Simple Summary
Post-weaning diarrhea (PWD) caused the destruction of tight junction and epithelial cells, resulting in the increased gut permeability of pathogenic E.coli, and the reduction of the growth performance. Stimbiotic (STB), while generating specific Xylo-oligosaccharides and a positive effect on gut health, has been introduced as an antibiotics alternative. This study evaluated the effect in weaned piglets of experimentally induced PWD. Our results showed that the pigs were challenged by Shiga toxigenic Escherichia coli (STEC). STB significantly increased the growth performance, immunity and intestinal health compared with the non-supplemented group. Therefore, STB can be used as an effective additive for weaned piglets.
Abstract
The aim of this study was to investigate the effects of stimbiotic (STB), a xylanase and xylo-oligosaccharide complex. A total of 36 male weaned pigs with initial body weights of 8.49 ± 0.10 kg were used in a 3-week experiment. The experiment was conducted in a 2 × 3 factorial arrangement (six replicates/treatment) of treatments consisting of two levels of challenge (challenge and non-challenge) and three levels of STB (0, 0.5, and 1 g/kg diet). Supplementations STB 0.5 g/kg (STB5) and STB 1 g/kg (STB10) improved the G:F (p = 0.04) in piglets challenged with STEC. STB supplementation, which also decreased (p < 0.05) the white blood cells, neutrophils, lymphocytes, and expression levels of tumor necrosis factor-alpha and interleukin-6. Supplementations STB5 and STB10 improved (p < 0.01) the lymphocytes and neutrophils in piglets challenged with STEC on 14 dpi. Additionally, supplementations STB5 and STB10 improved (p < 0.01) the tumor necrosis factor-alpha in piglets challenged with STEC on 3 dpi. Supplementations STB5 and STB10 also improved the villus height-to-crypt depth ratio (p < 0.01) in piglets challenged with STEC. Supplementation with STB reduced (p < 0.05) the expression levels of calprotectin. In conclusion, STB could alleviate a decrease of the performance, immune response, and inflammatory response induced by the STEC challenge.
1. Introduction
The gastrointestinal (GI) tract digests and absorbs nutrients while also acting as a barrier against harmful substances and pathogens ingested through the diet [1]. Pigs are exposed to a variety of pathogenic challenges, which causes the GI immune system to become activated [2]. Although a highly activated immune system may appear to be the best protective mechanism for animals, it can have a negative impact on animal performance [3]. For example, the overactivation of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) leads to the poor growth of pigs [4]. Weaned pigs face problems of post-weaning diarrhea (PWD) caused by pathogenic bacteria such as Shiga toxigenic Escherichia coli (STEC) and enterotoxigenic Escherichia coli (ETEC) during the 4 weeks of the postweaning period [5,6]. The small intestines of pigs show significant structural and functional changes after weaning. These changes lead to the vicissitude of protein conversion rates, microbiota composition, digestive barrier, and immune function [7]. In previous studies, PWD caused a lower growth performance through changes in the gut integrity, microbiome, and villus height, as well as proinflammatory cytokines [8,9]. Many researchers have been studying nonantibiotic biological methods, such as using essential oils, enzymes, probiotics, prebiotics, and organic acids, to prevent digestive diseases by controlling intestinal microbiota [10,11].
Diets in monogastric animals consist mostly of corn and non-starch polysaccharides such as arabinoxylans in Asia [12]. Endo-β-1,4-xylanase (XYL) is a carbohydrate-active enzyme that can hydrolyze the bonds of xylans, thus improving the availability of the antinutritive factors [13]. Xylo-oligosaccharide (XOS) produced through XYL can act as a prebiotic to increase the fermentation metabolites [14]. The concept of stimbiotic (STB), a complex of XYL and XOS, has been recently introduced as a nondigestible and fermentable additive that can improve the fermentation of the fiber microbiome [15,16]. The mechanism of action of STB is that it can stimulate the intestinal microbiota responsible for fiber degradation [17]. In a previous study, Bedford et al. [18] reported that STB can enhance the growth performance and production of short chain fatty acids (SCFAs). Likewise, the supplementation of STB can stimulate the fermentation of dietary fiber, thereby decreasing the digesta viscosity and improving energy utilization [19]. Petry et al. [20] reported that the supplementation of XYL and XOS can improve the intestinal barrier integrity and reduce oxidative stress, respectively. Additionally, poor sanitary conditions, which induce bacterial infection found in industrial pig production, can negatively affect the growth performance and TNF- α, which could alleviate the use of STB [16].
STEC is known to be a major pathogen causing diarrhea, but there is insufficient research validating what causes PWD [21]. Therefore, we conducted experiments to verify the effects of STEC, which induce PWD. In this experiment, we hypothesized that (1) the experimental induction of STEC infection could increase the inflammatory responses and reduce the growth performance of pigs and that (2) the supplementation of STB could attenuate the extent of the performance loss and inflammatory response caused by PWD induced by STEC challenge. Therefore, the purpose of this study was to determine the effects of adding STB on the growth performance, immune response, and inflammatory response when weaned pigs were orally administered with pathogenic E. coli.
2. Materials and Methods
2.1. Ethics
All experimental procedures received prior approval from the Animal Ethics Committee of Chungbuk National University (CBNUA-1618-21-02).
2.2. Bacterial Strains, Culture and Challenge
STEC F18 was provided in stock form. The F18 E. coli expressed heat labile toxin (LT) and shiga toxin type 2e (stx2e). Ten microliter of thawed E. coli stock was inoculated into 10 mL of nutrient broth and cultured at 37 °C for 24 h and then subcultured [22]. Thereafter, the subcultured E. coli was smeared on MacConkey agar to confirm the bacterial enumeration. A final concentration of 1.2 × 1010 CFU/mL was used in this study.
2.3. Animals, Experimental Design and Diets
A total of 36 male pigs (Duroc × Yorkshire × Landrace), weaned at 28 d (initial body weight of 8.49 ± 0.10 kg), were assigned to 6 treatments with 6 replicates per treatment. Pigs were individually placed in 45 × 55 × 45 stainless steel metabolism cages in an environmentally controlled room. Pigs were housed in individual pens for 21 days, including 7 days before and 14 days after the first E. coli challenge (0 dpi). The experiment was conducted in a 2 × 3 factorial arrangement of treatments consisting of two levels of challenge (challenge and non-challenge) and three levels of STB (0, 0.5, and 1 g/kg diet). Corn and soybean meal basal diets were formulated to meet or exceed the nutrient requirements for the weaned piglets by NRC (Table 1) [23]. The pigs were fed daily at 8:30 and 17:00 h and had ad libitum access to water. Feed residues were removed before the next meal and considered in the calculations. Figure 1 depicts the schematic diagram of the weaned piglets experimental design used for this study.

Table 1.
Compositions of basal diets (as-fed basis).
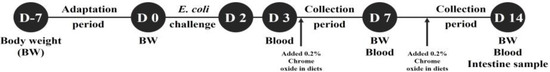
Figure 1.
Schematic diagram of the experimental design.
2.4. Growth Performance
All piglets were weighed every week during the experiment period, and feed consumption was recorded to calculate the average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratio (G:F).
2.5. Fecal Scores
The fecal scores were individually recorded at 08:00 and 17:00 by the same person during the entire experimental period. The fecal score was scored using a method used by Zhao et al. [24]. The fecal scores were as follows: 0, Normal feces; 1, Soft feces; 2, Mild diarrhea; and 3, Severe diarrhea.
2.6. Nutrient Digestibility
To estimate the digestibility, 0.2% chromium oxide (Cr2O3) was supplemented with the diets as an indigestible marker. Pigs were fed diets mixed with chromium oxide for 4 consecutive days from 4 dpi to 11, fresh excreta samples were collected in that period. At the end of the experiment, the fecal samples were stored at −20 °C and dried at 70 °C for 72 h and then ground up to pass through a 1-mm screen. All analysis items (feed and fecal) were analyzed for DM and CP. The procedures utilized for the determination of dry matter (DM) and crude protein (CP) digestibility were conducted with the methods by the AOAC [25]. Chromium was analyzed with an ultraviolet absorption spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan). The digestibility was calculated using the following formula: digestibility (%) = [1 − (Nf × Cd)/(Nd × Cf)] × 100, where Nf is the nutrient concentration in feces (% DM), Nd is the nutrient concentration in diet (% DM), Cd is the chromium concentration in diet (% DM), and Cf is the chromium concentration in feces (% DM).
2.7. Blood Profile
Blood samples were obtained from the anterior vena cava of 6 pigs per each treatment at 3, 7, and 14 dpi. At the time of collection, blood samples were collected into vacuum tubes containing K3EDTA for CBC analysis and nonheparinized tubes for serum analysis, respectively. After collection, blood samples were centrifuged (3000× g for 15 min at 4 °C). The white blood cells (WBC), basophils, neutrophils, and lymphocyte levels in the whole blood were measured using an automatic blood analyzer (ADVIA 120, Bayer, NY, USA). The immunoglobulin G (IgG) and immunoglobulin A (IgA) levels were gauged using an automatic biochemistry blood analyzer (Hitachi 747; Hitachi, Tokyo, Japan).
2.8. Morphological Analysis of Small Intestine
At the end of the experiment (14 dpi), the pigs were anesthetized with carbon dioxide gas after blood sampling and euthanized by exsanguination. Intestinal tissues of about 10 cm from the ileum (close to the ileocecal junction), were collected and fixed in 10% neutral buffered formalin (NBF; Sigma-Aldrich, St. Louis, MO, USA). After cutting the intestine sample, it was dehydrated and dealcoholized. The samples were then installed on slides, treated with paraffin, and stained with hematoxylin and eosin (ab245880, abcam). Villus height and crypt depth were measured under a light microscope (OLYMPUS DP71, BX50F-3, Olympus Optical Co. Ltd., Tokyo, Japan). Villus height (VH) was determined by measuring the distance between the tip of the villi to the villus crypt junction, and the crypt depth (CD) was determined by measuring the distance between adjacent villi. Mean values of 10 fields, 30 well-oriented, complete villus-crypt structures were calculated for each pig.
2.9. Measurements of Pro-Inflammatory Cytokine
The inflammatory biomarkers such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) were measured using commercially available ELISA kits according to manufacturer’s instructions (Quantikine, R&D Systems, Minneapolis, MN, USA).
2.10. Expression of Tight Junction Proteins
The expression of claudin-1 and calprotectin was determined via immunohistochemistry. Histologic tissues were deparaffinized, rehydrated, and rinsed using standard methods. The sections of slide were incubated with the primary antibody for claudin-1 (1:200; Novus Biologicals, Minneapolis, MN, USA) and calprotectin (1:800; Thermo Fisher Scientific, Waltham, MA, USA), followed by washing and incubation with the secondary antibody envision anti-rabbit for claudin-1 (Dako, Santa Clara, CA, USA) and calprotectin (Dako, Santa Clara, CA, USA) for 30 min. The stained samples were evaluated under a microscope (Axio Scan Z1; Carl Zeiss, Jena, Germany), and the images were analyzed via Zen 3.4 blue edition.2.11. Statistical Analysis.
Data for the effects of different levels of STB were added with a challenge or not. Data were subjected to two-way ANOVA. All data were statistically analyzed with a PROC General Linear Models (GLM) of SAS (SAS Institute, Cary, NC, USA). Differences between treatment groups were measured using Duncan’s multiple range test, with a p-value of less than 0.05 designating statistical significance.
3. Results
3.1. Growth Performance
A difference was observed in the BW on 7 dpi and 14 dpi among the treatments (Table 2). The challenged groups showed lesser (p < 0.01) BW compared with the non-challenged groups, and the supplementation of STB groups showed greater (p < 0.05) BW. STB supplementation showed greater (p < 0.05) on ADG in the whole experiment period. Additionally, the supplementation of STB showed greater (p < 0.05) G:F on 0–7 d and 0–14 d. There were interactions among the treatments and challenges in the BW, ADFI, and ADG. Supplementations STB 0.5 g/kg (STB5) and STB 1 g/kg (STB10) improved the BW (p < 0.01), ADFI (p < 0.01), and ADG (p < 0.01) in piglets challenged with E. coli.

Table 2.
Effects of stimbiotic supplementation on the growth performance in pigs challenged with STEC.
3.2. Fecal Score
The fecal score was greater (p < 0.05) in the challenged treatments compared with the non-challenged treatments on 1–7 dpi and 1–14 dpi (Table 3). There was no interaction among the treatments and challenge.

Table 3.
Effects of stimbiotic on the fecal score 1 of pigs challenged with STEC.
3.3. Nutrient Digestibility
The digestibility of DM showed a significant increase (p < 0.05) in the supplementations of STB5 and STB10 compared with the non-supplementation of STB (Table 4). The digestibility of DM and CP were significantly decreased (p < 0.05) and challenged with STEC on 14 dpi. There was no interaction among the treatments and challenge.

Table 4.
Effects of stimbiotic supplementation on the nutrient digestibility of pigs challenged with STEC.
3.4. Blood Profile
Table 5 showed the results of blood profiles at 3, 7, and 14 dpi. At 3 dpi, the counts of the white blood cells were lesser (p < 0.05) in the challenged groups compared to 3 dpi and 7 dpi (Table 5). Neutrophils showed up more in the challenged groups compared to the non-challenged groups on 3, 7, and 14 dpi. Lymphocytes showed up less (p < 0.05) in the challenged groups compared to the non-challenge groups on 3, 7, and 14 dpi. The supplementations of STB5 and STB10 showed reduced (p < 0.05) WBC, neutrophils, and greater (p < 0.05) lymphocytes compared to the non-supplementation of the STB group. There were interactions among the treatments and challenges in neutrophils and lymphocytes. Supplementations STB5 and STB10 improved (p < 0.01) the lymphocytes and neutrophils in piglets challenged with E. coli on d 14.

Table 5.
Effects of stimbiotic supplementation on the blood profile of pigs challenged with STEC.
3.5. Measurements of Proinflammatory Cytokine and Immunoglobulin
The non-challenged treatments were greater (p < 0.05) than the challenged treatments in IL-6 on 3 dpi (Table 6). The supplementations of STB5 and STB10 decreased (p < 0.05) the TNF-α on 3 dpi. On 7 and 14 dpi compared to non-supplementation of the STB group. There were interactions among the treatments and challenges in TNF-α. IgG showed more in the challenged groups compared with the non-challenged groups on 3, 7, and 14 dpi. Supplementations STB5 and STB10 improved (p < 0.01) TNF-α in piglets challenged with E. coli.

Table 6.
Effects of stimbiotic supplementation on the proinflammatory cytokines of pigs challenged with STEC.
3.6. Morphological Analysis of Small Intestine
The non-challenged treatments were longer (p < 0.05) than the challenged treatments in VH. CD was greater (p < 0.05) in the challenged treatments than the non-challenged treatments (Table 7). HDR was greater (p < 0.05) in the non-challenged treatments than the challenged treatments. The supplementation of STB5 and STB10 was increased the VH and HDR (p < 0.05) compared with the non-supplementation of STB group (Figure 2). There was an interaction among the treatments and challenges in the VH, CD, and HDR. Supplementations STB5 and STB10 improved the VH (p < 0.01) and HDR (p < 0.01) in piglets challenged with E. coli. Representative images of the intestinal morphology are captured in Figure 3.

Table 7.
Effects of stimbiotic supplementations on the villus height and crypt depth of pigs challenged with STEC.
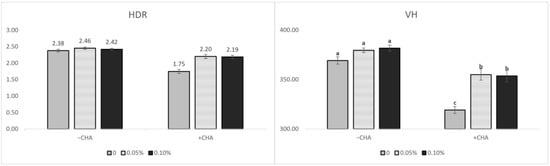
Figure 2.
Effects of stimbiotic on the villus height (VH) and height-to-depth ratio (HDR) in the ileum of weaned piglets. Data are represented as the mean ± standard error. a,b,c Different superscript letters indicate significant differences between groups (p < 0.05).
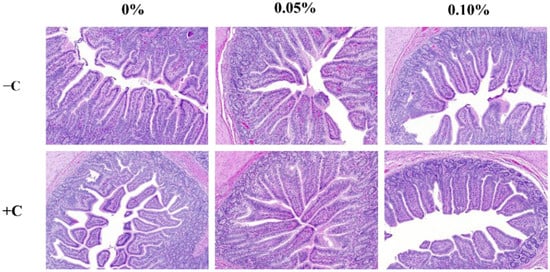
Figure 3.
Histological analysis of the intestinal morphology of weaned piglets. Figures display the morphology of ileum tissue from pigs in six dietary treatments.
3.7. Expression of Tight Junction Proteins
The non-challenged treatments were greater than the challenged treatments in the stained area of calprotectin (Calp) and claudin-1 (CLDN-1) (p < 0.05), and the supplementation with STB5 tended to reduce (p = 0.052) the stained area of the Calp (Table 8). However, the supplementation of STB did not affect CLDN-1. There was no interaction among the treatments and challenge. Representative images of immunohistochemistry staining of the protein expression are captured in Figure 4.

Table 8.
Effects of stimbiotic supplementation on the expression of tight junction proteins of pigs challenged with STEC.
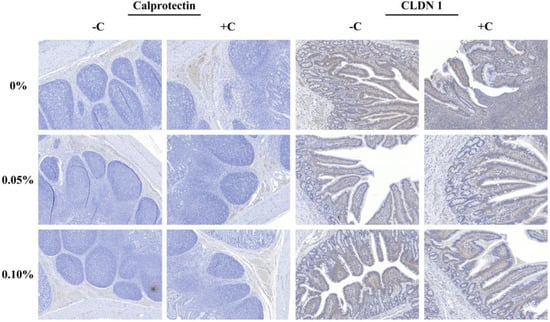
Figure 4.
Immunohistochemistry staining of the protein expression for Claudin-1 and Calprotectin of the ileum tissue. Positive staining indicated by a brown precipitate in cells.
4. Discussion
The small intestine is a major area to digest and absorb nutrients. It can serve as the first line of defense against various harmful substances or pathogens [26]. Weaning causes numerous changes, including enzymatic, morphological, and inflammatory changes that can, damage the intestinal integrity [27]. Harmful bacterial pathogens (i.e., Escherichia coli) can invade through the damaged intestine and lead to decreased nutrient digestion and absorption, consequently decreasing the growth rate [28,29]. In the present study, the STEC challenge decreased the growth performance. The ADG and G:F were reduced by 31% and 23%, respectively. These results were agreement with the results of He et al. [30], which reported that the challenge with E. coli decreased the BW, ADG, ADFI, and G:F compared to the non-challenged groups. A previous study showed that E. coli infection can increase the frequency of diarrhea [31,32]. This result is similar to those obtained in prior studies using an E. coli strain to inoculate pigs. In the present study, the VH and HDR were decreased, but the CD was increased in STEC-challenged pigs. These results are consistent with previous studies showing that pigs challenged with E. coli had a lower VH and HDR in the small intestine [29,33,34,35]. The E. coli challenge could cause a defect of the intestinal barrier integrity by downregulating the expression of tight junction (TJ) proteins. [36,37]. The STEC challenge significantly decreased the CLDN-1 and Calp expression compared to the non-challenge groups in the present, as in the previous study of Yu et al. [34]. TJ proteins, located in the intercellular structure, are junctional adhesion molecules and multi-protein complexes composed of transmembrane proteins [38,39]. They are commonly considered as a strong barrier against the absorption of endotoxin [39]. These results indicated that the oral administration of STEC successfully induced the PWD model, as we hypothesized before starting this study.
STB is a fermentable additive that can stimulate the development of a proportion of bacterial species involved in fiber degradation [16,20]. For example, XOS can improve the intestinal morphology and expression of TJ proteins by improving the gut microbiota communities [40]. Thus, we hypothesized that dietary STB supplementation could mitigate pathogenic E. coli-induced intestinal damage by improving the barrier integrity and suppressing inflammation in weaned pigs. In our study, the supplementation of STB improved the intestinal morphology and expression of TJ in pigs challenged with STEC. These results were in agreement with previous results showing that 0.01% and 0.05% XOS could increase the VH in the ileum and HDR in the jejunum [40,41]. Consistently, prebiotics (i.e., alginate-oligosaccharide, AOS; mannan-oligosaccharide, MOS) could mitigate the intestinal mucosa injury by improving the VH and HDR [42,43]. Additionally, the supplementation of MOS could elevate the expression levels of TJ proteins such as zonula occludens-1, CLDN-1, and Occludin reduced by the ETEC challenge [43]. These results were similar to the results of the present study. Previous studies have shown that prebiotics could upregulate the expression levels of the intestinal TJ proteins in piglets [44,45]. Through many studies, including ours, the consistent improvement of oligosaccharides might be associated with the production of SCFAs. SCFAs are volatile fatty acids (VFA). They mainly include acetic acid, propionic acid, and butyric acid [46]. SCFAs can promote the intestinal morphology and TJ proteins of broilers [47,48]. They can also be used as energy sources, leading to an improved absorption surface in the intestine via increased the proliferation of epithelial cells [49,50,51,52]. The exact mechanism of action of STB is currently unknown. It has been suggested that the improvement of the gut integrity is due to improved gut fermentation.
In the current study, STB supplementation alleviated the reduction of nutrient digestibility and growth performance caused by the STEC challenge. This effect of STB might be due to its ability to improve gut health, as mentioned above. It might also be due to its ability to improve the enzyme activities [53]. Previous studies have reported that MOS supplementation can increase the mucosal enzyme activities, including duodenal sucrase, ileal lactase, and ileal maltase activities in the ETEC challenged pigs [43].
The count of WBC is one of the most common diagnostic indications of infection. WBC are an important part of the immune system that fights against infections in the body [54]. While neutrophils are the first cells to move into infected tissues during inflammatory reactions and phagocytose bacteria with their particles, lymphocytes produce specialized cellular and humoral immune responses [55]. The neutrophils-to-lymphocytes ratio is often used as a biomarker to assess the systemic inflammation severity. According to Liu et al. [31], E. coli can cause inflammation in weaned piglets by boosting WBC counts and neutrophils. A previous study showed that Chito-oligosaccharides with a function similar to XOS can mitigated the increase in the value of neutrophils [33]. In the current study, during E. coli infection, pigs supplemented with STB had a lower neutrophils-to-lymphocytes ratio than non-supplemented with the STB groups, indicating that inflammation was decreased in piglets with STB supplementation. These results indicate that STB can reduce E. coli-induced intestinal inflammation in pigs potentially by lowering the bacterial growth and metabolism in gut bacterial environments [56].
When E. coli enters the bloodstream, the general immune response is triggered, and immune cells in tissues are activated by bacterial ligands, resulting in a fast burst of proinflammatory cytokines [57]. In a previous study, acute exposure to E. coli can increase the blood endotoxin levels, leukocyte numbers, and proinflammatory cytokine production [58]. Shiga toxins released by pathogenic E. coli could cause systemic inflammation, therefore increasing inflammatory cytokines [59]. Moreover, STEC could utilize hemoglobin as an iron source for their proliferation and virulence production [60]. A recent study showed that E. coli can increase the production of proinflammatory cytokines in piglets [61]. Yu et al. [43] reported that the supplementation of MOS can reduce the concentrations of inflammatory cytokines. Our study also showed that supplementation with STB reduced the levels of the proinflammatory cytokines. These results confirmed that the E. coli challenge induces elevated concentrations of inflammatory cytokines and that the supplementation with STB decreases the concentrations of inflammatory cytokines in piglets.
5. Conclusions
The challenge with E. coli decreased the growth performance and VH, increased the inflammatory response, and downregulated TJ proteins. However, supplementing STB, a complex of XYL and XOS, alleviated these negative effects of the E. coli challenge in this study. Supplementation with STB improved the growth performance, intestinal morphology, and inflammatory responses. These results suggest that STB might be effective in mitigating post-weaning diarrhea, a severe disease in weaning piglets.
Author Contributions
D.S., J.L., W.K. and M.S. conducted the experiment and wrote the manuscript. H.O., Y.K., J.A., S.C., Y.G. and H.C. helped to conduct animal trials and laboratory work and helped to revise the manuscript. J.C. and H.K. were the principal investigators and wrote the last version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2021R1I1A3051928).
Institutional Review Board Statement
The experimental protocol was approved (CBNUA-1618-21-02) by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kyoung, H.; Lee, J.J.; Cho, J.H.; Choe, J.; Kang, J.; Lee, H.; Song, M. Dietary glutamic acid modulates immune responses and gut health of weaned pigs. Animals 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluske, J.R.; Kim, J.C.; Black, J.L. Manipulating the immune system for pigs to optimise performance. Anim. Prod. Sci. 2018, 58, 666–680. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okello, E.; Moonens, K.; Erume, J.; De Greve, H. Orally Fed Recombinant Lactococcus lactis Displaying Surface Anti-Fimbrial Nanobodies Protects Piglets against Escherichia coli Causing Post-Weaning Diarrhea. Agriculture. 2021, 11, 186. [Google Scholar] [CrossRef]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 2005, 295, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef]
- Xu, X.; Duarte, M.E.; Kim, S.W. Postbiotics effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2022. [Google Scholar] [CrossRef]
- Sun, Y.; Duarte, M.E.; Kim, S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim. Nutr. 2021, 7, 326–333. [Google Scholar] [CrossRef]
- Betancur, C.; Martínez, Y.; Merino-Guzman, R.; Hernandez-Velasco, X.; Castillo, R.; Rodríguez, R.; Tellez-Isaias, G. Evaluation of oral administration of Lactobacillus plantarum CAM6 strain as an alternative to antibiotics in weaned pigs. Animals 2020, 10, 1218. [Google Scholar] [CrossRef]
- Hall, H.N.; Wilkinson, D.J.; Le Bon, M. Oregano essential oil improves piglet health and performance through maternal feeding and is associated with changes in the gut microbiota. Anim. Microbiome. 2021, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.B.; Hedemann, M.S.; Lærke, H.N. The role of carbohydrates in intestinal health of pigs. Anim. Feed Sci. Technol. 2012, 173, 41–53. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Nyachoti, C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013, 26, 71–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiao, K.; Yu, C.; Wang, L.; Liang, T.; Zhu, H.; Liu, Y. Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities. Anim. Nutr. 2021, 7, 609–620. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, G.; Olukosi, O.A.; Jurgens, G.; Apajalahti, J.; Bedford, M.R. Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase. Poult. Sci. 2020, 99, 2068–2077. [Google Scholar] [CrossRef]
- Cho, H.M.; González-Ortiz, G.; Melo-Durán, D.; Heo, J.M.; Cordero, G.; Bedford, M.R.; Kim, J.C. Stimbiotic supplementation improved performance and reduced inflammatory response via stimulating fiber fermenting microbiome in weaner pigs housed in a poor sanitary environment and fed an antibiotic-free low zinc oxide diet. PLoS ONE. 2020, 15, e0240264. [Google Scholar] [CrossRef] [PubMed]
- Bautil, A.; Verspreet, J.; Buyse, J.; Goos, P.; Bedford, M.R.; Courtin, C.M. Arabinoxylan-oligosaccharides kick-start arabinoxylan digestion in the aging broiler. Poult. Sci. 2020, 99, 2555–2565. [Google Scholar] [CrossRef]
- Bedford, M.R. The evolution and application of enzymes in the animal feed industry: The role of data interpretation. Br. Poult. Sci. 2018, 59, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Morgan, N.K.; Gomes, G.A.; Kim, J.C. Comparing the efficacy of stimbiotic and a combination of xylanase and beta-glucanase, in broilers fed wheat-barley based diets with high or low AME. Poult Sci. 2021, 100, 101383. [Google Scholar] [CrossRef]
- Petry, A.L.; Patience, J.F.; Koester, L.R.; Huntley, N.F.; Bedford, M.R.; Schmitz-Esser, S. Xylanase modulates the microbiota of ileal mucosa and digesta of pigs fed corn-based arabinoxylans likely through both a stimbiotic and prebiotic mechanism. PLoS ONE. 2021, 16, e0246144. [Google Scholar] [CrossRef]
- Baldo, V.; Salogni, C.; Giovannini, S.; D’Incau, M.; Boniotti, M.B.; Birbes, L.; Alborali, G.L. Pathogenicity of Shiga toxin type 2e Escherichia coli in pig colibacillosis. Front. Vet. Sci. 2020, 7, 545818. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Sureshkumar, S.; Kim, I.H. Effects of dietary lysozyme supplementation on growth performance, nutrient digestibility, intestinal microbiota, and blood profiles of weanling pigs challenged with Escherichia coli. J. Anim. Sci. Technol. 2021, 63, 501. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Zhao, P.Y.; Jung, J.H.; Kim, I.H. Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs. J. Anim. Sci. 2012, 90, 833–839. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2007. [Google Scholar]
- Wu, Z.; Feng, H.; Cao, Y.; Huang, Y.; Dai, C.; Wu, S.; Bao, W. New insight into the molecular mechanism of the FUT2 regulating Escherichia Coli F18 resistance in weaned piglets. Int. J. Mol. Sci. 2018, 19, 3301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Gao, Y.; Han, F.; Huang, X.; Rong, Y.; Yi, H.; Wang, Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: A comparative study. J. Anim. Sci. 2013, 91, 5614–5625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Jinno, C.; Kim, K.; Wu, Z.; Tan, B.; Li, X.; Liu, Y. Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J. Anim. Sci. Biotechnol. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.M.; Almeida, J.A.S.; Lee, J.J.; Bravo, D.; Pettigrew, J.E. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J. Anim. Sci. 2013, 91, 5294–5306. [Google Scholar] [CrossRef] [Green Version]
- Yi, G.F.; Carroll, J.A.; Allee, G.L.; Gaines, A.M.; Kendall, D.C.; Usry, J.L.; Izuru, S. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J. Anim. Sci. 2005, 83, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Meng, T.; He, W.; Huang, H.; Liu, C.; Fu, X.; Xiao, D. Dietary Chito-oligosaccharides Improve Intestinal Immunity via Regulating Microbiota and Th17/Treg Balance-Related Immune Signaling in Piglets Challenged by Enterotoxigenic E. coli. J. Agric. Food Chem. 2021, 69, 15195–15207. [Google Scholar] [CrossRef] [PubMed]
- Wellington, M.O.; Hamonic, K.; Krone, J.E.; Htoo, J.K.; Van Kessel, A.G.; Columbus, D.A. Effect of dietary fiber and threonine content on intestinal barrier function in pigs challenged with either systemic E. coli lipopolysaccharide or enteric Salmonella Typhimurium. J. Anim. Sci. Biotechnol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: An effective way to alter the barrier integrity. Microb. Pathog. 2017, 113, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; He, Y.; Xiong, X.; Ehrlich, A.; Li, X.; Raybould, H.; Liu, Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, K.K.; Shukla, P.K.; Mir, H.; Manda, B.; Gangwar, R.; Yadav, N.; Rao, R. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J. Nutr. Biochem. 2016, 27, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xie, Y.; Zhong, R.; Liu, L.; Lin, C.; Xiao, L.; Everaert, N. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front. Microbiol. 2021, 12, 355. [Google Scholar] [CrossRef]
- Yin, J.; Li, F.; Kong, X.; Wen, C.; Guo, Q.; Zhang, L.; Yin, Y. Dietary xylo-oligosaccharide improves intestinal functions in weaned piglets. Food Funct. 2019, 10, 2701–2709. [Google Scholar] [CrossRef]
- Su, J.; Zhang, W.; Ma, C.; Xie, P.; Blachier, F.; Kong, X. Dietary Supplementation with Xylo-oligosaccharides Modifies the Intestinal Epithelial Morphology, Barrier Function and the Fecal Microbiota Composition and Activity in Weaned Piglets. Front. Vet. Sci. 2021, 8, 680208. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; He, J. Alginate oligosaccharide alleviates enterotoxigenic Escherichia coli-induced intestinal mucosal disruption in weaned pigs. Food Funct. 2018, 9, 6401–6413. [Google Scholar] [CrossRef]
- Yu, E.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; He, J. Manno-oligosaccharide attenuates inflammation and intestinal epithelium injury in weaned pigs upon enterotoxigenic Escherichia coli K88 challenge. Br. J. Nutr. 2021, 126, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Kong, X.F.; Lian, G.Q.; Blachier, F.; Geng, M.M.; Yin, Y.L. Dietary supplementation with soybean oligosaccharides increases short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal content of weaned Huanjiang mini-piglets. Nutr. Res. 2014, 34, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.C.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; Xiangjie, K.; Hailong, J.; Guixin, Q.; Sossah, F.L.; Dongsheng, C. Prebiotic effects of alfalfa (Medicago sativa) fiber on cecal bacterial composition, short-chain fatty acids, and diarrhea incidence in weaning piglets. RSC Adv. 2019, 9, 13586–13599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Henningsson, Å.; Björck, I.; Nyman, M. Short-chain fatty acid formation at fermentation of indigestible carbohydrates. Näringsforskning 2001, 45, 165–168. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, Y.; Tao, S.; Zhang, G.; Wang, J.; Liu, L.; Zhang, S. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model. J. Funct. Foods. 2019, 57, 266–274. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes. Nutr. 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, J.; Yin, Q.; Lu, M.; Di, Y.; Wang, P.; Lu, F. Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Anim. Nutr. 2017, 3, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Ariyibi, S.; Antony, L.; Scaria, J.; Dilberger-Lawson, S.; Francis, D.; Woyengo, T.A. Growth performance and gut health of Escherichia coli-challenged weaned pigs fed canola meal-containing diet. J. Anim. Sci. 2021, 99, skab196. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; He, Y.; Jinno, C.; Kovanda, L.; Li, X.; Song, M.; Liu, Y. Trace amounts of antibiotic exacerbated diarrhea and systemic inflammation of weaned pigs infected with a pathogenic Escherichia coli. J. Anim. Sci. 2021, 99, skab073. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Looft, T.; Severin, A.J.; Bayles, D.O.; Nasko, D.J.; Wommack, K.E.; Allen, H.K. The in-feed antibiotic carbadox induces phage gene transcription in the swine gut microbiome. mBio. 2017, 8, e00709-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyachoti, C.M.; Kiarie, E.; Bhandari, S.K.; Zhang, G.; Krause, D.O. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J. Anim. Sci. 2012, 90, 252. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Cai, S.; Zhou, T.; Zhang, S.; Li, S.; Jin, E.; Qiao, S. Isoleucine attenuates infection induced by E. coli challenge through the modulation of intestinal endogenous antimicrobial peptide expression and the inhibition of the increase in plasma endotoxin and IL-6 in weaned pigs. Food Funct. 2019, 10, 3535–3542. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Law, D.; Kelly, J. Use of heme and hemoglobin by Escherichia coli O157 and other Shiga-like-toxin-producing E. coli serogroups. Infect. Immun. 1995, 63, 700–702. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Awji, E.G.; Lee, S.J.; Tassew, D.D.; Park, Y.B.; Park, K.S.; Park, S.C. Effect of Lactobacillus plantarum CJLP243 on the growth performance and cytokine response of weaning pigs challenged with enterotoxigenic Escherichia coli. J. Anim. Sci. 2012, 90, 3709–3717. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).