRef]
- Parodi, A.; Paolino, S.; Greco, A.; Drago, F.; Mansi, C.; Rebora, A.; Parodi, A.; Savarino, V. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clin. Gastroenterol. Hepatol. 2008, 6, 759–764. [Google Scholar] [CrossRef]
- Pelucchi, C.; Galeone, C.; Bach, J.F.; La Vecchia, C.; Chatenoud, L. Pet exposure and risk of atopic dermatitis at the pediatric age: A meta-analysis of birth cohort studies. J. Allergy Clin. Immunol. 2013, 132, 616–622.e7. [Google Scholar] [CrossRef] [Green Version]
- Vitaliti, G.; Pavone, P.; Guglielmo, F.; Spataro, G.; Falsaperla, R. The immunomodulatory effect of probiotics beyond atopy: An update. J. Asthma 2014, 51, 320–332. [Google Scholar] [CrossRef]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guidelines for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prelaud, P.; International Committee on Allergic Diseases of, A. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet. Res. 2015, 11, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saridomichelakis, M.N.; Olivry, T. An update on the treatment of canine atopic dermatitis. Vet. J. 2016, 207, 29–37. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Macri, F.; Fusco, R.; Siracusa, R.; D’Amico, R.; Cordaro, M.; Peritore, A.F.; Impellizzeri, D.; Genovese, T.; Cuzzocrea, S.; et al. The Protective Effect of Snail Secretion Filtrate in an Experimental Model of Excisional Wounds in Mice. Vet. Sci. 2021, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Siracusa, R.; Genovese, T.; D’Amico, R.; Impellizzeri, D.; Di Paola, R.; Cuzzocrea, S.; et al. Protective effect of snail secretion filtrate against ethanol-induced gastric ulcer in mice. Sci. Rep. 2021, 11, 3638. [Google Scholar] [CrossRef]
- Gentili, V.; Bortolotti, D.; Benedusi, M.; Alogna, A.; Fantinati, A.; Guiotto, A.; Turrin, G.; Cervellati, C.; Trapella, C.; Rizzo, R.; et al. HelixComplex snail mucus as a potential technology against O3 induced skin damage. PLoS ONE 2020, 15, e0229613. [Google Scholar] [CrossRef] [Green Version]
- Greistorfer, S.; Klepal, W.; Cyran, N.; Gugumuck, A.; Rudoll, L.; Suppan, J.; von Byern, J. Snail mucus-glandular origin and composition in Helix pomatia. Zoology (Jena) 2017, 122, 126–138. [Google Scholar] [CrossRef]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181, quiz 208. [Google Scholar] [CrossRef] [Green Version]
- Tsoutsos, D.; Kakagia, D.; Tamparopoulos, K. The efficacy of Helix aspersa Muller extract in the healing of partial thickness burns: A novel treatment for open burn management protocols. J. Dermatolog. Treat. 2009, 20, 219–222. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Palma, E.; Cordaro, M.; D’Amico, R.; Peritore, A.F.; Licata, P.; Crupi, R. Canine atopic dermatitis: Role of luteolin as new natural treatment. Vet. Med. Sci. 2020, 6, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Galfo, F.; Pallio, G.; Mannino, F.; D’Amore, A.; Pellegrino, E.; Ieni, A.; Russo, G.T.; Calapai, M.; et al. Effects of the antagomiRs 15b and 200b on the altered healing pattern of diabetic mice. Br. J. Pharmacol. 2018, 175, 644–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcique, M.A.; Bajwa, J. Atopic dermatitis in humans and dogs. Can Vet J 2020, 61, 82–84. [Google Scholar] [PubMed]
- Marsella, R.; De Benedetto, A. Atopic Dermatitis in Animals and People: An Update and Comparative Review. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Bizikova, P. A systematic review of randomized controlled trials for prevention or treatment of atopic dermatitis in dogs: 2008–2011 update. Vet. Dermatol. 2013, 24, 97-e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, D. Therapies in Canine Atopic Dermatitis: An Update. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 9–26. [Google Scholar] [CrossRef]
- Guidi, E.E.A.; Gramenzi, A.; Persico, P.; Di Prinzio, R.; Di Simone, D.; Cornegliani, L. Effects of Feeding a Hypoallergenic Diet with a Nutraceutical on Fecal Dysbiosis Index and Clinical Manifestations of Canine Atopic Dermatitis. Animals 2021, 11, 2985. [Google Scholar] [CrossRef]
- Tulstrup, M.V.; Christensen, E.G.; Carvalho, V.; Linninge, C.; Ahrne, S.; Hojberg, O.; Licht, T.R.; Bahl, M.I. Antibiotic Treatment Affects Intestinal Permeability and Gut Microbial Composition in Wistar Rats Dependent on Antibiotic Class. PLoS ONE 2015, 10, e0144854. [Google Scholar] [CrossRef]
- Marchegiani, A.; Fruganti, A.; Spaterna, A.; Dalle Vedove, E.; Bachetti, B.; Massimini, M.; Di Pierro, F.; Gavazza, A.; Cerquetella, M. Impact of Nutritional Supplementation on Canine Dermatological Disorders. Vet. Sci. 2020, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- de Roock, S.; van Elk, M.; van Dijk, M.E.; Timmerman, H.M.; Rijkers, G.T.; Prakken, B.J.; Hoekstra, M.O.; de Kleer, I.M. Lactic acid bacteria differ in their ability to induce functional regulatory T cells in humans. Clin. Exp. Allergy 2010, 40, 103–110. [Google Scholar] [CrossRef]
- Anturaniemi, J.; Zaldivar-Lopez, S.; Savelkoul, H.F.J.; Elo, K.; Hielm-Bjorkman, A. The Effect of Atopic Dermatitis and Diet on the Skin Transcriptome in Staffordshire Bull Terriers. Front. Vet. Sci. 2020, 7, 552251. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, S.M.; Aikawa, T.; Matsumoto, J.J. Antibacterial activity of snail mucus mucin. Comp. Biochem. Physiol. A Comp. Physiol. 1982, 72, 571–574. [Google Scholar] [CrossRef]
- Shah, J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef] [PubMed]
- El Mubarak, M.A.; Lamari, F.N.; Kontoyannis, C. Simultaneous determination of allantoin and glycolic acid in snail mucus and cosmetic creams with high performance liquid chromatography and ultraviolet detection. J. Chromatogr. A 2013, 1322, 49–53. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Naruke, A.; Nakano, R.; Nunomura, J.; Suwabe, Y.; Nakano, M.; Namba, S.; Kitanaka, T.; Kitanaka, N.; Sugiya, H.; Nakayama, T. Tpl2 contributes to IL-1beta-induced IL-8 expression via ERK1/2 activation in canine dermal fibroblasts. PLoS ONE 2021, 16, e0259489. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Lindley, I.; Aschauer, H.; Seifert, J.M.; Lam, C.; Brunowsky, W.; Kownatzki, E.; Thelen, M.; Peveri, P.; Dewald, B.; von Tscharner, V.; et al. Synthesis and expression in Escherichia coli of the gene encoding monocyte-derived neutrophil-activating factor: Biological equivalence between natural and recombinant neutrophil-activating factor. Proc. Natl. Acad. Sci. USA 1988, 85, 9199–9203. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Xu, S.; Cao, X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, M.; Leung, D.Y.; Molet, S.; Boguniewicz, M.; Taha, R.; Christodoulopoulos, P.; Fukuda, T.; Elias, J.A.; Hamid, Q.A. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J. Allergy Clin. Immunol. 2003, 111, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitoh, A.; Egawa, G.; Natsuaki, Y.; Nakamizo, S.; Moniaga, C.S.; Otsuka, A.; Honda, T.; Hanakawa, S.; Amano, W.; et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J. Investig. Dermatol. 2014, 134, 2122–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.M.; Lee, B.; Min, J.H.; Kim, E.Y.; Kim, J.H.; Hong, S.; Kim, J.J.; Sohn, Y.; Jung, H.S. Effect of peiminine on DNCB-induced atopic dermatitis by inhibiting inflammatory cytokine expression in vivo and in vitro. Int. Immunopharmacol. 2018, 56, 135–142. [Google Scholar] [CrossRef]
- Munoz, F.C.; Cervantes, M.M.; Cervantes-Garcia, D.; Jimenez, M.; Ventura-Juarez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef]
- Weller, C.L.; Collington, S.J.; Hartnell, A.; Conroy, D.M.; Kaise, T.; Barker, J.E.; Wilson, M.S.; Taylor, G.W.; Jose, P.J.; Williams, T.J. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc. Natl. Acad. Sci. USA 2007, 104, 11712–11717. [Google Scholar] [CrossRef] [Green Version]
- Hundley, T.R.; Prasad, A.R.; Beaven, M.A. Elevated levels of cyclooxygenase-2 in antigen-stimulated mast cells is associated with minimal activation of p38 mitogen-activated protein kinase. J. Immunol. 2001, 167, 1629–1636. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, L.; Bruno, F.; Licata, P.; Paola, D.D.; Franco, G.; Marino, Y.; Peritore, A.F.; Cuzzocrea, S.; Gugliandolo, E.; Crupi, R. Snail Mucus Filtrate Reduces Inflammation in Canine Progenitor Epidermal Keratinocytes (CPEK). Animals 2022, 12, 1848. https://doi.org/10.3390/ani12141848
Messina L, Bruno F, Licata P, Paola DD, Franco G, Marino Y, Peritore AF, Cuzzocrea S, Gugliandolo E, Crupi R. Snail Mucus Filtrate Reduces Inflammation in Canine Progenitor Epidermal Keratinocytes (CPEK). Animals. 2022; 12(14):1848. https://doi.org/10.3390/ani12141848
Chicago/Turabian StyleMessina, Laura, Fabio Bruno, Patrizia Licata, Davide Di Paola, Gianluca Franco, Ylenia Marino, Alessio Filippo Peritore, Salvatore Cuzzocrea, Enrico Gugliandolo, and Rosalia Crupi. 2022. "Snail Mucus Filtrate Reduces Inflammation in Canine Progenitor Epidermal Keratinocytes (CPEK)" Animals 12, no. 14: 1848. https://doi.org/10.3390/ani12141848
APA StyleMessina, L., Bruno, F., Licata, P., Paola, D. D., Franco, G., Marino, Y., Peritore, A. F., Cuzzocrea, S., Gugliandolo, E., & Crupi, R. (2022). Snail Mucus Filtrate Reduces Inflammation in Canine Progenitor Epidermal Keratinocytes (CPEK). Animals, 12(14), 1848. https://doi.org/10.3390/ani12141848









