Duodenal Metabolic Profile Changes in Heat-Stressed Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
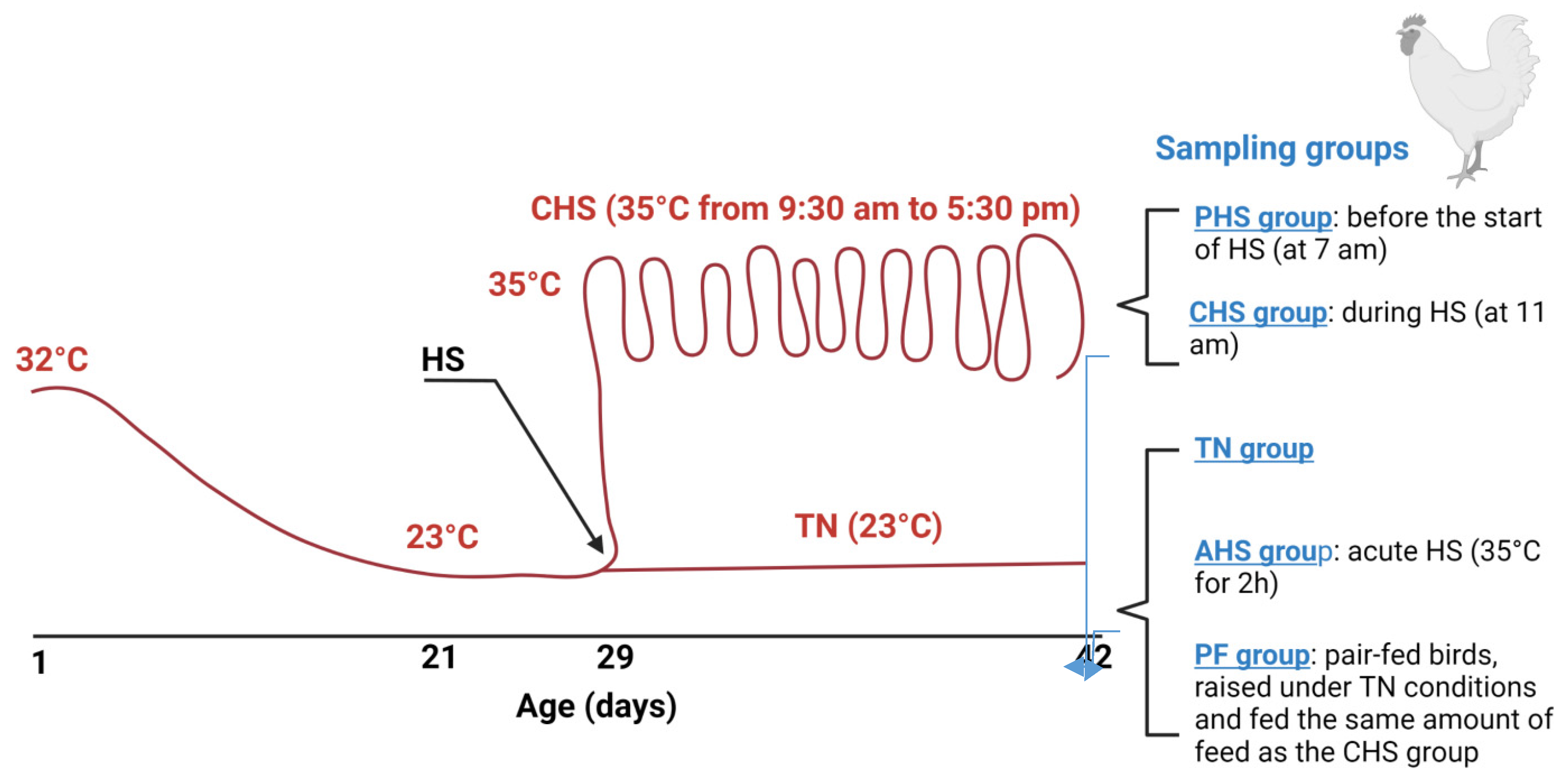
2.2. Birds, Diets, and Heat Stress Challenge
2.3. Sample Collection and Preparation
2.4. Ultra-High Performance Liquid Chromatography—High Resolution Mass Spectrometry (UHPLC–HRMS) Metabolomics Analysis
2.5. Ingenuity Pathway Analysis (IPA)
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Immunoblot Analysis
2.8. Data Processing and Statistical Analysis
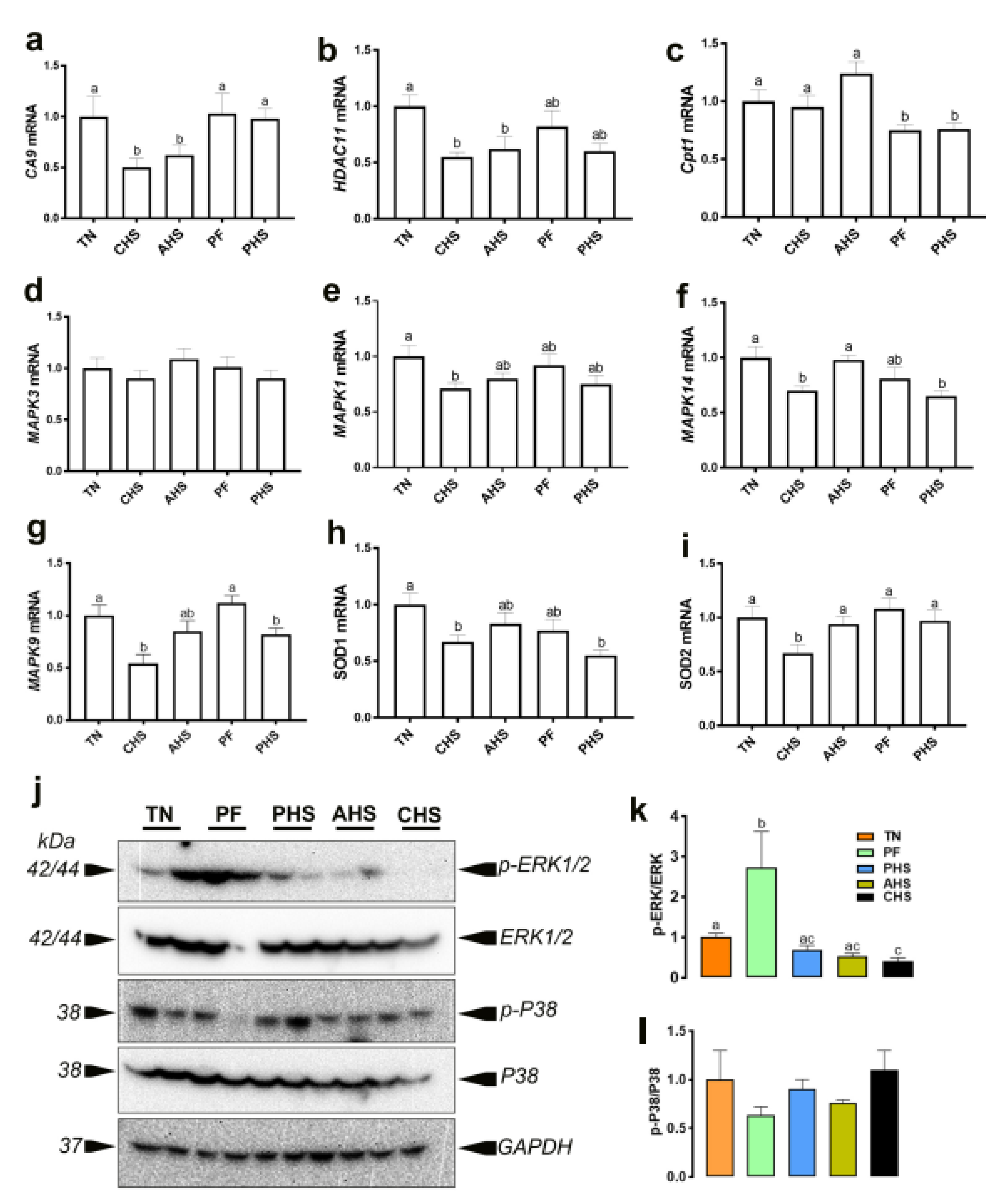
3. Results
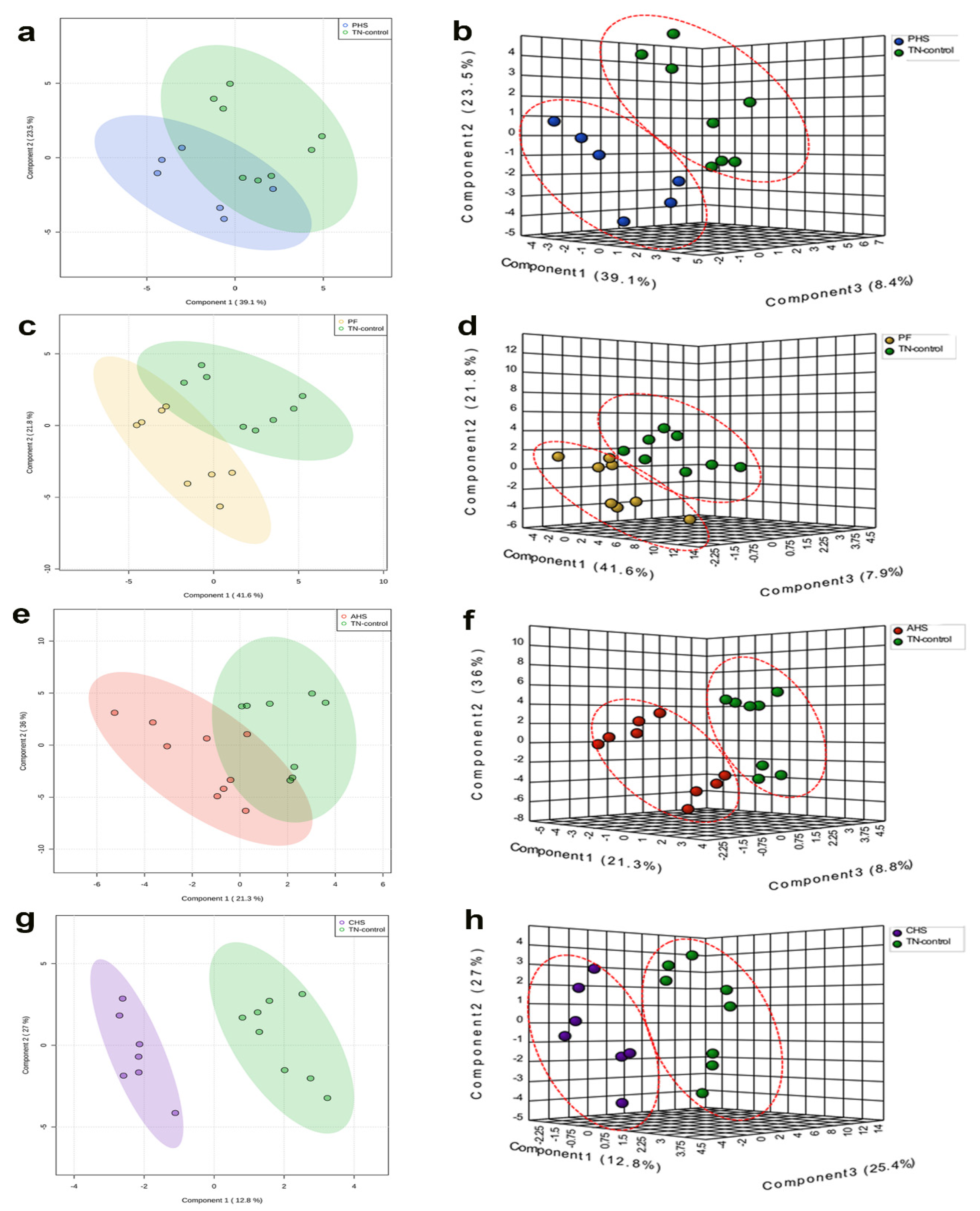
3.1. Global Analysis of the Duodenal Dynamic Metabolic Profiling
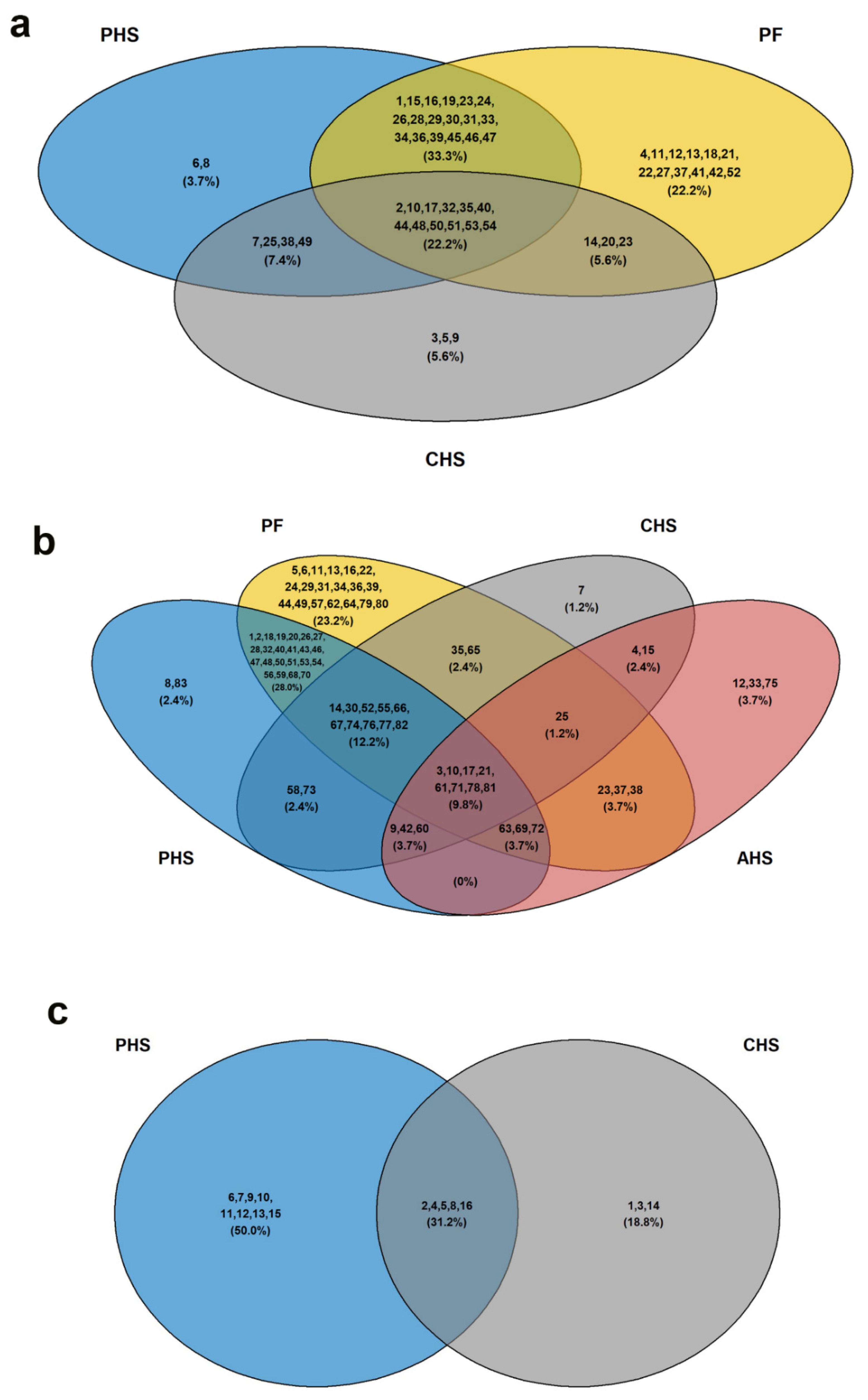
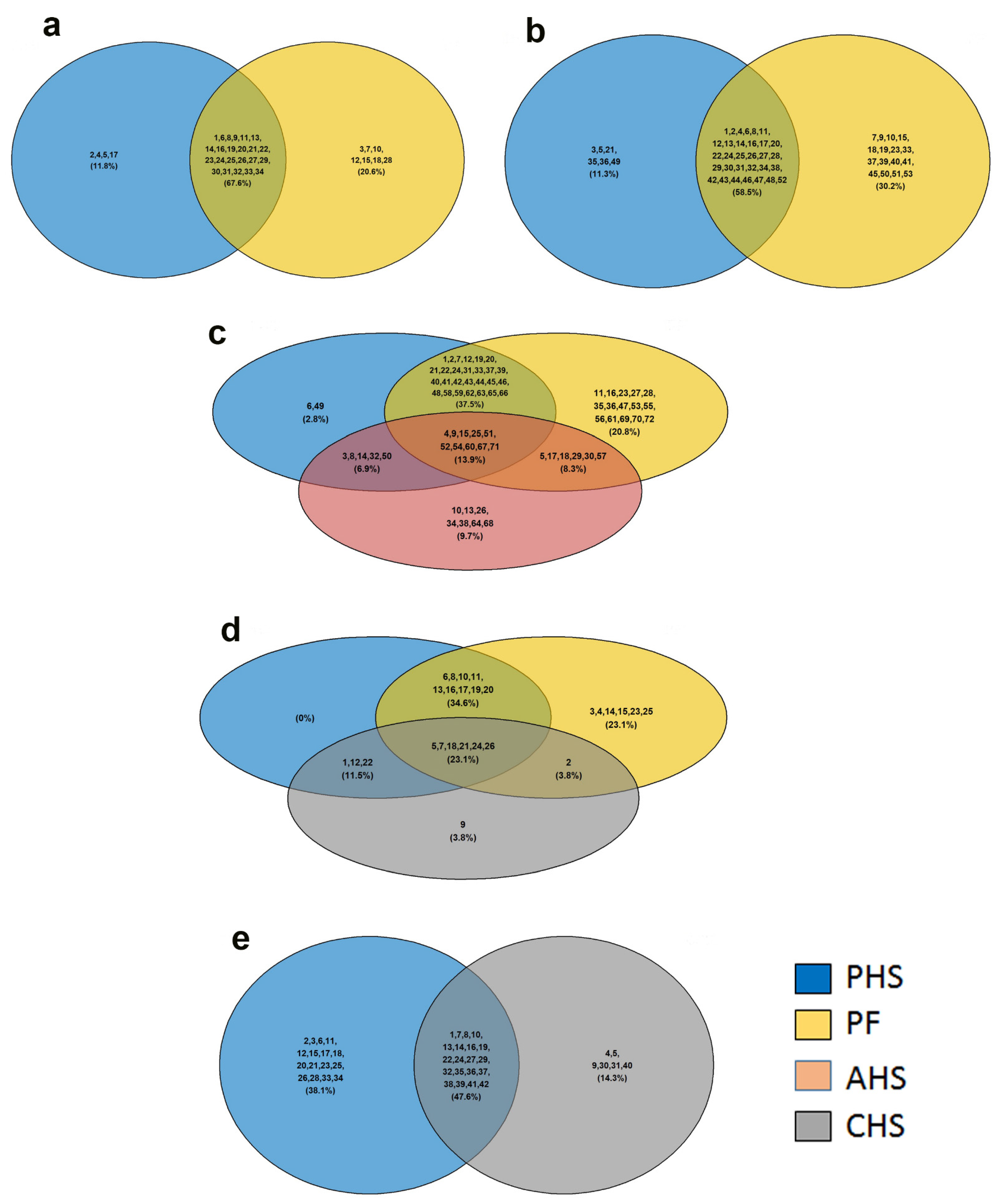
3.2. Identification of Potential Metabolic Signatures
3.3. Metabolic Pathway and Network Analysis
3.3.1. Top Canonical Pathways
3.3.2. Top Diseases and Disorders
3.3.3. Top Molecular and Cellular Functions
3.3.4. Top Up- and Downstream Regulators
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture, Foreign Agricultural Service. Livestock and Poultry: World Markets and Trade; United States Department of Agriculture, Foreign Agricultural Service: Washington, DC, USA, 2022; p. 16.
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry Response to Heat Stress: Its Physiological, Metabolic, and Genetic Implications on Meat Production and Quality Including Strategies to Improve Broiler Production in a Warming World. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef] [PubMed]
- Alley, R.B.; Clark, P.U.; Huybrechts, P.; Joughin, I. Ice-sheet and sea-level changes. Science 2005, 310, 456–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karn, M.; Sharma, M. Climate change, natural calamities and the triple burden of disease. Nat. Clim. Chang. 2021, 11, 796–797. [Google Scholar] [CrossRef]
- Baker, J.S.; Havlik, P.; Beach, R.; Leclere, D.; Schmid, E.; Valin, H.; Cole, J.; Creason, J.; Ohrel, S.; McFarland, J. Evaluating the effects of climate change on US agricultural systems: Sensitivity to regional impact and trade expansion scenarios. Environ. Res. Lett. 2018, 13, 064019. [Google Scholar] [CrossRef] [Green Version]
- Moore, F.C.; Baldos, U.; Hertel, T.; Diaz, D. New science of climate change impacts on agriculture implies higher social cost of carbon. Nat. Commun. 2017, 8, 1607. [Google Scholar] [CrossRef]
- Nelson, G.C.; Valin, H.; Sands, R.D.; Havlik, P.; Ahammad, H.; Deryng, D.; Elliott, J.; Fujimori, S.; Hasegawa, T.; Heyhoe, E.; et al. Climate change effects on agriculture: Economic responses to biophysical shocks. Proc. Natl. Acad. Sci. USA 2014, 111, 3274–3279. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Muller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [Green Version]
- Stevanovic, M.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Muller, C.; Bonsch, M.; Schmitz, C.; Bodirsky, B.L.; Humpenoder, F.; Weindl, I. The impact of high-end climate change on agricultural welfare. Sci. Adv. 2016, 2, e1501452. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat stress impacts on broiler performance: A systematic review and meta-analysis. Poult. Sci. 2020, 99, 6205–6211. [Google Scholar] [CrossRef] [PubMed]
- Deeb, N.; Shlosberg, A.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 4. Association between responses to heat stress and to cold-induced ascites. Poult. Sci. 2002, 81, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Narinc, D.; Erdogan, S.; Tahtabicen, E.; Aksoy, T. Effects of thermal manipulations during embryogenesis of broiler chickens on developmental stability, hatchability and chick quality. Animal 2016, 10, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Greene, E.S.; Kogut, M.H.; Dridi, S. Heat Stress and Feed Restriction Distinctly Affect Performance, Carcass and Meat Yield, Intestinal Integrity, and Inflammatory (Chemo)Cytokines in Broiler Chickens. Front. Physiol. 2021, 12, 707757. [Google Scholar] [CrossRef]
- Orlowski, S.K.; Cauble, R.; Tabler, T.; Hiltz, J.Z.; Greene, E.S.; Anthony, N.B.; Dridi, S. Processing evaluation of random bred broiler populations and a common ancestor at 55 days under chronic heat stress conditions. Poult. Sci. 2020, 99, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.S.; Cauble, R.; Kadhim, H.; de Almeida Mallmann, B.; Gu, I.; Lee, S.O.; Orlowski, S.; Dridi, S. Protective effects of the phytogenic feed additive "comfort" on growth performance via modulation of hypothalamic feeding- and drinking-related neuropeptides in cyclic heat-stressed broilers. Domest. Anim. Endocrinol. 2021, 74, 106487. [Google Scholar] [CrossRef]
- Baxter, M.F.A.; Greene, E.S.; Kidd, M.T.; Tellez-Isaias, G.; Orlowski, S.; Dridi, S. Water amino acid-chelated trace mineral supplementation decreases circulating and intestinal HSP70 and proinflammatory cytokine gene expression in heat-stressed broiler chickens. J. Anim. Sci. 2020, 98, skaa049. [Google Scholar] [CrossRef]
- Cahaner, A.; Leenstra, F. Effects of high temperature on growth and efficiency of male and female broilers from lines selected for high weight gain, favorable feed conversion, and high or low fat content. Poult. Sci. 1992, 71, 1237–1250. [Google Scholar] [CrossRef]
- Leenstra, F.; Cahaner, A. Effects of low, normal, and high temperatures on slaughter yield of broilers from lines selected for high weight gain, favorable feed conversion, and high or low fat content. Poult. Sci. 1992, 71, 1994–2006. [Google Scholar] [CrossRef]
- Dale, N.M.; Fuller, H.L. Effect of diet composition on feed intake and growth of chicks under heat stress. II. Constant vs. cycling temperatures. Poult. Sci. 1980, 59, 1434–1441. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sa, L.R.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.A.; Carlisle, A.J. The effects of chronic exposure to elevated environmental temperature on intestinal morphology and nutrient absorption in the domestic fowl (Gallus domesticus). Comp. Biochem. Physiol. Part A Comp. Physiol. 1992, 101, 137–142. [Google Scholar] [CrossRef]
- Jones, M.P.; Dilley, J.B.; Drossman, D.; Crowell, M.D. Brain-gut connections in functional GI disorders: Anatomic and physiologic relationships. Neurogastroenterol. Motil. 2006, 18, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, B.; Mazur-Bialy, A.; Pajdo, R.; Kwiecien, S.; Bilski, J.; Zwolinska-Wcislo, M.; Mach, T.; Brzozowski, T. Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis. Curr. Neuropharmacol. 2016, 14, 892–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhtar, K.; Nawaz, H.; Abid, S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J. Gastroenterol. 2019, 25, 552–566. [Google Scholar] [CrossRef]
- Lambert, G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 2009, 87, E101–E108. [Google Scholar] [CrossRef] [Green Version]
- Tur, J.A.; Rial, R.V. The effect of temperature and relative humidity on the gastrointestinal motility of young broilers. Comp. Biochem. Physiol. Part. A Physiol. 1985, 80, 481–486. [Google Scholar] [CrossRef]
- Hai, L.; Rong, D.; Zhang, Z.Y. The effect of thermal environment on the digestion of broilers. Anim. Physiol. Anim. Nutr. 2000, 83, 57–64. [Google Scholar] [CrossRef]
- Hall, D.M.; Baumgardner, K.R.; Oberley, T.D.; Gisolfi, C.V. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 1999, 276, G1195–G1203. [Google Scholar] [CrossRef]
- Wolfenson, D. Blood flow through arteriovenous anastomoses and its thermal function in the laying hen. J. Physiol. 1983, 334, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Ophir, E.; Arieli, Y.; Marder, J.; Horowitz, M. Cutaneous blood flow in the pigeon Columba livia: Its possible relevance to cutaneous water evaporation. J. Exp. Biol. 2002, 205, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.O.; Walsberg, G.E. The role of the plumage in heat transfer processes of birds. Am. Zool. 2000, 40, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in Susceptibility to Heat Stress along the Chicken Intestine and the Protective Effects of Galacto-Oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [Green Version]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef]
- Alhenaky, A.; Abdelqader, A.; Abuajamieh, M.; Al-Fataftah, A.R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017, 70, 9–14. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Gomes, A.V.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-de-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012, 41, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2018, 47, 616–624. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Benestad, S.L.; Lovland, A. Epidemiologic aspects of necrotic enteritis in broiler chickens—Disease occurrence and production performance. Avian Pathol. 2016, 45, 271–274. [Google Scholar] [CrossRef]
- Kadykalo, S.; Roberts, T.; Thompson, M.; Wilson, J.; Lang, M.; Espeisse, O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 2018, 51, 304–310. [Google Scholar] [CrossRef]
- Skinner, J.T.; Bauer, S.; Young, V.; Pauling, G.; Wilson, J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010, 54, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The projected timing of climate departure from recent variability. Nature 2013, 502, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Tabler, T.W.; Greene, E.S.; Orlowski, S.K.; Hiltz, J.Z.; Anthony, N.B.; Dridi, S. Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild Jungle Fowl. Front. Vet. Sci. 2020, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Barros, T.L.; Tellez, G., Jr.; Blankenship, J.; Lester, H.; Graham, B.D.; Selby, C.A.M.; Vuong, C.N.; Dridi, S.; Greene, E.S.; et al. Research Note: Evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poult. Sci. 2020, 99, 1687–1692. [Google Scholar] [CrossRef]
- Greene, E.; Cauble, R.; Dhamad, A.E.; Kidd, M.T.; Kong, B.; Howard, S.M.; Castro, H.F.; Campagna, S.R.; Bedford, M.; Dridi, S. Muscle Metabolome Profiles in Woody Breast-(un)Affected Broilers: Effects of Quantum Blue Phytase-Enriched Diet. Front. Vet. Sci. 2020, 7, 458. [Google Scholar] [CrossRef]
- Clemmons, B.A.; Martino, C.; Powers, J.B.; Campagna, S.R.; Voy, B.H.; Donohoe, D.R.; Gaffney, J.; Embree, M.M.; Myer, P.R. Rumen Bacteria and Serum Metabolites Predictive of Feed Efficiency Phenotypes in Beef Cattle. Sci. Rep. 2019, 9, 19265. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef] [Green Version]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, 37, 14.11.1–14.11.23. [Google Scholar] [CrossRef] [Green Version]
- Bazurto, J.V.; Dearth, S.P.; Tague, E.D.; Campagna, S.R.; Downs, D.M. Untargeted metabolomics confirms and extends the understanding of the impact of aminoimidazole carboxamide ribotide (AICAR) in the metabolic network of Salmonella enterica. Microb. Cell 2017, 5, 74–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastings, J.; Owen, G.; Dekker, A.; Ennis, M.; Kale, N.; Muthukrishnan, V.; Turner, S.; Swainston, N.; Mendes, P.; Steinbeck, C. ChEBI in 2016: Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016, 44, D1214–D1219. [Google Scholar] [CrossRef]
- Rajaei-Sharifabadi, H.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Noni (Morinda citrifolia) Modulates the Hypothalamic Expression of Stress- and Metabolic-Related Genes in Broilers Exposed to Acute Heat Stress. Front. Genet. 2017, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Piekarski, A.; Nagarajan, G.; Ishola, P.; Flees, J.; Greene, E.S.; Kuenzel, W.J.; Ohkubo, T.; Maier, H.; Bottje, W.G.; Cline, M.A.; et al. AMP-Activated Protein Kinase Mediates the Effect of Leptin on Avian Autophagy in a Tissue-Specific Manner. Front. Physiol. 2018, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Dhamad, A.E.; Greene, E.; Sales, M.; Nguyen, P.; Beer, L.; Liyanage, R.; Dridi, S. 75-kDa glucose-regulated protein (GRP75) is a novel molecular signature for heat stress response in avian species. Am. J. Physiol. Cell Physiol. 2020, 318, C289–C303. [Google Scholar] [CrossRef]
- Ferver, A.; Dridi, S. Regulation of avian uncoupling protein (av-UCP) expression by cytokines and hormonal signals in quail myoblast cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 248, 110747. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Dridi, S.; Hirano, Y.; Tarallo, V.; Kim, Y.; Fowler, B.J.; Ambati, B.K.; Bogdanovich, S.; Chiodo, V.A.; Hauswirth, W.W.; Kugel, J.F.; et al. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 13781–13786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Hoon, M.J.; Imoto, S.; Nolan, J.; Miyano, S. Open source clustering software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldanha, A.J. Java Treeview--extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef] [Green Version]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality?—a review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat Stress Causes Immune Abnormalities via Massive Damage to Effect Proliferation and Differentiation of Lymphocytes in Broiler Chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Piestun, Y.; Patael, T.; Yahav, S.; Velleman, S.G.; Halevy, O. Early posthatch thermal stress affects breast muscle development and satellite cell growth and characteristics in broilers. Poult. Sci. 2017, 96, 2877–2888. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, S.; Yin, B.; Xu, J.; Di, L.; Zhang, J.; Bao, E. Heat stress-induced renal damage in poultry and the protective effects of HSP60 and HSP47. Cell Stress Chaperones 2018, 23, 1033–1040. [Google Scholar] [CrossRef]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Hermes, J.D.; Tipton, P.A.; Fisher, M.A.; O’Leary, M.H.; Morrison, J.F.; Cleland, W.W. Mechanisms of enzymatic and acid-catalyzed decarboxylations of prephenate. Biochemistry 1984, 23, 6263–6275. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Manandhar, M.; Cronan, J.E. Pimelic acid, the first precursor of the Bacillus subtilis biotin synthesis pathway, exists as the free acid and is assembled by fatty acid synthesis. Mol. Microbiol. 2017, 104, 595–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Jiang, H.; Zheng, Q.; Chen, X.; Wang, R.; Yang, S.; Zhao, G.; Liu, J.; Norvienyeku, J.; Wang, Z. Isopropylmalate isomerase MoLeu1 orchestrates leucine biosynthesis, fungal development, and pathogenicity in Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2019, 103, 327–337. [Google Scholar] [CrossRef]
- He, Y.; Chen, B.; Pang, Q.; Strul, J.M.; Chen, S. Functional specification of Arabidopsis isopropylmalate isomerases in glucosinolate and leucine biosynthesis. Plant. Cell Physiol. 2010, 51, 1480–1487. [Google Scholar] [CrossRef] [Green Version]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Gallardo, M.E.; Desviat, L.R.; Rodriguez, J.M.; Esparza-Gordillo, J.; Perez-Cerda, C.; Perez, B.; Rodriguez-Pombo, P.; Criado, O.; Sanz, R.; Morton, D.H.; et al. The molecular basis of 3-methylcrotonylglycinuria, a disorder of leucine catabolism. Am. J. Hum. Genet. 2001, 68, 334–346. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, K.; Ng, H.; Leonard, J.V. A combined defect of three mitochondrial carboxylases presenting as biotin-responsive 3-methylcrotonyl glycinuria and 3-hydroxyisovaleric aciduria. Clin. Chim. Acta 1980, 100, 183–186. [Google Scholar] [CrossRef]
- Liebich, H.M.; Forst, C. Hydroxycarboxylic and oxocarboxylic acids in urine: Products from branched-chain amino acid degradation and from ketogenesis. J. Chromatogr. 1984, 309, 225–242. [Google Scholar] [CrossRef]
- Tamura, Y.; Kitaoka, Y.; Matsunaga, Y.; Hoshino, D.; Hatta, H. Daily heat stress treatment rescues denervation-activated mitochondrial clearance and atrophy in skeletal muscle. J. Physiol. 2015, 593, 2707–2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujahid, A.; Akiba, Y.; Toyomizu, M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult. Sci. 2007, 86, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiang, Z.; Chang, X.; Xue, H.; Yahefu, W.; Zhang, X. 4-Hydroxyphenylacetic Acid Prevents Acute APAP-Induced Liver Injury by Increasing Phase II and Antioxidant Enzymes in Mice. Front. Pharmacol. 2018, 9, 653. [Google Scholar] [CrossRef]
- Dangl, J. Innate immunity. Plants just say NO to pathogens. Nature 1998, 394, 525. [Google Scholar] [CrossRef]
- Gamble-George, J.C.; Baldi, R.; Halladay, L.; Kocharian, A.; Hartley, N.; Silva, C.G.; Roberts, H.; Haymer, A.; Marnett, L.J.; Holmes, A.; et al. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. Elife 2016, 5, e14137. [Google Scholar] [CrossRef]
- Guo, J.Y.; Li, C.Y.; Ruan, Y.P.; Sun, M.; Qi, X.L.; Zhao, B.S.; Luo, F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur. J. Pharmacol. 2009, 612, 54–60. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, A.; Dhir, A. Protective effect of non-selective and selective COX−2-inhibitors in acute immobilization stress-induced behavioral and biochemical alterations. Pharmacol. Rep. 2007, 59, 699–707. [Google Scholar]
- Rossi, A.; Coccia, M.; Trotta, E.; Angelini, M.; Santoro, M.G. Regulation of cyclooxygenase-2 expression by heat: A novel aspect of heat shock factor 1 function in human cells. PLoS ONE 2012, 7, e31304. [Google Scholar] [CrossRef] [Green Version]
- Moon, E.J.; Sonveaux, P.; Porporato, P.E.; Danhier, P.; Gallez, B.; Batinic-Haberle, I.; Nien, Y.C.; Schroeder, T.; Dewhirst, M.W. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. USA 2010, 107, 20477–20482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, A.W.; Abo, A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem. Sci. 1993, 18, 43–47. [Google Scholar] [CrossRef]
- Hirst, J. Towards the molecular mechanism of respiratory complex I. Biochem. J. 2009, 425, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Luo, P.; Chen, S.J.; Deng, Z.C.; Fu, X.L.; Xu, D.N.; Tian, Y.B.; Huang, Y.M.; Liu, W.J. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021, 100, 101459. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Kikusato, M.; Sudo, S.; Amo, T.; Toyomizu, M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Mracek, T.; Drahota, Z.; Houstek, J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. BioChim. Biophys. Acta 2013, 1827, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mracek, T.; Holzerova, E.; Drahota, Z.; Kovarova, N.; Vrbacky, M.; Jesina, P.; Houstek, J. ROS generation and multiple forms of mammalian mitochondrial glycerol-3-phosphate dehydrogenase. Biochim. Biophys. Acta 2014, 1837, 98–111. [Google Scholar] [CrossRef] [Green Version]
- McCormack, J.G.; Denton, R.M. The role of Ca2+ in the regulation of intramitochondrial energy production in heart. Biomed BioChim. Acta 1987, 46, S487–S492. [Google Scholar]
- Furukawa, A.; Tada-Oikawa, S.; Kawanishi, S.; Oikawa, S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell. Physiol. Biochem. 2007, 20, 45–54. [Google Scholar] [CrossRef]
- Ying, W. NAD+ and NADH in brain functions, brain diseases and brain aging. Front. Biosci. 2007, 12, 1863–1888. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Wei, G.; Wang, D.; Wang, Q.; Tang, X.; Shi, J.; Zhang, P.; Lu, H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front. Biosci. 2007, 12, 2728–2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houtkooper, R.H.; Canto, C.; Wanders, R.J.; Auwerx, J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010, 31, 194–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaud, C.; Joyeux-Faure, M.; Godin-Ribuot, D.; Ribuot, C. COX−2: An in vivo evidence of its participation in heat stress-induced myocardial preconditioning. Cardiovasc. Res. 2003, 58, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, C.; Godin-Ribuot, D.; Bottari, S.; Peinnequin, A.; Joyeux, M.; Demenge, P.; Ribuot, C. iNOS is a mediator of the heat stress-induced preconditioning against myocardial infarction in vivo in the rat. Cardiovasc. Res. 2003, 58, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Canto, C.; Menzies, K.J.; Auwerx, J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef] [Green Version]
- Cerutti, R.; Pirinen, E.; Lamperti, C.; Marchet, S.; Sauve, A.A.; Li, W.; Leoni, V.; Schon, E.A.; Dantzer, F.; Auwerx, J.; et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014, 19, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Koch, F.; Albrecht, D.; Gors, S.; Kuhla, B. Jejunal mucosa proteomics unravel metabolic adaptive processes to mild chronic heat stress in dairy cows. Sci. Rep. 2021, 11, 12484. [Google Scholar] [CrossRef]
- Milanese, C.; Bombardieri, C.R.; Sepe, S.; Barnhoorn, S.; Payan-Gomez, C.; Caruso, D.; Audano, M.; Pedretti, S.; Vermeij, W.P.; Brandt, R.M.C.; et al. DNA damage and transcription stress cause ATP-mediated redesign of metabolism and potentiation of anti-oxidant buffering. Nat. Commun. 2019, 10, 4887. [Google Scholar] [CrossRef] [Green Version]
- Horecker, B.L. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef] [Green Version]
- Ralser, M.; Wamelink, M.M.; Kowald, A.; Gerisch, B.; Heeren, G.; Struys, E.A.; Klipp, E.; Jakobs, C.; Breitenbach, M.; Lehrach, H.; et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralser, M.; Wamelink, M.M.; Latkolik, S.; Jansen, E.E.; Lehrach, H.; Jakobs, C. Metabolic reconfiguration precedes transcriptional regulation in the antioxidant response. Nat. Biotechnol. 2009, 27, 604–605. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Grieco, D.; Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011, 30, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.M.; Iqbal, S.; Naseem, I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch. Biochem. Biophys. 2015, 584, 10–19. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruss, A.; Georgieva, R.; Baumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [Green Version]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Ippolito, D.L.; Lewis, J.A.; Yu, C.; Leon, L.R.; Stallings, J.D. Alteration in circulating metabolites during and after heat stress in the conscious rat: Potential biomarkers of exposure and organ-specific injury. BMC Physiol. 2014, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Knochel, J.P.; Dotin, L.N.; Hamburger, R.J. Heat stress, exercise, and muscle injury: Effects on urate metabolism and renal function. Ann. Intern. Med. 1974, 81, 321–328. [Google Scholar] [CrossRef]
- Massot, C.; Bancel, D.; Lopez Lauri, F.; Truffault, V.; Baldet, P.; Stevens, R.; Gautier, H. High temperature inhibits ascorbate recycling and light stimulation of the ascorbate pool in tomato despite increased expression of biosynthesis genes. PLoS ONE 2013, 8, e84474. [Google Scholar] [CrossRef]
- Fredholm, B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007, 14, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Calefi, A.S.; Fonseca, J.; Nunes, C.A.Q.; Lima, A.P.N.; Quinteiro-Filho, W.M.; Florio, J.C.; Zager, A.; Ferreira, A.J.P.; Palermo-Neto, J. Heat Stress Modulates Brain Monoamines and Their Metabolites Production in Broiler Chickens Co-Infected with Clostridium perfringens Type A and Eimeria spp. Vet. Sci. 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.J.; File, S.E. Cold restraint alters dopamine metabolism in frontal cortex, nucleus accumbens and neostriatum. Physiol. Behav. 1983, 31, 511–513. [Google Scholar] [CrossRef]
- Gradinaru, D.; Minn, A.L.; Artur, Y.; Minn, A.; Heydel, J.M. Effect of oxidative stress on UDP-glucuronosyltransferases in rat astrocytes. Toxicol. Lett. 2012, 213, 316–324. [Google Scholar] [CrossRef]
- Livernois, A.M.; Mallard, B.A.; Cartwright, S.L.; Canovas, A. Heat stress and immune response phenotype affect DNA methylation in blood mononuclear cells from Holstein dairy cows. Sci. Rep. 2021, 11, 11371. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.B.; Williams, S.R.O.; Wales, W.J.; Marett, L.C.; DiGiacomo, K.; Leury, B.J.; Hayes, B.J. Responses of dairy cows to short-term heat stress in controlled-climate chambers. Anim. Prod. Sci. 2017, 57, 1233–1241. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fandriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef]
- Adell, A.; Garcia-Marquez, C.; Armario, A.; Gelpi, E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J. Neurochem. 1988, 50, 1678–1681. [Google Scholar] [CrossRef]
- Ouzzine, M.; Gulberti, S.; Ramalanjaona, N.; Magdalou, J.; Fournel-Gigleux, S. The UDP-glucuronosyltransferases of the blood-brain barrier: Their role in drug metabolism and detoxication. Front. Cell. Neurosci. 2014, 8, 349. [Google Scholar] [CrossRef] [Green Version]
- Geng, G.; Lv, C.; Stevanato, P.; Li, R.; Liu, H.; Yu, L.; Wang, Y. Transcriptome Analysis of Salt-Sensitive and Tolerant Genotypes Reveals Salt-Tolerance Metabolic Pathways in Sugar Beet. Int. J. Mol. Sci. 2019, 20, 5910. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.W.; Verma, R.; Kim, M.; Lee, J.Y.; Kim, Y.K.; Bang, J.W.; Reiter, W.D.; Pai, H.S. Depletion of UDP-D-apiose/UDP-D-xylose synthases results in rhamnogalacturonan-II deficiency, cell wall thickening, and cell death in higher plants. J. Biol. Chem. 2006, 281, 13708–13716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, A.; Aziz, M.A.; Mahri, S.A.; Gabere, M.N.; Dlamy, M.A.; Mohammad, S.; Abbad, M.A.; Hussein, M. A Model of Exposure to Extreme Environmental Heat Uncovers the Human Transcriptome to Heat Stress. Sci. Rep. 2017, 7, 9429. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; Refaat, B.; Qasem, A.H.; Khan, A.; Ghaith, M.; Almasmoum, H.; Mahbub, A.; Almaimani, R.A. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 22736–22746. [Google Scholar] [CrossRef]
- Davis, M.T.; Holmes, S.E.; Pietrzak, R.H.; Esterlis, I. Neurobiology of Chronic Stress-Related Psychiatric Disorders: Evidence from Molecular Imaging Studies. Chronic Stress 2017, 1, 2470547017710916. [Google Scholar] [CrossRef]
- Smith, K.E.; Pollak, S.D. Early life stress and development: Potential mechanisms for adverse outcomes. J. Neurodev. Disord. 2020, 12, 34. [Google Scholar] [CrossRef]
- Yi, G.; Li, L.; Luo, M.; He, X.; Zou, Z.; Gu, Z.; Su, L. Heat stress induces intestinal injury through lysosome- and mitochondria-dependent pathway in vivo and in vitro. Oncotarget 2017, 8, 40741–40755. [Google Scholar] [CrossRef] [Green Version]
- Duncan, R.F.; Hershey, J.W. Protein synthesis and protein phosphorylation during heat stress, recovery, and adaptation. J. Cell Biol. 1989, 109, 1467–1481. [Google Scholar] [CrossRef]
- Thompson, S.M.; Callstrom, M.R.; Butters, K.A.; Knudsen, B.; Grande, J.P.; Roberts, L.R.; Woodrum, D.A. Heat stress induced cell death mechanisms in hepatocytes and hepatocellular carcinoma: In vitro and in vivo study. Lasers Surg. Med. 2014, 46, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, C.; Joyeux, M.; Garrel, C.; Godin-Ribuot, D.; Demenge, P.; Ribuot, C. Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br. J. Pharmacol. 2002, 135, 1776–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.T.; Ma, L.; Zhou, Z.; Zhou, Z.K.; Baumgard, L.H.; Jiang, D.; Bionaz, M.; Bu, D.P. Heat stress negatively affects the transcriptome related to overall metabolism and milk protein synthesis in mammary tissue of midlactating dairy cows. Physiol. Genom. 2019, 51, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Victoria Sanz Fernandez, M.; Johnson, J.S.; Abuajamieh, M.; Stoakes, S.K.; Seibert, J.T.; Cox, L.; Kahl, S.; Elsasser, T.H.; Ross, J.W.; Isom, S.C.; et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 2015, 3, e12315. [Google Scholar] [CrossRef] [PubMed]
- Kantidze, O.L.; Velichko, A.K.; Luzhin, A.V.; Razin, S.V. Heat Stress-Induced DNA Damage. Acta Nat. 2016, 8, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Febbraio, M.A. Alterations in energy metabolism during exercise and heat stress. Sports Med. 2001, 31, 47–59. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, S.; Xu, L.; Liu, X.; Ding, W.; Wang, Q.; Chen, Y.; Deng, H. CA9 Silencing Promotes Mitochondrial Biogenesis, Increases Putrescine Toxicity and Decreases Cell Motility to Suppress ccRCC Progression. Int. J. Mol. Sci. 2020, 21, 5939. [Google Scholar] [CrossRef]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Marin de Evsikova, C.; Bian, K.; Achille, A.; Telles, E.; Pei, H.; Seto, E. Programming and Regulation of Metabolic Homeostasis by HDAC11. EBioMedicine 2018, 33, 157–168. [Google Scholar] [CrossRef]
- Kumar, R.; Jain, V.; Kushwah, N.; Dheer, A.; Mishra, K.P.; Prasad, D.; Singh, S.B. HDAC inhibition prevents hypobaric hypoxia-induced spatial memory impairment through PIota3K/GSK3beta/CREB pathway. J. Cell. Physiol. 2021, 236, 6754–6771. [Google Scholar] [CrossRef]
- Odom, T.W.; Harrison, P.C.; Bottje, W.G. Effects of thermal-induced respiratory alkalosis on blood ionized calcium levels in the domestic hen. Poult. Sci. 1986, 65, 570–573. [Google Scholar] [CrossRef]
- Cottrell, J.J.; Furness, J.B.; Wijesiriwardana, U.A.; Ringuet, M.; Liu, F.; DiGiacomo, K.; Leury, B.J.; Clarke, I.J.; Dunshea, F.R. The Effect of Heat Stress on Respiratory Alkalosis and Insulin Sensitivity in Cinnamon Supplemented Pigs. Animals 2020, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Q.; Si, X.; Xie, Y.; Chen, L.; Liu, Z.; Liu, L.; Tang, X.; Zhang, H. Effects of acute heat stress at different ambient temperature on hepatic redox status in broilers. Poult. Sci. 2020, 99, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Harsini, S.; Habibiyan, M.; Moeini, M.M.; Abdolmohammadi, A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012, 148, 322–330. [Google Scholar] [CrossRef]
- Xue, B.; Song, J.; Liu, L.; Luo, J.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and antioxidant capacity in heat-stressed broilers. Arch. Anim. Nutr. 2017, 71, 362–372. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.J.; Wang, D.Q.; Li, G.Q.; Wang, G.L.; Lu, L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: Evidence for differential thermal sensitivities. Cell Stress Chaperones 2014, 19, 895–901. [Google Scholar] [CrossRef]
- Gourgou, E.; Aggeli, I.K.; Beis, I.; Gaitanaki, C. Hyperthermia-induced Hsp70 and MT20 transcriptional upregulation are mediated by p38-MAPK and JNKs in Mytilus galloprovincialis (Lamarck); a pro-survival response. J. Exp. Biol. 2010, 213, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Murai, H.; Hiragami, F.; Kawamura, K.; Motoda, H.; Koike, Y.; Inoue, S.; Kumagishi, K.; Ohtsuka, A.; Kano, Y. Differential response of heat-shock-induced p38 MAPK and JNK activity in PC12 mutant and PC12 parental cells for differentiation and apoptosis. Acta Med. Okayama 2010, 64, 55–62. [Google Scholar] [CrossRef]
- Qi, Z.; Qi, S.; Gui, L.; Shen, L.; Feng, Z. Daphnetin protects oxidative stress-induced neuronal apoptosis via regulation of MAPK signaling and HSP70 expression. Oncol. Lett. 2016, 12, 1959–1964. [Google Scholar] [CrossRef] [Green Version]
- English, J.G.; Shellhammer, J.P.; Malahe, M.; McCarter, P.C.; Elston, T.C.; Dohlman, H.G. MAPK feedback encodes a switch and timer for tunable stress adaptation in yeast. Sci. Signal. 2015, 8, ra5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banton, M.C.; Tunnacliffe, A. MAPK phosphorylation is implicated in the adaptation to desiccation stress in nematodes. J. Exp. Biol. 2012, 215, 4288–4298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehart, H.; Kumpf, S.; Ittner, A.; Ricci, R. MAPK signalling in cellular metabolism: Stress or wellness? EMBO Rep. 2010, 11, 834–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogozelski, A.R.; Geng, T.; Li, P.; Yin, X.; Lira, V.A.; Zhang, M.; Chi, J.T.; Yan, Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS ONE 2009, 4, e7934. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Brecher, P. Salicylate inhibition of extracellular signal-regulated kinases and inducible nitric oxide synthase. Hypertension 1999, 34, 1259–1264. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Y.; Yang, C.; Zhu, S.; Wang, X.; Zhang, Z.; Deng, H. Decreased NAD Activates STAT3 and Integrin Pathways to Drive Epithelial-Mesenchymal Transition. Mol. Cell. Proteom. 2018, 17, 2005–2017. [Google Scholar] [CrossRef] [Green Version]
- Oeckler, R.A.; Arcuino, E.; Ahmad, M.; Olson, S.C.; Wolin, M.S. Cytosolic NADH redox and thiol oxidation regulate pulmonary arterial force through ERK MAP kinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L1017–L1025. [Google Scholar] [CrossRef]
- Huwiler, A.; Wartmann, M.; van den Bosch, H.; Pfeilschifter, J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br. J. Pharmacol. 2000, 129, 612–618. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef] [Green Version]



| Gene | Accession Number a | Primer Sequence (5′→3′) | Orientation | Product Size (bp) |
|---|---|---|---|---|
| CA9 HDAC11 CPT1 MAPK3 MAPK1 MAPK14 MAPK9 SOD1 SOD2 18S | XM_004937157 NM_001277141 AY675193 NM_204150 AY033635 XM_419263 NM_205095 NM_205064 NM_204211 AF173612 | GGGATGTGCTTGCTGTGCTAT AGGAAAGCCAGCATTGTGATG ACCAGTCCTCTTTCTTCCCAACT GGGTTCGCAGAGGTTTCAAA GCCCTGATGCCTTCATTCAA ATTTTCCCATGTCTCGGTAGTGA CGGACCATGATCACACAGGAT CAGGAGCCCTGTACCAACGT CGGACCATGATCACACAGGAT CAGGAGCCCTGTACCAACGT AGCTGGAGATTGAGGAATGGAA CGGTGGCACAAAGCTGATTA GCCGATGATCAGCCAGGAT GGCCCAATGGAAGCAAGAG TGGCTTCCATGTGCATGAAT AGCACCTGCGCTGGTACAC GCTGGAGCCCCACATCAGT GGTGGCGTGGTGTTTGCT TCCCCTCCCGTTACTTGGAT GCGCTCGTCGGCATGTA | Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse | 58 63 60 63 63 62 62 58 61 60 |
| HMDB ID | PHS | PF | AHS | CHS | |
|---|---|---|---|---|---|
| Cystathionine | HMDB0000099 | + | - | ||
| N-Acetylornithine | HMDB0003357 | + | - | ||
| Dihydroorotate | HMDB0003349 | + | - | ||
| Allantoate | HMDB0001209 | + | |||
| Trehalose | HMDB0000975 | + | |||
| Glucosamine | HMDB0001514 | + | + | ||
| sn-Glycerol 3-phosphate | HMDB0000126 | + | + | ||
| NAD+ | HMDB0000902 | + | + | ||
| Cysteate | HMDB0002757 | - | - | + | |
| Inosine | HMDB0000195 | - | |||
| Acetylphosphate | HMDB0001494 | + | + | ||
| Histidine | HMDB0000177 | + | - | - | |
| Homocysteic acid | HMDB0002205 | + | |||
| 3-Phosphoserine | HMDB0000272 | + | |||
| N-Acetyl-beta-alanine | HMDB0061880 | + | |||
| Cystine | HMDB0000192 | + | - | ||
| Methionine sulfoxide | HMDB0002005 | + | |||
| Arginine | HMDB0000517 | + | |||
| D-glucarate | HMDB0000663 | + | |||
| Xylose | HMDB0000098 | + | |||
| Glucose phosphate | HMDB0001254 | + | - | ||
| Myo-inositol | HMDB0000211 | + | |||
| N-Acetylglucosamine 1/6-phosphate | HMDB0002817 | + | |||
| 3-Phosphoglycerate | HMDB0000807 | + | |||
| 2-Oxo-4-methylthiobutanoate | HMDB0001553 | + | |||
| FAD | HMDB0001248 | + | + | ||
| Hypoxanthine | HMDB0000157 | + | |||
| Orotate | HMDB0000226 | + | - | ||
| Octulose bisphosphate | N/A | + | |||
| Allantoin | HMDB0000462 | + | |||
| D-gluconate | HMDB0000625 | + | - | ||
| Ribose phosphate | HMDB0001548 | + | |||
| Sedoheptulose 1/7-phosphate | HMDB0060509 | + | |||
| Aconitate | HMDB0000072 | + | - | ||
| Pyridoxine | HMDB0000239 | + | |||
| AICAR | HMDB0001517 | - | - | ||
| Prephenate | HMDB0012283 | + | + | ||
| pimelic acid | HMDB0000857 | + | + | ||
| 2-Isopropylmalate | HMDB0000402 | + | + | ||
| 3-Hydroxyisovalerate | HMDB0000754 | + | |||
| ADP-glucose | HMDB0006557 | + | + | ||
| Homocarnosine | HMDB0000745 | - | - | ||
| N-Carbamoyl-L-aspartate | HMDB0000828 | - | |||
| Homocysteine | HMDB0000742 | - | |||
| dAMP | HMDB0000905 | - | |||
| dTMP | HMDB0001227 | - | |||
| Glycinamide ribotide (GAR) | HMDB0002022 | - | - | ||
| S-Adenosyl-L-homocysteine | HMDB0000939 | - | |||
| Homocitrulline | HMDB0000679 | - | |||
| Citrate/isocitrate | HMDB0000193 | - | |||
| Hydroxyphenylacetate | HMDB0000020 | + | |||
| Salicylate | HMDB0000500 | + | |||
| NADH | HMDB0001487 | + | |||
| UDP | HMDB0000295 | + | |||
| NADP+ | HMDB0000217 | + | |||
| CDP | HMDB0001546 | + | |||
| D-Erythrose 4-phosphate | HMDB0001321 | + | |||
| Riboflavin | HMDB0000244 | + | |||
| N-Acetylputrescine | HMDB0002064 | - | |||
| 1-Methylhistidine | HMDB0000001 | - | |||
| Phosphothreonine | HMDB0011185 | - | |||
| Pantothenate | HMDB0000210 | - |
| Canonical Pathways | Molecules | Treatments 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PHS | AHS | CHS | PF | ||||||
| p-Value | Ratio | p-Value | Ratio | p-Value | Ratio | p-Value | Ratio | ||
| Purine Nucleotides Degradation | Hypoxanthine *, inosine, NAD+, NADH, uric acid, xanthosine, xanthosine monophosphate, adenosine †, GMP † | - | - | 7.7 × 10−8 | 0.412 | 5.0 × 10−7 | 0.412 | - | - |
| Urate Biosynthesis/Inosine 5′-phosphate degradation | NAD+, NADH, uric acid, xanthosine, xanthosine monophosphate | - | - | 1.0 × 10−6 | 0.556 | 4.0 × 10−6 | 0.556 | - | - |
| Adenosine Nucleotides Degradation | Hypoxanthine, inosine, NAD+, NADH, uric acid | - | - | 3.8 × 10−6 | 0.455 | - | - | - | - |
| Dopamine Degradation | 3′,5′-ADP, 3,4-dihydroxyphenylacetic, NAD+, NADH | - | - | 1.5 × 10−5 | 0.357 | - | - | - | - |
| Ascorbate Recycling | Ascorbic acid, NAD+, NADH, NADP, glutathione † | - | - | 2.5 × 10−5 | 0.5 | 1.8 × 10−6 | 0.625 | - | - |
| UDP-D-xylose and UDP-D-glucuronate Biosynthesis | NAD+, NADH, UDP-D-glucose, UDP-glucuronic acid | - | - | - | - | 5.5 × 10−6 | 0.8 | - | - |
| Salvage Pathways of Pyrimidine Deoxyribonucleotides | Deoxyuridine, dTMP, dUMP, thymidine, thymine, deoxycytidine ‡, uracil ‡ | - | - | - | - | 2.3 × 10−5 | 0.417 | 1.6 × 10−6 | 0.583 |
| Glycine Betaine Degradation | Dimethylglycine, glycine, L-homocysteine, L-methionine, L-serine, Pyruvic acid, sarcosine | 6.1 × 10−7 | 0.538 | - | - | - | - | 3.3 × 10−6 | 0.538 |
| tRNA Charging | Glycine, L-arginine, l-asparagine, L-aspartic acid, L-histidine, L-methionine, L-phenylalanine, L-proline, l-serine, L-tryptophan, L-tyrosine | 2.5 × 10−6 | 0.256 | - | - | - | - | - | - |
| Creatine Biosynthesis | Creatine, glycine, glycocyamine, L-arginine, L-ornithine, S-adenosylhomocysteine ‡ | 4.3 × 10−6 | 0.714 | - | - | - | - | 3.0 × 10−7 | 0.857 |
| Pyruvate Fermentation to Lactate | L-lactic acid, NAD+, NADH, Pyruvic acid | 4.8 × 10−6 | 1 | - | - | - | - | - | - |
| Diseases and Functions | Treatments 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| PHS | AHS | CHS | PF | |||||
| p-Value | # Mol. | p-Value | # Mol. | p-Value | # Mol. | p-Value | # Mol. | |
| Cancer | 4.7 × 10−2–8.8 × 10−9 | 36 | - | - | 3.3 × 10−2–2.2 × 10−5 | 22 | 4.9 × 10−2–4.7 × 10−10 | 45 |
| Organismal Injury and abnormalities | 4.7 × 10−2–8.8 × 10−9 | 51 | 4.9 × 10−2–6.5 × 10−4 | 23 | 3.3 × 10−2–2.2 × 10−5 | 29 | 4.9 × 10−2–4.7 × 10−10 | 70 |
| Hepatic system disease | 4.7 × 10−2–1.7 × 10−5 | 22 | - | - | - | - | - | - |
| Hematological disease | 4.7 × 10−2–1.3 × 10−4 | 13 | - | 3.3 × 10−2–2.9 × 10−4 | 8 | - | - | |
| Ophthalmic disease | - | - | 2.5 × 10−2–6.5 × 10−4 | 3 | - | - | - | - |
| Cardiovascular disease | - | - | 4.8 × 10−2–1.9 × 10−3 | 3 | - | - | - | - |
| Developmental disorders | - | - | 4.2 × 10−2–1.9 × 10−3 | 4 | - | - | - | - |
| Hereditary disorders | - | - | 4.2 × 10−2–1.9 × 10−3 | 6 | - | - | - | - |
| Neurological disease | - | - | - | - | 3.3 × 10−2–1.2 × 10−4 | 21 | - | - |
| Psychological disorders | - | - | - | - | 3.3 × 10−2–8.3 × 10−4 | 15 | - | - |
| Inflammatory disease | - | - | - | - | - | - | 4.9 × 10−2–1.4 × 10−6 | 21 |
| Inflammatory response | - | - | - | - | - | - | 4.7 × 10−2–1.4 × 10−6 | 32 |
| Treatments 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| PHS | AHS | CHS | PF | |||||
| Molecular and Cellular Functions | p-Value | # Mol. | p-Value | # Mol. | p-Value | # Mol. | p-Value | # Mol. |
| Amino acid metabolism | 4.7 × 10−2–1.1 × 10−8 | 27 | - | - | - | - | 4.9 × 10−2–8.9 × 10−9 | 31 |
| Molecular transport | 4.7 × 10−2–1.1 × 10−8 | 37 | - | - | - | - | 4.9 × 10−2–2.5 × 10−8 | 48 |
| Small molecule biochemistry | 4.7 × 10−2–1.1 × 10−8 | 44 | 4.6 × 10−2–8.4 × 10−5 | 28 | - | - | 4.9 × 10−2–2.5 × 10−8 | 58 |
| Protein synthesis | 2.6 × 10−2–1.7 × 10−7 | 18 | - | - | 3.3 × 10−2–2.0 × 10−4 | 11 | 1.4 × 10−2–3.6 × 10−7 | 22 |
| Cell death and survival | 4.7 × 10−2–7.5 × 10−7 | 36 | - | 3.3 × 10−2–1.2 × 10−4 | 26 | - | - | |
| Nucleic acid metabolism | - | - | 4.5 × 10−2–1.8 × 10−5 | 21 | - | - | - | - |
| DNA replication, damage, and repair | - | - | 4.2 × 10−2–3.0 × 10−4 | 11 | - | - | - | - |
| Energy production | - | - | 4.2 × 10−2–3.0 × 10−4 | 12 | - | - | - | - |
| Carbohydrate metabolism | - | - | 4.3 × 10−2–6.5 × 10−4 | 10 | - | - | - | - |
| Free radical scavenging | - | - | - | - | 3.3 × 10−2–1.4 × 10−4 | 12 | - | - |
| Upstream Regulators 2 | Treatments 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| PHS | AHS | CHS | PF | |||||
| p-Value | Z Score | p-Value | Z Score | p-Value | Z Score | p-Value | Z Score | |
| GNMT | 4.1 × 10−4 | −2.23 | - | - | - | - | 8.0 × 10−7 | −2.82 |
| CPT1B | 1.9 × 10−9 | −2.49 | 1.3 × 10−4 | −2.44 | 1.1 × 10−5 | −2.82 | 1.9 × 10−17 | −3.71 |
| GATA4 | 2.8 × 10−6 | 2.82 | - | - | - | - | 1.8 × 10−4 | 2.64 |
| MMP11 | 2.2 × 10−5 | 2.00 | - | - | - | - | - | - |
| IL37 | - | - | - | 9.9 × 10−4 | −2.23 | 2.9 × 10−10 | −2.49 | |
| CA9 | 1.5 × 10−11 | 3.31 | - | - | 1.6 × 10−3 | 2.00 | 6.9 × 10−9 | 3.16 |
| HDAC11 | 2.1 × 10−4 | 2.00 | - | - | - | - | 5.9 × 10−4 | 2.0 |
| CTH | 7.9 × 10−5 | −2.0 | - | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dridi, J.S.; Greene, E.S.; Maynard, C.W.; Brugaletta, G.; Ramser, A.; Christopher, C.J.; Campagna, S.R.; Castro, H.F.; Dridi, S. Duodenal Metabolic Profile Changes in Heat-Stressed Broilers. Animals 2022, 12, 1337. https://doi.org/10.3390/ani12111337
Dridi JS, Greene ES, Maynard CW, Brugaletta G, Ramser A, Christopher CJ, Campagna SR, Castro HF, Dridi S. Duodenal Metabolic Profile Changes in Heat-Stressed Broilers. Animals. 2022; 12(11):1337. https://doi.org/10.3390/ani12111337
Chicago/Turabian StyleDridi, Jalila S., Elizabeth S. Greene, Craig W. Maynard, Giorgio Brugaletta, Alison Ramser, Courtney J. Christopher, Shawn R. Campagna, Hector F. Castro, and Sami Dridi. 2022. "Duodenal Metabolic Profile Changes in Heat-Stressed Broilers" Animals 12, no. 11: 1337. https://doi.org/10.3390/ani12111337
APA StyleDridi, J. S., Greene, E. S., Maynard, C. W., Brugaletta, G., Ramser, A., Christopher, C. J., Campagna, S. R., Castro, H. F., & Dridi, S. (2022). Duodenal Metabolic Profile Changes in Heat-Stressed Broilers. Animals, 12(11), 1337. https://doi.org/10.3390/ani12111337







