Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Genotyping, Identification of Recapture, and Genetic Variation
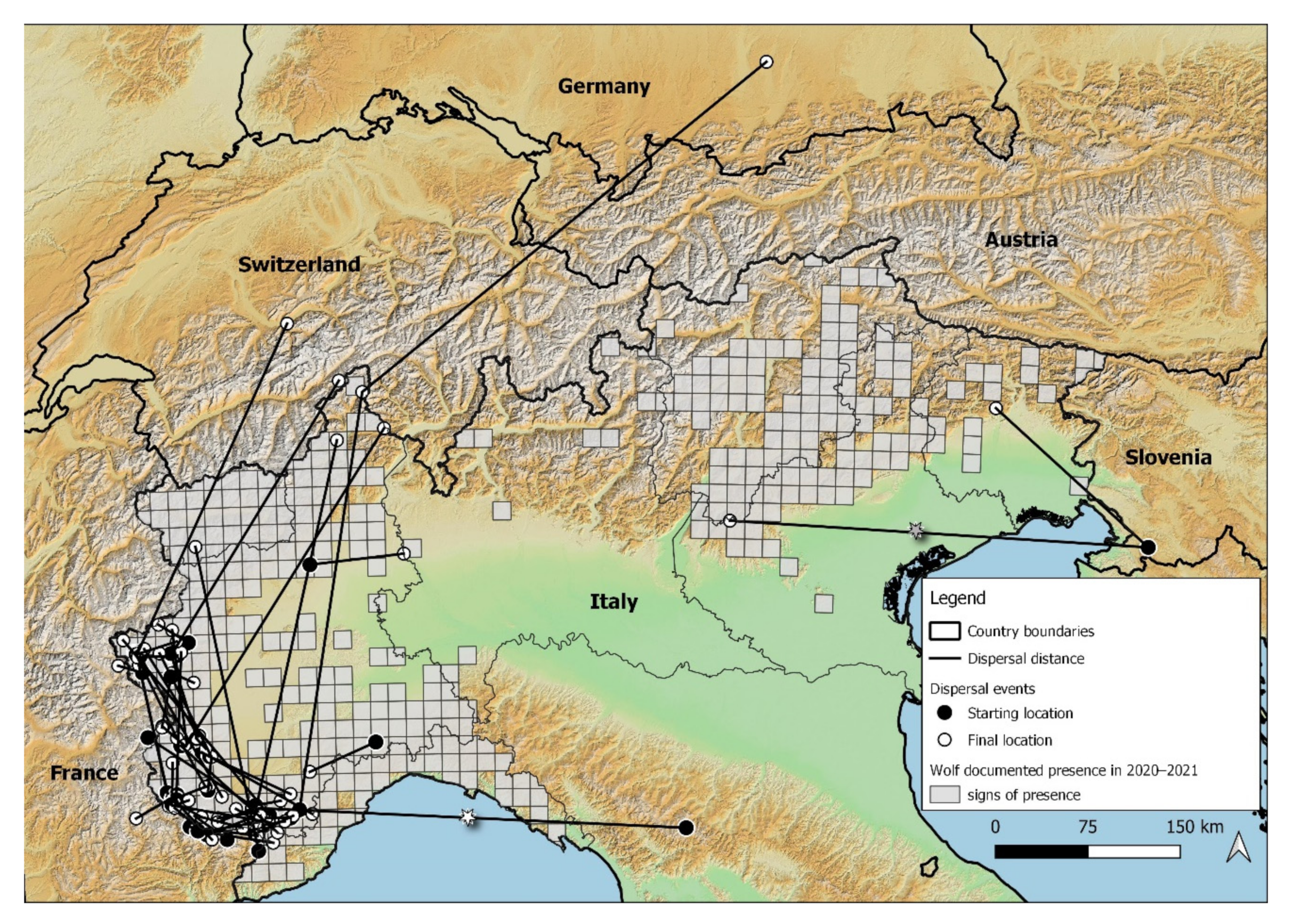
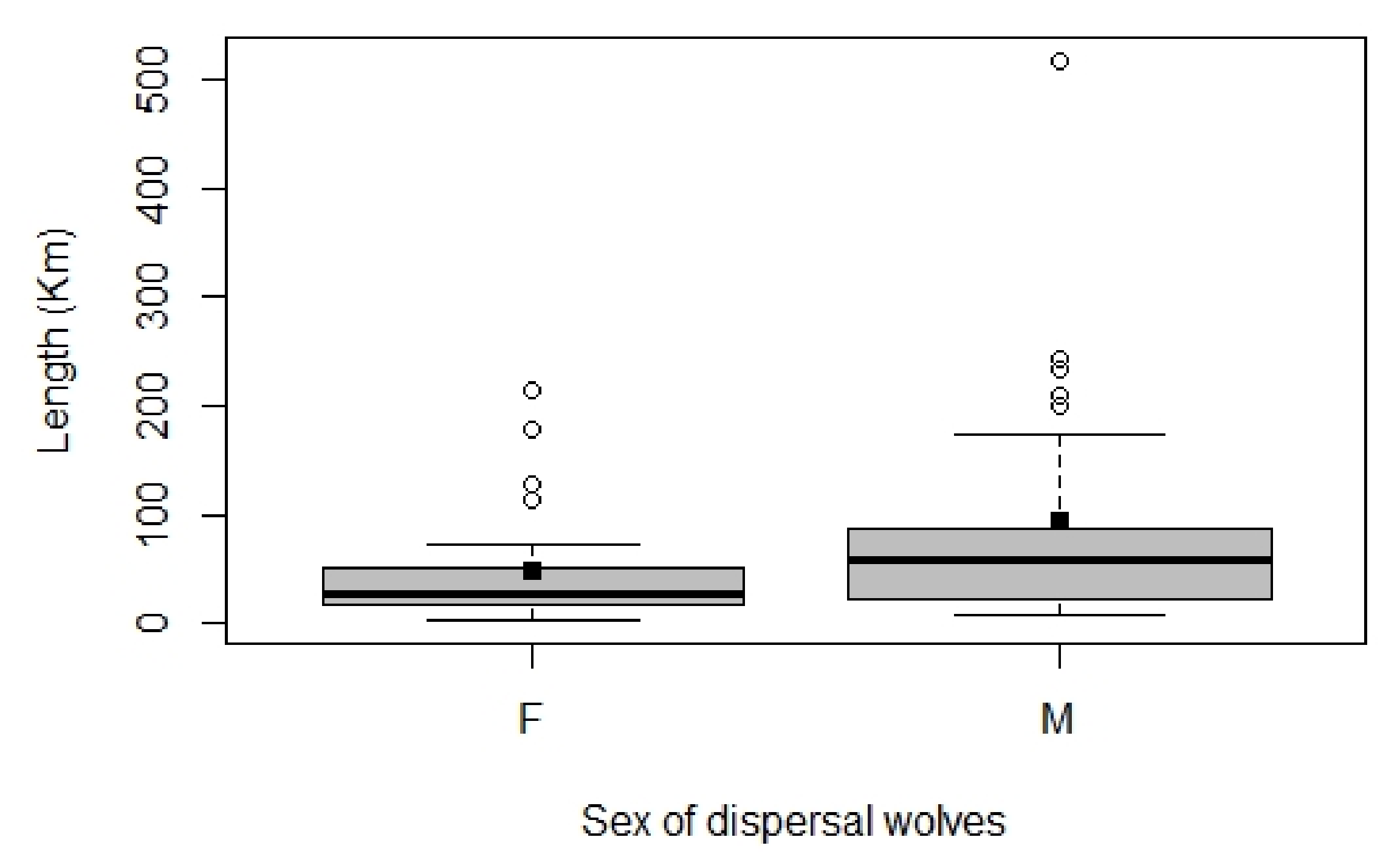
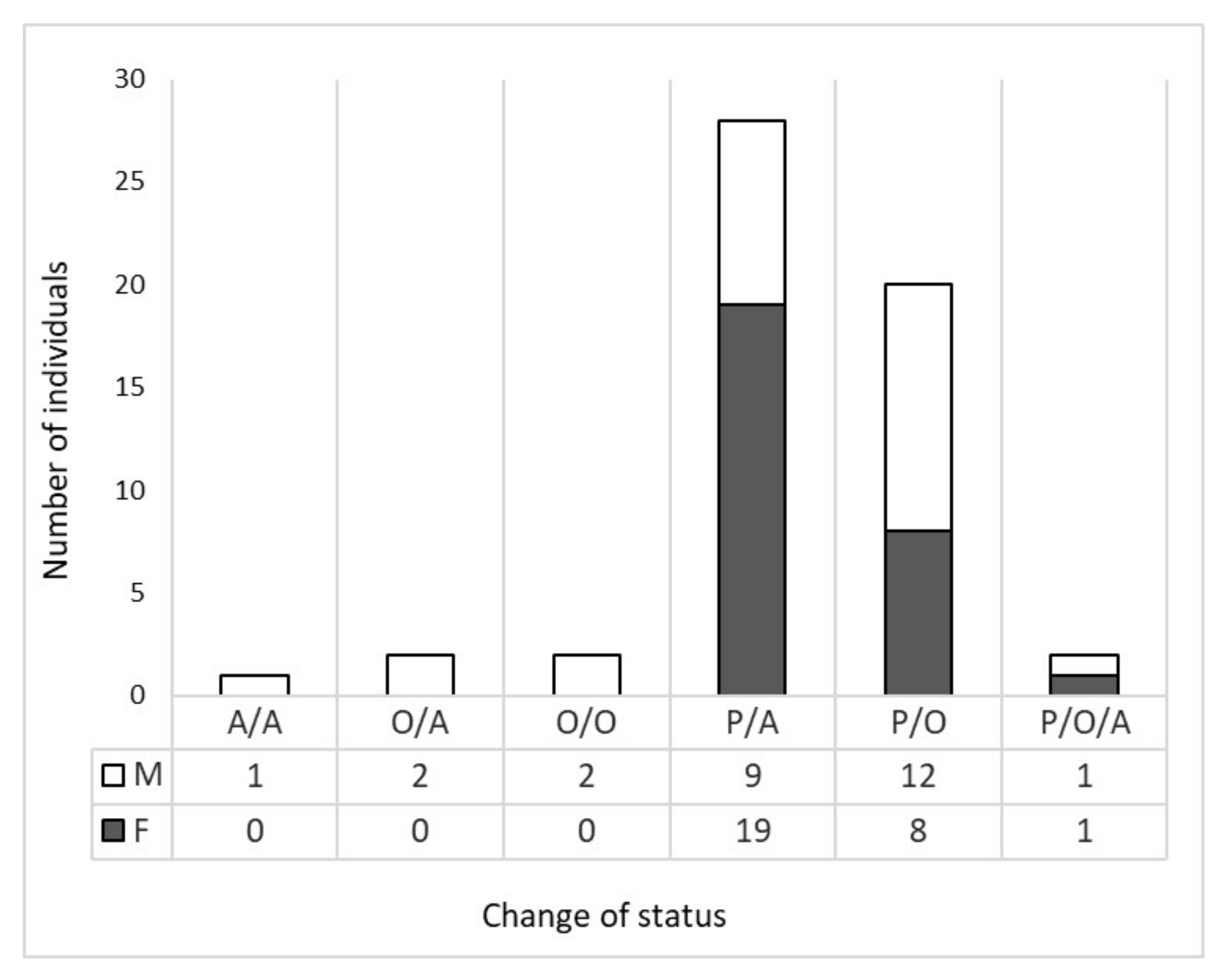
3.2. Wolf Dispersal Spatial and Individual Characteristics in the Italian Alps
4. Discussion
4.1. Wolf Dispersal Patterns in the Italian Alps Documented with Non-Invasive Genetic Analysis
4.2. Implications for Wildlife Diseases Spreading
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohrer, G.; Nathan, R.; Volis, S. Effects of long-distance dispersal for metapopulation survival and genetic structure at ecological time and spatial scales. J. Ecol. 2005, 93, 1029–1040. [Google Scholar] [CrossRef]
- Clobert, J.; Le Galliard, J.-F.; Cote, J.; Meylan, S.; Massot, M. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 2009, 12, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T. Integrating effects of hunting policy, catastrophic events, and inbreeding depression, in PVA simulation: The Scandinavian wolf population as an example. Biol. Conserv. 2004, 115, 227–239. [Google Scholar] [CrossRef]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 2014, 346, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Miquel, C.; Lucchini, V.; Santini, A.; Caniglia, R.; Duchamp, C.; Weber, J.-M.; Lequette, B.; Marucco, F.; Boitani, L.; et al. From the Apennines to the Alps: Colonization genetics of the naturally expanding Italian wolf (Canis lupus) population. Mol. Ecol. 2007, 16, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Marucco, F.; Avanzinelli, E.; Bassano, B.; Bionda, R.; Bisi, F.; Calderola, S.; Chioso, C.; Fattori, U.; Pedrotti, L.; Righetti, D.; et al. La Popolazione di Lupo sulle Alpi Italiane 2014–2018 (The Wolf Population in the Italian Alps 2014–2018); Report, Project LIFE 12 NAT/IT/00080 WOLFALPS; Centro Grandi Carnivori: Valdieri, Italy, 2018. [Google Scholar]
- Fabbri, E.; Caniglia, R.; Kusak, J.; Galov, A.; Gomerčić, T.; Arbanasić, H.; Huber, D.; Randi, E. Genetic structure of expanding wolf (Canis lupus) populations in Italy and Croatia, and the early steps of the recolonization of the Eastern Alps. Mamm. Biol. 2014, 79, 138–148. [Google Scholar] [CrossRef]
- Ražen, N.; Brugnoli, A.; Castagna, C.; Groff, C.; Kaczensky, P.; Kljun, F.; Knauer, F.; Kos, I.; Krofel, M.; Luštrik, R.; et al. Long-distance dispersal connects Dinaric-Balkan and Alpine grey wolf (Canis lupus) populations. Eur. J. Wildl. Res. 2015, 62, 137–142. [Google Scholar] [CrossRef]
- Wydeven, P.A.; Fuller, K.T.; Weber, W.; Macdonald, K. The Potential for Wolf Recovery in the Northeastern United States via Dispersal from Southeastern Canada. Wildl. Soc. Bull. 1998, 26, 776–784. Available online: https://www.jstor.org/stable/3783551 (accessed on 13 April 2022).
- Boyd, D.K.; Pletscher, D.H. Characteristics of Dispersal in a Colonizing Wolf Population in the Central Rocky Mountains. J. Wildl. Manag. 1999, 63, 1094. [Google Scholar] [CrossRef]
- Jimenez, M.D.; Bangs, E.E.; Boyd, D.K.; Smith, D.W.; Becker, S.A.; Ausband, D.E.; Woodruff, S.P.; Bradley, E.H.; Holyan, J.; Laudon, K. Wolf dispersal in the Rocky Mountains, Western United States: 1993–2008. J. Wildl. Manag. 2017, 81, 581–592. [Google Scholar] [CrossRef]
- Kojola, I.; Aspi, J.; Hakala, A.; Heikkinen, S.; Ilmoni, C.; Ronkainen, S. Dispersal in an Expanding Wolf Population in Finland. J. Mammal. 2006, 87, 281–286. Available online: www.mammalogy.org (accessed on 13 April 2022).
- Kojola, I.; Kaartinen, S.; Hakala, A.; Heikkinen, S.; Voipio, H.-M. Dispersal Behavior and the Connectivity Between Wolf Populations in Northern Europe. J. Wildl. Manag. 2009, 73, 309–313. [Google Scholar] [CrossRef]
- Blanco, J.C.; Cortés, Y. Dispersal patterns, social structure and mortality of wolves living in agricultural habitats in Spain. J. Zool. 2007, 273, 114–124. [Google Scholar] [CrossRef]
- Wabakken, P.; Sand, H.; Kojola, I.; Zimmermann, B.; Arnemo, J.M.; Pedersen, H.C.; Liberg, O. Multistage, Long-Range Natal Dispersal by a Global Positioning System—Collared Scandinavian Wolf. J. Wildl. Manag. 2007, 71, 1631–1634. [Google Scholar] [CrossRef]
- Barry, T.; Gurarie, E.; Cheraghi, F.; Kojola, I.; Fagan, W.F. Does dispersal make the heart grow bolder? Avoidance of anthropogenic habitat elements across wolf life history. Anim. Behav. 2020, 166, 219–231. [Google Scholar] [CrossRef]
- Valière, N.; Fumagalli, L.; Gielly, L.; Miquel, C.; Lequette, B.; Poulle, M.-L.; Weber, J.-M.; Arlettaz, R.; Taberlet, P. Long-distance wolf recolonization of France and Switzerland inferred from non-invasive genetic sampling over a period of 10 years. Anim. Conserv. 2003, 6, 83–92. [Google Scholar] [CrossRef]
- Stansbury, C.R.; Ausband, D.E.; Zager, P.; Mack, C.M.; Waits, L.P. Identifying gray wolf packs and dispersers using noninvasive genetic samples. J. Wildl. Manag. 2016, 80, 1408–1419. [Google Scholar] [CrossRef]
- Bassing, S.B.; Ausband, D.E.; Mitchell, M.S.; Schwartz, M.K.; Nowak, J.J.; Hale, G.C.; Waits, L.P. Immigration does not offset harvest mortality in groups of a cooperatively breeding carnivore. Anim. Conserv. 2020, 23, 750–761. [Google Scholar] [CrossRef]
- Morales-González, A.; Fernández-Gil, A.; Quevedo, M.; Revilla, E. Patterns and determinants of dispersal in grey wolves (Canis lupus). Biol. Rev. 2021, 97, 466–480. [Google Scholar] [CrossRef]
- Mech, L.D.; Boitani, L. Wolves: Behavior, Ecology, and Conservation; The University of Chicago Press: Chicago, IL, USA, 2003; 448p, ISBN 0-226-51696-2. [Google Scholar]
- Geffen, E.; Anderson, M.J.; Wayne, R.K. Climate and habitat barriers to dispersal in the highly mobile grey wolf. Mol. Ecol. 2004, 13, 2481–2490. [Google Scholar] [CrossRef]
- Marucco, F.; Pletscher, D.H.; Boitani, L.; Schwartz, M.K.; Pilgrim, K.L.; Lebreton, J.-D. Wolf survival and population trend using non-invasive capture-recapture techniques in the Western Alps. J. Appl. Ecol. 2009, 46, 1003–1010. [Google Scholar] [CrossRef]
- Marucco, F.; McIntire, E.J.B. Predicting spatio-temporal recolonization of large carnivore populations and livestock depredation risk: Wolves in the Italian Alps. J. Appl. Ecol. 2010, 47, 789–798. [Google Scholar] [CrossRef]
- Mills, L.S.; Pilgrim, K.L.; Schwartz, M.K.; McKelvey, K. Identifying lynx and other North American felids based on MtDNA analysis. Conserv. Genet. 2000, 1, 285–288. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Paabo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef] [PubMed]
- Randi, E.; Lucchini, V.; Christensen, M.F.; Mucci, N.; Funk, S.M.; Dolf, G.; Loeschcke, V. Mitochondrial DNA Variability in Italian and East European Wolves: Detecting the Consequences of Small Population Size and Hybridization. Conserv. Biol. 2000, 14, 464–473. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z. DAMBE: Software Package for Data Analysis in Molecular Biology and Evolution. J. Hered. 2001, 94, 371–373. [Google Scholar] [CrossRef]
- Ostrander, E.; Sprague, G.F.; Rine, J. Identification and Characterization of Dinucleotide Repeat (CA)n Markers for Genetic Mapping in Dog. Genomics 1993, 16, 207–213. [Google Scholar] [CrossRef]
- Fredholm, M.; Winteroe, A.K. Variation of short tandem repeats within and between species belonging to the Canidae family. Mamm. Genome 1995, 6, 11–18. [Google Scholar] [CrossRef]
- Francisco, L.V.; Langsten, A.A.; Mellersh, C.S.; Neal, C.L.; Ostrander, E. A class of highly polymorphic tetranucleotide repeats for canine genetic mapping. Mamm. Genome 1996, 7, 359–362. [Google Scholar] [CrossRef]
- Neff, M.W.; Broman, K.W.; Mellersh, C.S.; Ray, K.; Ackland, G.M.; Aguirre, G.D.; Ziegle, J.S.; Ostrander, E.A.; Rine, J. A second-generation genetic linkage map of the domestic dog, Canis familiaris. Genetics 1999, 151, 803–820. [Google Scholar] [CrossRef]
- McKelvey, K.S.; Schwartz, M.K. Genetic errors associated with population estimation using non-invasive molecular tagging: Problems and new solutions. J. Wildl. Manag. 2004, 68, 439–448. [Google Scholar] [CrossRef]
- Lucchini, V.; Fabbri, E.; Marucco, F.; Ricci, S.; Boitani, L.; Randi, E. Noninvasive molecular tracking of colonizing wolf (Canis lupus) packs in the western Italian Alps. Mol. Ecol. 2002, 11, 857–868. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.S.; Schwartz, M.K. Dropout: A program to identify problem loci and samples for noninvasive genetic samples in a capture-mark-recapture framework. Mol. Ecol. Notes 2005, 5, 716–718. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Wagner, A.P.; Taper, M.L. ml-relate: A computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. Notes 2006, 6, 576–579. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2022. Available online: http://qgis.osgeo.org (accessed on 13 April 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 13 April 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 13 April 2022).
- Paetkau, D.; Strobeck, C. Microsatellite analysis of genetic variation in black bear populations. Mol. Ecol. 1994, 3, 489–495. [Google Scholar] [CrossRef]
- Evett, I.W.; Weir, B.S. Interpreting DNA Evidence: Statistical Genetics for Forensic Scientists; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Montana, L.; Caniglia, R.; Galaverni, M.; Fabbri, E.; Randi, E. A new mitochondrial haplotype confirms the distinctiveness of the Italian wolf (Canis lupus) population. Mamm. Biol. 2017, 84, 30–34. [Google Scholar] [CrossRef]
- Ciucci, P.; Reggioni, W.; Maiorano, L.; Boitani, L. Long-Distance Dispersal of a Rescued Wolf From the Northern Apennines to the Western Alps. J. Wildl. Manag. 2009, 73, 1300–1306. [Google Scholar] [CrossRef]
- Bowler, D.E.; Benton, T. Causes and consequences of animal dispersal strategies: Relating individual behaviour to spatial dynamics. Biol. Rev. 1999, 80, 205–225. [Google Scholar] [CrossRef]
- Sanz-Pérez, A.; Ordiz, A.; Sand, H.; Swenson, J.E.; Wabakken, P.; Wikenros, C.; Zimmermann, B.; Åkesson, M.; Milleret, C. No place like home? A test of the natal habitat-biased dispersal hypothesis in Scandinavian wolves. R. Soc. Open Sci. 2018, 5, 181379. [Google Scholar] [CrossRef] [PubMed]
- Cimatti, M.; Ranc, N.; Benítez-López, A.; Maiorano, L.; Boitani, L.; Cagnacci, F.; Čengić, M.; Ciucci, P.; Huijbregts, M.A.J.; Krofel, M.; et al. Large carnivore expansion in Europe is associated with human population density and land cover changes. Divers. Distrib. 2021, 27, 602–617. [Google Scholar] [CrossRef]
- Berggren, Å.; Birath, B.; Kindvall, O. Effect of Corridors and Habitat Edges on Dispersal Behavior, Movement Rates, and Movement Angles in Roesel’s Bush-Cricket (Metrioptera roeseli). Conserv. Biol. 2002, 16, 1562–1569. [Google Scholar] [CrossRef]
- Tallmon, D.A.; Luikart, G.; Waples, R.S. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004, 19, 489–496. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Miller, A.D.; Weeks, A.R. Genetic mixing for population management: From genetic rescue to provenancing. Evol. Appl. 2020, 14, 634–652. [Google Scholar] [CrossRef]
- Bell, D.A.; Robinson, Z.L.; Funk, W.C.; Fitzpatrick, S.W.; Allendorf, F.W.; Tallmon, D.A.; Whiteley, A.R. The Exciting Potential and Remaining Uncertainties of Genetic Rescue. Trends Ecol. Evol. 2019, 34, 1070–1079. [Google Scholar] [CrossRef]
- Vilà, C.; Sundqvist, A.; Flagstad, Ø.; Seddon, J.; Rnerfeldt, S.B.; Kojola, I.; Casulli, A.; Sand, H.; Wabakken, P.; Ellegren, H. Rescue of a severely bottlenecked wolf (Canis lupus) population by a single immigrant. Proc. R. Soc. B Boil. Sci. 2003, 270, 91–97. [Google Scholar] [CrossRef]
- Åkesson, M.; Liberg, O.; Sand, H.; Wabakken, P.; Bensch, S.; Flagstad, Ø. Genetic rescue in a severely inbred wolf population. Mol. Ecol. 2016, 25, 4745–4756. [Google Scholar] [CrossRef]
- Schwartz, M.K.; Luikart, G.; Waples, R.S. Genetic monitoring as a promising tool for conservation and management. Trends Ecol. Evol. 2007, 22, 25–33. [Google Scholar] [CrossRef]
- Robinson, Z.L.; Bell, D.A.; Dhendup, T.; Luikart, G.; Whiteley, A.R.; Kardos, M. Evaluating the outcomes of genetic rescue attempts. Conserv. Biol. 2020, 35, 666–677. [Google Scholar] [CrossRef]
- Kruse, H.; Kirkemo, A.-M.; Handeland, K. Wildlife as Source of Zoonotic Infections. Emerg. Infect. Dis. 2004, 10, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Cantacessi, C.; Pfeffer, M.; Torres, F.D.; Brianti, E.; Deplazes, P.; Genchi, C.; Guberti, V.; Capelli, G. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Veter. Parasitol. 2015, 213, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Toma, B.; Andral, L. Epidemiology of Fox Rabies. Adv. Virus Res. 1977, 21, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Blancou, J. Ecology and Epidemiology of Fox Rabies. Clin. Infect. Dis. 1988, 10 (Suppl. 4), S606–S609. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, F.; Müller, M.S.; Grimm, V.; Wissel, C.; Brandl, R. Pattern formation triggered by rare events: Lessons from the spread of rabies. Proc. R. Soc. B Boil. Sci. 1997, 264, 495–503. [Google Scholar] [CrossRef]
- Mulatti, P.; Bonfanti, L.; Patregnani, T.; Lorenzetto, M.; Ferrè, N.; Gagliazzo, L.; Casarotto, C.; Ponti, A.M.; Ferri, G.; Marangon, S. 2008–2011 sylvatic rabies epidemic in Italy: Challenges and experiences. Pathog. Glob. Health 2013, 107, 346–353. [Google Scholar] [CrossRef][Green Version]
- Lojkić, I.; Šimić, I.; Bedeković, T.; Krešić, N. Current Status of Rabies and Its Eradication in Eastern and Southeastern Europe. Pathogens 2021, 10, 742. [Google Scholar] [CrossRef]
- Linnell, J.D.C.; Kovtun, E.; Rouart, I. Wolf Attacks on Humans: An Update for 2002–2020; NINA Report 1944; Norwegian Institute for Nature Research: Oslo, Norway, 2021. [Google Scholar]
- Oksanen, A.; Siles-Lucas, M.; Karamon, J.; Possenti, A.; Conraths, F.J.; Romig, T.; Wysocki, P.; Mannocci, A.; Mipatrini, D.; La Torre, G.; et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: A systematic review and meta-analysis. Parasites Vectors 2016, 9, 1–23. [Google Scholar] [CrossRef]
- Combes, B.; Comte, S.; Raton, V.; Raoul, F.; Boué, F.; Umhang, G.; Favier, S.; Dunoyer, C.; Woronoff, N.; Giraudoux, P. Westward Spread ofEchinococcus multilocularisin Foxes, France, 2005–2010. Emerg. Infect. Dis. 2012, 18, 2059–2062. [Google Scholar] [CrossRef]
- Citterio, C.V.; Obber, F.; Trevisiol, K.; Dellamaria, D.; Celva, R.; Bregoli, M.; Ormelli, S.; Sgubin, S.; Bonato, P.; Da Rold, G.; et al. Echinococcus multilocularis and other cestodes in red foxes (Vulpes vulpes) of northeast Italy, 2012–2018. Parasites Vectors 2021, 14, 29. [Google Scholar] [CrossRef]
- De Macedo, M.; Zanet, S.; Bruno, S.; Tolosano, A.; Marucco, F.; Rossi, L.; Muller, G.; Ferroglio, E. Gastrointestinal helminths of wolves (Canis lupus Linnaeus, 1758) in Piedmont, north-western Italy. J. Helminthol. 2019, 94, e88. [Google Scholar] [CrossRef] [PubMed]
- Massolo, A.; Valli, D.; Wassermann, M.; Cavallero, S.; D’Amelio, S.; Meriggi, A.; Torretta, E.; Serafini, M.; Casulli, A.; Zambon, L.; et al. Unexpected Echinococcus multilocularis infections in shepherd dogs and wolves in south-western Italian Alps: A new endemic area? Int. J. Parasitol. Parasites Wildl. 2018, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kapel, C.; Torgerson, P.; Thompson, R.; Deplazes, P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006, 36, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Garippa, G.; Manfredi, M.T. Cystic echinococcosis in Europe and in Italy. Veter. Res. Commun. 2009, 33 (Suppl. 1), 35–39. [Google Scholar] [CrossRef] [PubMed]
- Gori, F.; Armua-Fernandez, M.T.; Milanesi, P.; Serafini, M.; Magi, M.; Deplazes, P.; Macchioni, F. The occurrence of taeniids of wolves in Liguria (northern Italy). Int. J. Parasitol. Parasites Wildl. 2015, 4, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Poglayen, G.; Gori, F.; Morandi, B.; Galuppi, R.; Fabbri, E.; Caniglia, R.; Milanesi, P.; Galaverni, M.; Randi, E.; Marchesi, B.; et al. Italian wolves (Canis lupus italicus Altobello, 1921) and molecular detection of taeniids in the Foreste Casentinesi National Park, Northern Italian Apennines. Int. J. Parasitol. Parasites Wildl. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Macchioni, F.; Coppola, F.; Furzi, F.; Gabrielli, S.; Baldanti, S.; Boni, C.B.; Felicioli, A. Taeniid cestodes in a wolf pack living in a highly anthropic hilly agro-ecosystem. Parasite 2021, 28, 10. [Google Scholar] [CrossRef]
- Eckert, J.; Gemmell, M.A.; Meslin, F.X.; Pawlowski, Z. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; WHO/OIE: Geneva, Switzerland, 2001; pp. 195–229. [Google Scholar]
- Paoletti, B.; Della Salda, L.; Di Cesare, A.; Iorio, R.; Vergara, A.; Fava, C.; Olivastri, A.; Dessì, G.; Scala, A.; Varcasia, A. Epidemiological survey on cystic echinococcosis in wild boar from Central Italy. Parasitol. Res. 2019, 118, 43–46. [Google Scholar] [CrossRef]
- Desmecht, D.; Gerbier, G.; Schmidt, C.G.; Grigaliuniene, V.; Helyes, G.; Kantere, M.; Korytarova, D.; Linden, A.; Miteva, A.; Neghirla, I.; et al. Epidemiological analysis of African swine fever in the European Union (September 2019 to August 2020). EFSA J. 2021, 19, e06572. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; et al. African Swine Fever in Wild Boar in Europe—A Review. Viruses 2021, 13, 1717. [Google Scholar] [CrossRef]
- Szewczyk, M.; Łepek, K.; Nowak, S.; Witek, M.; Bajcarczyk, A.; Kurek, K.; Stachyra, P.; Mysłajek, R.W.; Szewczyk, B. Evaluation of the Presence of ASFV in Wolf Feces Collected from Areas in Poland with ASFV Persistence. Viruses 2021, 13, 2062. [Google Scholar] [CrossRef] [PubMed]
- Boklund, A.; Cay, B.; Depner, K.; Földi, Z.; Guberti, V.; Masiulis, M.; Miteva, A.; More, S.; Olsevskis, E.; Šatrán, P.; et al. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA J. 2018, 16, e05494. [Google Scholar] [CrossRef] [PubMed]
- Brandell, E.E.; Cross, P.C.; Craft, M.E.; Smith, D.W.; Dubovi, E.J.; Gilbertson, M.L.J.; Wheeldon, T.; Stephenson, J.A.; Barber-Meyer, S.; Borg, B.L.; et al. Patterns and processes of pathogen exposure in gray wolves across North America. Sci. Rep. 2021, 11, 3722, Erratum in Sci. Rep. 2021, 11, 22579. [Google Scholar] [CrossRef] [PubMed]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R.J. Canine Distemper In Terrestrial Carnivores: A Review. J. Zoo Wildl. Med. 2000, 31, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Trogu, T.; Canziani, S.; Salvato, S.; Bianchi, A.; Bertoletti, I.; Gibelli, L.R.; Alborali, G.L.; Barbieri, I.; Gaffuri, A.; Sala, G.; et al. Canine Distemper Outbreaks in Wild Carnivores in Northern Italy. Viruses 2021, 13, 99. [Google Scholar] [CrossRef]
- Di Sabatino, D.; Lorusso, A.; Di Francesco, C.E.; Gentile, L.; Di Pirro, V.; Bellacicco, A.L.; Giovannini, A.; Di Francesco, G.; Marruchella, G.; Marsilio, F.; et al. Arctic Lineage-Canine Distemper Virus as a Cause of Death in Apennine Wolves (Canis lupus) in Italy. PLoS ONE 2014, 9, e82356. [Google Scholar] [CrossRef]
- Moroni, B.; Rossi, L.; Meneguz, P.G.; Orusa, R.; Zoppi, S.; Robetto, S.; Marucco, F.; Tizzani, P. Dirofilaria immitis in wolves recolonizing northern Italy: Are wolves competent hosts? Parasites Vectors 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Bezerra-Santos, M.A.; Moroni, B.; Mendoza-Roldan, J.A.; Perrucci, S.; Cavicchio, P.; Cordon, R.; Cianfanelli, C.; Lia, R.P.; Rossi, L.; Otranto, D. Wild carnivores and Thelazia callipaeda zoonotic eyeworms: A focus on wolves. Int. J. Parasitol. Parasites Wildl. 2022, 17, 239–243. [Google Scholar] [CrossRef]

| ID Genotype | Sex | Length of Dispersal (km) | Area of Dispersal | Change of Status | Recovered Dead | ||
|---|---|---|---|---|---|---|---|
| From | To | From | To | ||||
| CN-46 | M | 174.4 | CN (IT) | AO (IT) | P | O | No |
| TO-46 | F | 177.7 | TO (IT) | Swiss | P | O | Yes |
| TO-41 | M | 199.5 | TO (IT) | Swiss | P | O | Yes |
| CN-123 | M | 208.6 | CN (IT) | VB (IT) | P | O | No |
| CN-31 | F | 214.1 | CN (IT) | VB (IT) | P | O | No |
| SLO-01—Slavc | M | 233.0 | Slovenia | VR (IT) | P | A | No |
| CN-95-Ligabue | M | 239.0 | PR (IT) | CN (IT) | P | O | Yes |
| CN-100 | M | 517.2 | CN (IT) | Germany | P | O | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marucco, F.; Pilgrim, K.L.; Avanzinelli, E.; Schwartz, M.K.; Rossi, L. Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading. Animals 2022, 12, 1260. https://doi.org/10.3390/ani12101260
Marucco F, Pilgrim KL, Avanzinelli E, Schwartz MK, Rossi L. Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading. Animals. 2022; 12(10):1260. https://doi.org/10.3390/ani12101260
Chicago/Turabian StyleMarucco, Francesca, Kristine L. Pilgrim, Elisa Avanzinelli, Michael K. Schwartz, and Luca Rossi. 2022. "Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading" Animals 12, no. 10: 1260. https://doi.org/10.3390/ani12101260
APA StyleMarucco, F., Pilgrim, K. L., Avanzinelli, E., Schwartz, M. K., & Rossi, L. (2022). Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading. Animals, 12(10), 1260. https://doi.org/10.3390/ani12101260






