Triacylglycerides and Cholesterol in Organic Milk from Chiapas, Mexico
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source
2.2. Sample Collection
2.3. Milk Fat Extraction
2.4. Analysis of Triacylglycerides by Gas Chromatography with a Flame Ionization Detector
2.5. Chromatographic Conditions
2.6. Cholesterol Analysis by Gas Chromatography with a Flame Ionization Detector
2.7. Chromatographic Conditions
2.8. Statistical Analysis
3. Results and Discussion
3.1. Triacylglycerides
3.2. Cholesterol and Other Sterols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CANILEC (Cámara Nacional de la Industria de la Leche). Estadísticas del Sector Lácteo 2010–2018; CANILEC: Ciudad de México, Mexico, 2019. [Google Scholar]
- SADER (Secretaría de Agricultura y Desarrollo Rural). Servicio de Información Agroalimentaria y Pesquera-Secretaría de Desarrollo Rural. Panorama de la leche en México. Boletín Informativo. México. 2019. Available online: https://www.inforural.com.mx/panorama-de-la-leche-en-mexico-6/ (accessed on 12 December 2020).
- Kourimska, L.; Legarova, V.; Panovzka, Z.; Panek, J. Quality of cow´s from organic and conventional farming. Czech J. Food Sci. 2014, 32, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.A.; Braun, H.; Brown, N.; Um, C.; Ehrel, K.; Figueroa, J.; Barr, D.B. Production-related contaminants (pesticides, antibiotics and hormones) in organic and conventionally produced milk samples sold in USA. Public Health Nutr. 2019, 22, 2972–2980. [Google Scholar] [CrossRef] [Green Version]
- Luzardo, O.P.; Almeida-González, M.; Henríquez-Hernández, L.A.; Zumbado, M.; Álvarez-León, E.E.; Boada, L.D. Polychlorobiphenyls and organochlorine pesticides in conventional and organic brands of milk: Occurrence and dietary intake in the population of the Canary Islands (Spain). Chemosphere 2012, 88, 307–315. [Google Scholar] [CrossRef]
- Markiewicz-Keszicka, M.; Czyzak-Runowska, G.; Lipinska, P.; Wojtowski, J. Fatty acid profile of milk–A review. Bull. Vet. Inst. Polawy. 2013, 57, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Alcalá, L.M.; Castro-Gómez, M.P.; Pimentel, L.L.; Fontecha, J. Milk fat components with potential anticancer activity–A review. Biosci. Rep. 2017, 37, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Butler, G.; Stergiades, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef]
- INEGI (Instituto Nacional de Estadística, Geografía e Informática). Prontuario de Información Geográfica Municipal de los Estados Unidos Mexicanos. Tecpatán, Chiapas. Clave 07092. 2021. Available online: http://www3.inegi.org.mx (accessed on 9 April 2021).
- COFOCALEC (Consejo para el Fomento de la Calidad de la Leche y sus Derivados, A.C.). NMX-F-718-COFOCALEC-2006. In Sistema Producto Alimento-Lácteos-Guía Para el Muestreo de Leche y Productos Lácteos; COFOCALEC: Guadalajara, Mexico, 2006. [Google Scholar]
- Frank, C.; Smith, E.H.; Brauwn, H.E.; Holdrinet, A.; Mc Wade, J.W. Organochlorine insecticides and industrial pollu tants in the milk supply of the Southern Region of Ontario, Canada. J. Milk. Food Technol. 1975, 38, 65–72. [Google Scholar] [CrossRef]
- Jensen, R.J. The composition of bovine milk lipids: January 1995 to december 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Firestone, P. Triglycerides in fats and oil gas chromatography method, IUPAC-AOAC method. J. Assoc. Anal. Chem. 1986, 69, 346–365. [Google Scholar]
- Gutiérrez, T.R.; Vega y León, S.; Díaz, G.G.; Delgadillo, G.H.J.; Urbán, C.G.; Ramírez, A.A.; González, C.C.; Méndez, R.I. Detección de grasa extraña en grasa láctea por cromatografía de gases y estadística multivariable. Agrociencia 2007, 41, 733–742. [Google Scholar]
- AOAC (Association of Analytical Communities International). Official Method AOAC 976.26-1977. In Cholesterol in Multicomponent Foods–Gas Chromatographic Method; AOAC: Rockville, MD, USA, 2010. [Google Scholar]
- IBM SPSS. IBM SPSS Statistics for Windows, Version 24.0; SPSS Inst. Inc.: Armonk, NY, USA, 2016. [Google Scholar]
- Molkentin, J.; Precht, D. Comparison of packed and capillary columns for quantitative gas chromatography of triglycerides in milk fat. Chromatographia 1994, 39, 265–270. [Google Scholar] [CrossRef]
- Fontecha, J.; Díaz, V.; Fraga, M.J.; Juárez, M. Triglyceride analysis by gas chromatography in assessment of authenticity of goat milk fat. J. Am. Oil Chem. Soc. 1998, 75, 1893–1896. [Google Scholar] [CrossRef]
- Pathania, P.; Sharma, V.; Singh, R.P.; Arora, A.; Raju, N.P. A distinction of cow and buffalo ghee using principal component analysis of triglyceride composition. Int. J. Dairy Technol. 2021, 74, 352–358. [Google Scholar] [CrossRef]
- Pustjens, A.M.; Boerrigter-Eenling, R.; Koot, A.H.; Rozijn, M.; van Ruth, S.M. Characterization of retail conventional, organic, and grass full-fat butters by their fat contents, free fatty acid contents, and triglyceride and fatty acid profiling. Foods. 2017, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Larsen, M.K.; Andersen, K.K.; Kaufmann, N.; Wiking, L. Seasonal variation in the composition and melting behavior of milk fat. J. Dairy Sci. 2014, 97, 4703–4712. [Google Scholar] [CrossRef] [Green Version]
- Capuano, E.; Gravink, R.; Boerrigter-Eenling, R.; van Ruth, S.M. Fatty acid and triglycerides profiling and retail organic, conventional and pasture milk: Implication for health and authenticity. Int. Dairy J. 2015, 42, 58–63. [Google Scholar] [CrossRef]
- Bonini, M.; Tateo, F.; Tateo, A. Triglycerides variability in donkey milk. Ital. J. Food Sci. 2017, 29, 233–242. [Google Scholar]
- Castro-Gómez, P.; Montero, O.; Fontecha, J. In-Depth lipidomic analysis of molecular species of triacylglycerides, diacylglycerides, glycerophospholipids and, sphingolipids of buttermilk by GCMS/FID, HPLC-ELSD and, UPLC-QToFMS. Int. J. Mol. Sci. 2017, 18, 605. [Google Scholar] [CrossRef]
- Capuano, E.; Boerrigter-Eenling, R.; Elgersma, A.; van Ruth, S.M. Effect of fresh grass feeding, pasture grazing and organic/biodynamic farming on bovine milk triglyceride profile and implications for authentication. Eur. Food Res. Technol. 2014, 238, 573–580. [Google Scholar] [CrossRef]
- Karrar, E.; Mohamed, A.I.A.; Huppertz, T.; Wei, W.; Jin, J.; Wang, X. Fatty acid composition and stereospecificity and sterol composition of milk fat from different species. Int. Dairy J. 2022, 128, 1–9. [Google Scholar] [CrossRef]
- Fontecha, J.; Calvo, M.V.; Juárez, M.; Gil, A.; Martínez-Vizcaíno, V. Milk and dairy products consumption and cardiovascular diseases: An overview of systematic reviews and meta-analyses. Adv. Nutr. 2019, 10, S164–S189. [Google Scholar] [CrossRef]
- Nurseitova, M.M.; Amutova, F.B.; Zhakupbecova, A.A.; Omarova, A.S.; Kondybayeb, A.B.; Bayandy, G.A.; Akhmetsadykov, N.N.; Faye, B.; Konuspayeva, G.S. Comparative study of fatty acid and sterol profiles for the investigation of potential milk fat adulteration. J. Dairy Sci. 2019, 102, 7723–7733. [Google Scholar] [CrossRef]


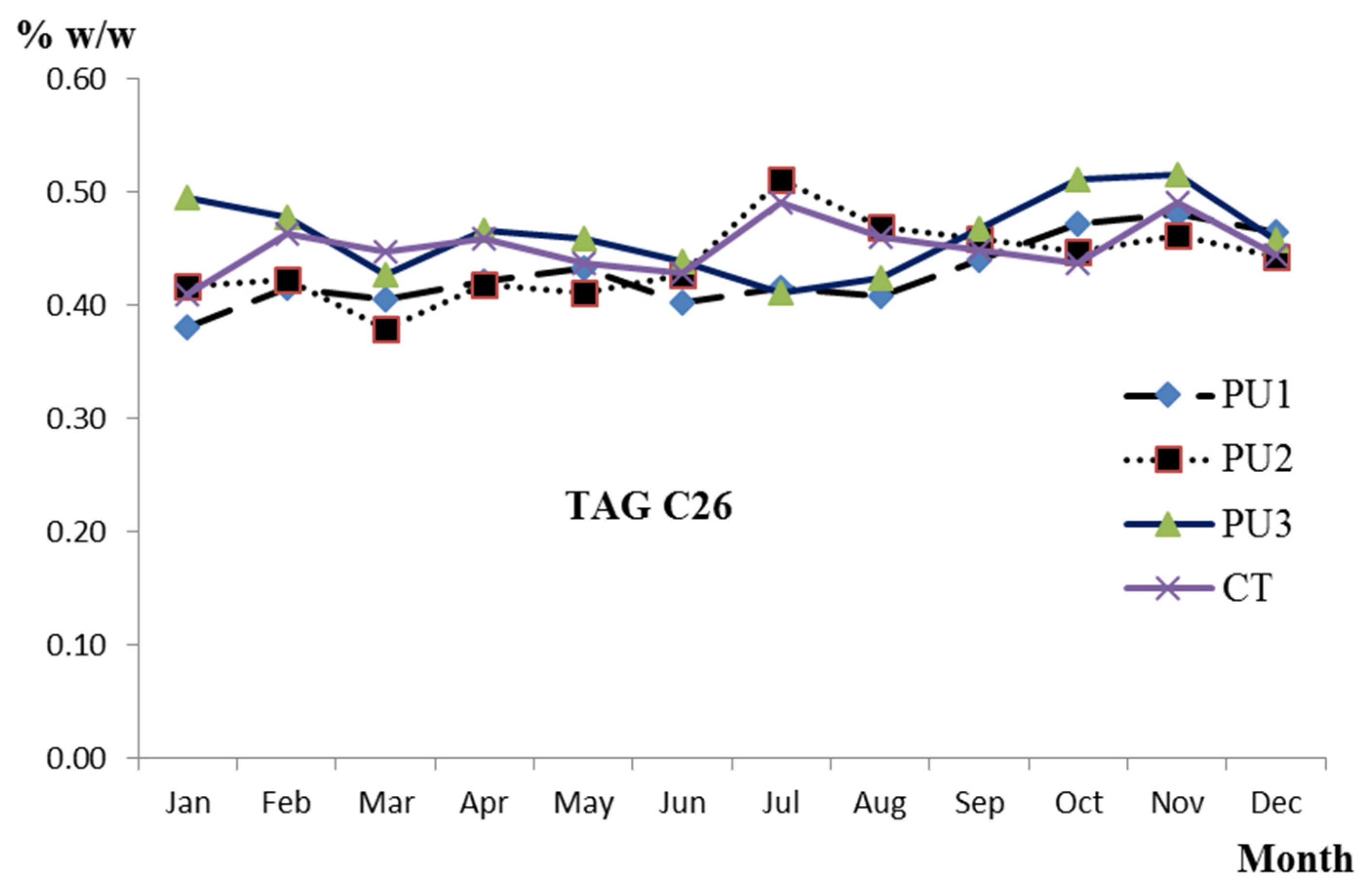

| TAG | PU1 | PU2 | PU3 | Collector Tank | ||||
|---|---|---|---|---|---|---|---|---|
| § ±SD | CI | ±SD | CI | ±SD | CI | ±SD | CI | |
| C26 | 0.43 a/0.29 | (0.41,0.45) | 0.44 ab/0.34 | (0.42,0.46) | 0.46 ab/0.35 | (0.44,0.49) | 0.45 b/0.23 | (0.44,0.47) |
| C28 | 0.49 a/0.96 | (0.43,0.55) | 0.46 a/0.32 | (0.44,0.48) | 0.43 a/0.78 | (0.38.0.48) | 0.47 a/0.54 | (0.44,0.51) |
| C30 | 0.87 a/0.15 | (0.77,0.97) | 0.88 a/0.09 | (0.83,0.94) | 0.82 a/0.16 | (0.72,0.92) | 0.90 a/0.13 | (0.82,0.98) |
| C32 | 1.83 a/0.33 | (1.52,2.03) | 1.89 a/0.17 | (1.78,2.00) | 1.79 a/0.32 | (1.59,1.99) | 1.89 a/0.28 | (1.71,2.06) |
| C34 | 4.77 a/0.87 | (4.22,5.33) | 4.81 a/0.44 | (4.53,5.09) | 4.74 a/0.65 | (4.31,5.15) | 4.74 a/0.57 | (4.38,5.10) |
| C36 | 10.66 a/1.18 | (9.90,11.41) | 10.79 a/0.71 | (10.34,11.24) | 10.60 a/0.81 | (10.08,11.11) | 10.69 a/0.84 | (10.15,11.22) |
| C38 | 14.27 a/0.74 | (13.81,14.74) | 14.72 a/0.58 | (14.34,15.09) | 14.36 a/0.37 | (14.12,14.59) | 14.56 a/0.30 | (14.37,14.75) |
| C40 | 11.08 a/1.03 | (10.42,11.74) | 11.31 a/0.31 | (11.11,11.51) | 10.92 a/0.48 | (10.61.11.22) | 11.43 a/0.51 | (11.10,11.75) |
| C42 | 6.37 a/0.58 | (6.0,6.7) | 6.59 a/0.36 | (6.36,6.82) | 6.33 a/0.53 | (5.99,6.67) | 6.49 a/0.51 | (6.17,6.81) |
| C44 | 6.01 a/0.92 | (5.42,6.59) | 6.02 a/0.45 | (5.74,6.31) | 5.95 a/0.67 | (5.52,6.37) | 5.88 a/0.72 | (5.42,6.33) |
| C46 | 6.83 a/1.01 | (6.19,7.48) | 6.55 a/0.43 | (6.28,6.82) | 6.70 a/0.55 | (6.35,7.05) | 6.48 a/0.67 | (6.05,6.90) |
| C48 | 9.29 a/0.98 | (8.66,9.91) | 8.72 a/0.34 | (8.50,8.94) | 9.12 a/0.54 | (8.78,9.47) | 8.72 a/0.52 | (8.38,9.04) |
| C50 | 11.73 a/0.71 | (11.27,12.17) | 11.31 a/0.39 | (11.06,11.56) | 11.82 a/0.98 | (11.19,12.44) | 11.36 a/0.52 | (11.03,11.70) |
| C52 | 11.35 a/2.42 | (9.81,12.88) | 11.54 a/1.31 | (10.71,12.38) | 11.83 a/1.89 | (10.63,13.03) | 11.68 a/1.88 | (10.48,12.87) |
| C54 | 4.34 a/1.71 | (3.26,5.43) | 4.29 a/0.84 | (3.76,4.83) | 4.42 a/1.15 | (3.69,5.15) | 4.61 a/1.37 | (3.74,5.48) |
| TAG | This Study | Denmark a | The Netherlands b | India c | Mexico d |
|---|---|---|---|---|---|
| C24 to C30 | - | 0.30 ± 0.16 | - | - | - |
| C26 | 0.45 ± 0.03 | - | 0.34 ± 0.02 | 0.26 ± 0.01 | - |
| C28 | 0.46 ± 0.07 | - | 0.78 ± 0.04 | 0.56 ± 0.01 | 0.45 ± 0.19 |
| C30 | 0.87 ± 0.13 | - | 0.43 ± 0.10 | 0.95 ± 0.01 | 0.58 ± 0.51 |
| C32 | 1.85 ± 0.28 | 2.60 ± 0.22 | 2.86 ± 0.15 | 1.87 ± 0.03 | 1.45 ± 0.52 |
| C34 | 4.77 ± 0.63 | 5.80 ± 0.25 | 6.13 ± 0.25 | 4.49 ± 0.03 | 3.41 ± 0.97 |
| C36 | 10.68 ± 0.88 | 10.50 ± 0.58 | 10.91 ± 0.29 | 9.29 ± 0.02 | 7.11 ± 1.57 |
| C38 | 14.48 ± 0.54 | 12.00 ± 0.34 | 12.27 ± 0.14 | 12.62 ± 0.04 | 11.26 ± 1.98 |
| C40 | 11.18 ± 0.65 | 9.30 ± 0.35 | 9.91 ± 0.17 | 10.64 ± 0.04 | 10.68 ± 3.24 |
| C42 | 6.44 ± 0.50 | 6.50 ± 0.58 | 7.11 ± 0.17 | 6.14 ± 0.07 | 7.26 ± 3.26 |
| C44 | 5.96 ± 0.69 | 6.70 ± 0.53 | 6.68 ± 0.24 | 5.34 ± 0.07 | 5.27 ± 1.67 |
| C46 | 6.64 ± 0.69 | 7.50 ± 0.51 | 7.34 ± 0.23 | 6.29 ± 0.05 | 6.59 ± 1.38 |
| C48 | 8.96 ± 0.67 | 9.2 ± 0.34 | 8.79 ± 0.20 | 8.59 ± 0.03 | 9.34 ± 1.53 |
| C50 | 11.55 ± 0.70 | 11.60 ± 0.56 | 10.93 ± 0.31 | 12.17 ± 0.07 | 13.43 ± 3.27 |
| C52 | 11.60 ± 1.86 | 11.10 ± 1.27 | 9.44 ± 0.56 | 12.87 ± 0.11 | 14.64 ± 4.98 |
| C54 | 4.41 ± 1.27 | 4.10 ± 0.85 | 4.67 ± 1.56 | 7.65 ± 0.07 | 8.61 ± 3.52 |
| TAG | C26 | C28 | C30 | C32 | C34 | C36 | C38 | C40 | C42 | C44 | C46 | C48 | C50 | C52 | C54 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C26 | 1 | ||||||||||||||
| C28 | −0.3 * | 1 | |||||||||||||
| C30 | −0.3 * | 0.8 ** | 1 | ||||||||||||
| C32 | −0.2 | 0.8 ** | 0.9 ** | 1 | |||||||||||
| C34 | −0.1 | 0.8 ** | 0.9 ** | 0.9 ** | 1 | ||||||||||
| C36 | −0.2 | 0.6 ** | 0.8 ** | 0.9 ** | 0.9 ** | 1 | |||||||||
| C38 | −0.5 ** | 0.1 | 0.3 * | 0.2 | 0.2 | 0.4 ** | 1 | ||||||||
| C40 | −0.3 * | −0.1 | −0.1 | −0.2 | −0.5** | −0.4** | 0.5** | 1 | |||||||
| C42 | −0.1 | 0.7 ** | 0.9 ** | 0.9 ** | 0.9 ** | 0.8 ** | 0.2 | −0.3 * | 1 | ||||||
| C44 | 0.02 | 0.6 ** | 0.8 ** | 0.8 ** | 0.9 ** | 0.8 ** | −0.05 | −0.6 ** | 0.9 ** | 1 | |||||
| C46 | 0.2 | 0.5 ** | 0.5 ** | 0.7 ** | 0.8 ** | 0.7 ** | −0.3 | −0.8 ** | 0.7 ** | 0.9 ** | 1 | ||||
| C48 | 0.4** | 0.1 | 0.1 | 0.2 | 0.4 * | 0.3 * | −0.6 ** | −0.9 ** | 0.3 | 0.6 ** | 0.8 ** | 1 | |||
| C50 | 0.4** | −0.7 ** | −0.9 ** | −0.8 ** | −0.7 ** | −0.7 ** | −0.6 ** | −0.2 | −0.8 ** | −0.5 ** | −0.2 | 0.3 * | 1 | ||
| C52 | 0.1 | −0.7 ** | −0.9 ** | −0.9 ** | −0.9 ** | −0.9 ** | −0.2 | 0.5 ** | −0.9 ** | −0.9 ** | −0.9 ** | −0.5 ** | 0.6 ** | 1 | |
| C54 | 0.04 | −0.6 ** | −0.7 ** | −0.8 ** | −0.9 ** | −0.9 ** | −0.1 | 0.6 ** | −0.8 ** | −0.9 ** | −0.9 ** | −0.6 ** | 0.4 ** | 0.9 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, J.J.P.; Romero, M.G.O.; Bermúdez, B.S.; Rojas, J.L.R.; Vázquez, C.C.R.; Frechero, N.M.; Tolentino, R.G. Triacylglycerides and Cholesterol in Organic Milk from Chiapas, Mexico. Animals 2022, 12, 1292. https://doi.org/10.3390/ani12101292
González JJP, Romero MGO, Bermúdez BS, Rojas JLR, Vázquez CCR, Frechero NM, Tolentino RG. Triacylglycerides and Cholesterol in Organic Milk from Chiapas, Mexico. Animals. 2022; 12(10):1292. https://doi.org/10.3390/ani12101292
Chicago/Turabian StyleGonzález, José Jesús Pérez, Marcela Guillermina Ortiz Romero, Beatriz Schettino Bermúdez, Jorge Luis Ruíz Rojas, Claudia Cecilia Radilla Vázquez, Nelly Molina Frechero, and Rey Gutiérrez Tolentino. 2022. "Triacylglycerides and Cholesterol in Organic Milk from Chiapas, Mexico" Animals 12, no. 10: 1292. https://doi.org/10.3390/ani12101292
APA StyleGonzález, J. J. P., Romero, M. G. O., Bermúdez, B. S., Rojas, J. L. R., Vázquez, C. C. R., Frechero, N. M., & Tolentino, R. G. (2022). Triacylglycerides and Cholesterol in Organic Milk from Chiapas, Mexico. Animals, 12(10), 1292. https://doi.org/10.3390/ani12101292






