Canine Neonatal Assessment by Vitality Score, Amniotic Fluid, Urine, and Umbilical Cord Blood Analysis of Glucose, Lactate, and Cortisol: Possible Influence of Parturition Type?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cesarean Section
Puppy Resuscitation
2.3. Vaginal Parturition
2.4. Evaluation of the Puppy, Apgar Score, and Neonatal Reflexes
2.5. Amniotic Fluid, Umbilical Blood, and Urine Samples
2.6. Growth Rate of Newborn Puppies
2.7. Statistical Analysis
3. Results
3.1. Basic Information
3.2. Survival, Apgar Score, and Neonatal Reflexes
3.3. Amniotic Fluid, Umbilical Blood, and Urine Samples
3.4. Growth Rate of Newborn Puppies
3.5. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vannucchi, C.I.; Silva, L.C.; Lúcio, C.F.; Regazzi, F.M.; Veiga, G.A.; Angrimani, D.S. Prenatal and neonatal adaptations with a focus on the respiratory system. Reprod. Domest. Anim. 2012, 47, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.A. Perinatal and Late Neonatal Mortality in the Dog. Ph.D. Thesis, University of Sydney, Sydney, Australia, March 2001. Available online: https://core.ac.uk/download/pdf/41232423.pdf (accessed on 1 October 2021).
- Münnich, A. The pathological newborn in small animals: The neonate is not a small adult. Vet. Res. Commun. 2008, 32 (Suppl. S1), S81–S85. [Google Scholar] [CrossRef]
- Mosier, J.E. Canine pediatrics: The neonate. In Proceedings of the 48th American Animal Hospital Association Annual Meeting, Montreal, Canada, 11 March 1981; pp. 339–347. [Google Scholar]
- Moon, P.F.; Erb, H.N.; Ludders, J.W.; Gleed, R.D.; Pascoe, P.J. Perioperative risk factors for puppies delivered by cesarean section in the United States and Canada. J. Am. Anim. Hosp. Assoc. 2000, 36, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Indrebø, A.; Trangerud, C.; Moe, L. Canine neonatal mortality in four large breeds. Acta Vet. Scand. 2007, 49, S2. [Google Scholar] [CrossRef] [Green Version]
- Tønnessen, R.; Borge, K.S.; Nødtvedt, A.; Indrebø, A. Canine perinatal mortality: A cohort study of 224 breeds. Theriogenology 2012, 77, 1788–1801. [Google Scholar] [CrossRef]
- Münnich, A.; Küchenmeister, U. Causes, diagnosis and therapy of common diseases in neonatal puppies in the first days of life: Cornerstones of practical approach. Reprod. Domest. Anim. 2014, 49 (Suppl. S2), 64–74. [Google Scholar] [CrossRef]
- England, G.C.W. Care of the Neonate and Fading Pups. In Textbook of Veterinary Internal Medicine, 7th ed.; Ettinger, S.J., Feldman, E.C., Eds.; Saunders: St. Louis, MO, USA, 2010; Volume 2, pp. 1949–1954. [Google Scholar]
- Bolis, B.; Prandi, A.; Rota, A.; Faustini, M.; Veronesi, M.C. Cortisol fetal fluid concentrations in term pregnancy of small-sized purebred dogs and its preliminary relation to first 24 hours survival of newborns. Theriogenology 2017, 88, 264–269. [Google Scholar] [CrossRef]
- Roos, J.; Maenhoudt, C.; Zilberstein, L.; Mir, F.; Borges, P.; Furthner, E.; Niewiadomska, Z.; Nudelmann, N.; Fontbonne, A. Neonatal puppy survival after planned caesarean section in the bitch using aglepristone as a primer: A retrospective study on 74 cases. Reprod. Domest. Anim. 2018, 53 (Suppl. S3), 85–95. [Google Scholar] [CrossRef]
- Vassalo, F.G.; Simões, C.R.; Sudano, M.J.; Prestes, N.C.; Lopes, M.D.; Chiacchio, S.B.; Lourenço, M.L. Topics in the routine assessment of newborn puppy viability. Top. Companion. Anim. Med. 2015, 30, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, M.C.; Panzani, S.; Faustini, M.; Rota, A. An Apgar scoring system for routine assessment of newborn puppy viability and short-term survival prognosis. Theriogenology 2009, 72, 401–407. [Google Scholar] [CrossRef]
- Veronesi, M.C. Assessment of canine neonatal viability-the Apgar score. Reprod. Domest. Anim. 2016, 51 (Suppl. S1), 46–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, L.; Stenson, B.J. Use of umbilical cord blood gas analysis in the assessment of the newborn. Arch. Dis. Child. Fetal. Neonatal. 2007, 92, F430–F434. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, C.F.; Silva, L.C.; Rodrigues, J.A.; Veiga, G.A.; Vannucchi, C.I. Acid-base changes in canine neonates following normal birth or dystocia. Reprod. Domest. Anim. 2009, 44 (Suppl. S2), 208–210. [Google Scholar] [CrossRef] [PubMed]
- Groppetti, D.; Pecile, A.; Del Carro, A.P.; Copley, K.; Minero, M.; Cremonesi, F. Evaluation of newborn canine viability by means of umbilical vein lactate measurement, Apgar score and uterine tocodynamometry. Theriogenology 2010, 74, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Groppetti, D.; Martino, P.A.; Ravasio, G.; Bronzo, V.; Pecile, A. Prognostic potential of amniotic fluid analysis at birth on canine neonatal outcomes. Vet. J. 2015, 206, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, C.F.; Silva, L.C.G.; Vannucchi, C.I. Perinatal cortisol and blood glucose concentrations in bitches and neonatal puppies: Effects of mode of whelping. Domest. Anim. Endocrinol. 2021, 74, 106483. [Google Scholar] [CrossRef]
- Pang, D.S.; Boysen, S. Lactate in veterinary critical care: Pathophysiology and management. J. Am. Anim. Hosp. Assoc. 2007, 43, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Castagnetti, C.; Cunto, M.; Bini, C.; Mariella, J.; Capolongo, S.; Zambelli, D. Time-dependent changes and prognostic value of lactatemia during the first 24 h of life in brachycephalic newborn dogs. Theriogenology 2017, 94, 100–104. [Google Scholar] [CrossRef]
- Antończyk, A.; Ochota, M.; Niżański, W. Umbilical cord blood gas parameters and Apgar scoring in assessment of new-born dogs delivered by cesarean section. Animals 2021, 11, 685. [Google Scholar] [CrossRef]
- Bolis, B.; Scarpa, P.; Rota, A.; Vitiello, T.; Veronesi, M.C. Association of amniotic uric acid, glucose, lactate and creatinine concentrations and lactate/creatinine ratio with newborn survival in small-sized dogs-prelimi-nary results. Acta Vet. Hung. 2018, 66, 125–136. [Google Scholar] [CrossRef]
- Dysart, K.C. Neonatal hypoglycemia. In Merck Manual Professional Version; Merck Sharp & Dohme Corp.: Kenilworth, NJ, USA, 2021; pp. e1–e5. Available online: https://www.merckmanuals.com/professional/pediatrics/metabolic,-electrolyte,-and-toxic-disorders-in-neonates/neonatal-hypoglycemia (accessed on 8 October 2021).
- Thompson-Branch, A.; Havranek, T. Neonatal Hypoglycemia. Pediatr. Rev. 2017, 38, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Smith, F.O. Challenges in small animal parturition: Timing elective and emergency cesarian sections. Theriogenology 2007, 68, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Traas, A.M. Surgical management of canine and feline dystocia. Theriogenology 2008, 70, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Meloni, T. Some Perinatal Endocrine and Morphological Aspects of Canine Species. Ph.D. Thesis, University of Milan, Milan, Italy, 2015. Available online: https://air.unimi.it/retrieve/handle/2434/265723/370688/phd_unimi_R09461.pdf (accessed on 1 October 2021).
- Batista, M.; Moreno, C.; Vilar, J.; Golding, M.; Brito, C.; Santana, M.; Alamo, D. Neonatal viability evaluation by Apgar score in puppies delivered by cesarean section in two brachycephalic breeds (English and French bulldog). Anim. Reprod. Sci. 2014, 146, 218–226. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 16 January 2022).
- Zakošek Pipan, M.; Švara, T.; Zdovc, I.; Papić, B.; Avberšek, J.; Kušar, D.; Mrkun, J. Staphylococcus pseudintermedius septicemia in puppies after elective cesarean section: Confirmed transmission via dam’s milk. BMC Vet. Res. 2019, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Fusi, J.; Faustini, M.; Bolis, B.; Veronesi, M.C. Apgar score or birthweight in Chihuahua dogs born by elective Caesarean section: Which is the best predictor of the survival at 24 h after birth? Acta. Vet. Scand. 2020, 62, 39. [Google Scholar] [CrossRef]
- Mila, H.; Grellet, A.; Feugier, A.; Chastant-Maillard, S. Differential impact of birth weight and early growth on neonatal mortality in puppies. J. Anim. Sci. 2015, 93, 4436–4442. [Google Scholar] [CrossRef]
- Mugnier, A.; Mila, H.; Guiraud, F.; Brévaux, J.; Lecarpentier, M.; Martinez, C.; Mariani, C.; Adib-Lesaux, A.; Chastant-Maillard, S.; Saegerman, C.; et al. Birth weight as a risk factor for neonatal mortality: Breed-specific approach to identify at-risk puppies. Prev. Vet. Med. 2019, 171, 104746. [Google Scholar] [CrossRef]
- Doebeli, A.; Michel, E.; Bettschart, R.; Hartnack, S.; Reichler, I.M. Apgar score after induction of anesthesia for canine cesarean section with alfaxalone versus propofol. Theriogenology 2013, 80, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Titkova, R.; Fialkovicova, M.; Karasova, M.; Hajurka, J. Puppy Apgar scores after vaginal delivery and caesarean section. Vet. Med. 2017, 62, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Vilar, J.M.; Batista, M.; Pérez, R.; Zagorskaia, A.; Jouanisson, E.; Díaz-Bertrana, L.; Rosales, S. Comparison of 3 anesthetic protocols for the elective cesarean-section in the dog: Effects on the bitch and the newborn puppies. Anim. Reprod. Sci. 2018, 190, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Siena, G.; Milani, C. Usefulness of maternal and fetal parameters for the prediction of parturition date in dogs. Animals 2021, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Zaigham, M.; Helfer, S.; Kristensen, K.H.; Isberg, P.E.; Wiberg, N. Maternal arterial blood gas values during delivery: Effect of mode of delivery, maternal characteristics, obstetric interventions and correlation to fetal umbilical cord blood. Acta Obstet. Gynecol. Scand. 2020, 99, 1674–1681. [Google Scholar] [CrossRef]
- Bakker, P.C.; Kurver, P.H.; Kuik, D.J.; Van Geijn, H.P. Elevated uterine activity increases the risk of fetal acidosis at birth. Am. J. Obstet. Gynecol. 2007, 196, 313.e1-6. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Dollberg, S.; Mimouni, F.B.; Berger, I.; Mordechayev, N.; Ochshorn, Y.; Mandel, D. Neonatal blood glucose concentrations in caesarean and vaginally delivered term infants. Acta Paediatr. 2010, 99, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Balogh, O.; Bruckmaier, R.; Keller, S.; Reichler, I.M. Effect of maternal metabolism on fetal supply: Glucose, non-esterified fatty acids and beta-hydroxybutyrate concentrations in canine maternal serum and fetal fluids at term pregnancy. Anim. Reprod. Sci. 2018, 193, 209–216. [Google Scholar] [CrossRef]
- Newfield, A. Reproductive Emergencies. In Small Animal Emergency and Critical Care for Veterinary Technicians, 4th ed; Battaglia, A., Steele, A.M., Eds.; Elsevier Health Sciences: St. Louis, MO, USA, 2020; pp. 271–282. [Google Scholar]
- Bergström, A.; Fransson, B.; Lagerstedt, A.S.; Olsson, K. Primary uterine inertia in 27 bitches: Aetiology and treatment. J. Small Anim. Pract. 2006, 47, 456–460. [Google Scholar] [CrossRef]
- Mila, H.; Grellet, A.; Delebarre, M.; Mariani, C.; Feugier, A.; Chastant-Maillard, S. Monitoring of the newborn dog and prediction of neonatal mortality. Prev. Vet. Med. 2017, 143, 11–20. [Google Scholar] [CrossRef]
- Aoki, T.; Ishii, M. Hematologic and biochemical profiles in peripartum mares and neonatal foals (heavy draft horse). J. Eq. Vet. Sci. 2012, 32, 170–176. [Google Scholar] [CrossRef]
- Knowles, T.G.; Edwards, J.E.; Bazeley, K.J.; Brown, S.N.; Butterworth, A.; Warriss, P.D. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 2000, 147, 593–598. [Google Scholar] [CrossRef]
- Mastorakos, G.; Ilias, I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N. Y. Acad. Sci. 2003, 997, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Fusi, J.; Carluccio, A.; Peric, T.; Faustini, M.; Prandi, A.; Veronesi, M.C. Effect of delivery by emergency or elective Cesarean section on nitric oxide metabolites and cortisol amniotic concentrations in at term normal newborn dogs: Preliminary results. Animals 2021, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Balogh, O.; Roch, M.; Keller, S.; Michel, E.; Reichler, I.M. The use of semi-quantitative tests at Cesarean section delivery for the differentiation of canine fetal fluids from maternal urine on the basis of biochemical characteristics. Theriogenology 2017, 88, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusi, J.; Bolis, B.; Probo, M.; Faustini, M.; Carluccio, A.; Veronesi, M.C. Clinical trial on the usefulness of on-site evaluation of canine fetal fluids by reagent test strip in puppies at elective Caesarean section. Biology 2021, 11, 38. [Google Scholar] [CrossRef]



| Breed Size | Breed | Number of Parturitions (%) | Age of Bitches (Years) | Number of Parity | Number of Female Puppies (%) | Number of Male Puppies (%) | Number of Puppies (%) |
|---|---|---|---|---|---|---|---|
| Miniature Schnauzer | 2 (9.1) | 3–4 | 1–2 | 6 (4.9) | 4 (3.2) | 10 (8.1) | |
| Yorkshire Terrier | 1 (4.5) | 2.25 | 1 | 1 (0.8) | 3 (2.4) | 4 (3.3) | |
| Maltese | 1 (4.5) | 3.25 | 2 | 2 (1.6) | 3 (2.4) | 5 (4.1) | |
| Miniature Poodle | 1 (4.5) | 7 | 3 | 1 (0.8) | 1 (0.8) | 2 (1.6) | |
| Boston Terrier | 4 (18.2) | 2–5.5 | 1–3 | 11 (8.9) | 5 (4.1) | 16 (13) | |
| Jack Russell Terrier–Maltese mix | 1 (4.5) | 5 | 1 | 3 (2.4) | 4 (3.3) | 7 (5.7) | |
| Total small breeds(<10 kg) | 10 (45.4) | 24 (19.5) | 20 (16.3) | 44 (35.8) | |||
| Pembroke Welsh Corgi | 2 (9.1) | 2.5–2.75 | 1 | 3 (2.4) | 7 (5.7) | 10 (8.1) | |
| English Bulldog | 1 (4.5) | 2.5 | 1 | 2 (1.6) | 2 (1.6) | 4 (3.3) | |
| French Bulldog | 2 (9.1) | 2–2.5 | 1 | 5 (4.1) | 8 (6.5) | 13 (10.6) | |
| Beagle | 1 (4.5) | 4.25 | 3 | 3 (2.4) | 4 (3.3) | 7 (5.7) | |
| Dachshund | 1 (4.5) | 3 | 2 | 7 (5.7) | 2 (1.6) | 9 (7.3) | |
| Whippet | 1 (4.5) | 2.5 | 1 | 4 (3.3) | 3 (2.4) | 7 (7.3) | |
| Total medium breeds (10–25 kg) | 8 (36.4) | 24 (19.5) | 26 (21.1) | 50 (40.6) | |||
| Greater Swiss Mountain Dog | 1 (4.5) | 3.5 | 2 | 1 (0.8) | 5 (4.1) | 6 (4.9) | |
| Golden Retriever | 1 (4.5) | 2.5 | 1 | 5 (4.1) | 6 (4.9) | 11 (8.9) | |
| Labrador Retriever | 1 (4.5) | 2.75 | 2 | 8 (6.5) | 2 (1.6) | 10 (8.1) | |
| German Shepherd | 1 (4.5) | 3 | 2 | 1 (0.8) | 1 (0.8) | 2 (1.6) | |
| Total large and giant breeds (>25 kg) | 4 (18.2) | 15 (12.2) | 14 (11.4) | 29 (23.6) |
| Parameter | Points | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Mucous membrane color | Pale or cyanotic | Pink | Reddish |
| Heart rate (bpm 1) | Absent or <120 bpm | 120–180 bpm | >180 bpm |
| Respiratory rate | Absent or <6/min | Weak, irregular, 6–15/min | >15/min, rhythmic |
| Activity, muscle tone | Flaccid | Some flexions | Active motion |
| Reflexes irritability | Absent | Weak vocalization and weak reflex | Vigorous vocalization and immediate reflex |
| Type of Parturition | Number of Puppies | Survival | Body Weight in Grams at Birth (Mean ± SEM) | Body Weight in Grams on Day 7 (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Born Alive | Stillborn | Died after Discharge | Female | Male | Female | Male | |
| VP | 36 | 32 | 63 | 5 | 3 | 279 ± 12 (n = 33) | 283 ± 17 (n = 28) | 466 ± 23 (n = 31) | 464 ± 32 (n = 27) |
| EL-CS | 18 | 20 | 36 | 2 | 4 | 243 ± 16 (n = 17) | 234 ± 18 (n = 19) | 382 ± 32 (n = 14) | 335 ± 32 (n = 16) |
| EM-CS | 9 | 8 | 15 | 2 | 0 | 252 ± 48 (n = 8) | 306 ± 49 (n = 7) | 439 ± 64 (n = 5) | 454 ± 47 (n = 6) |
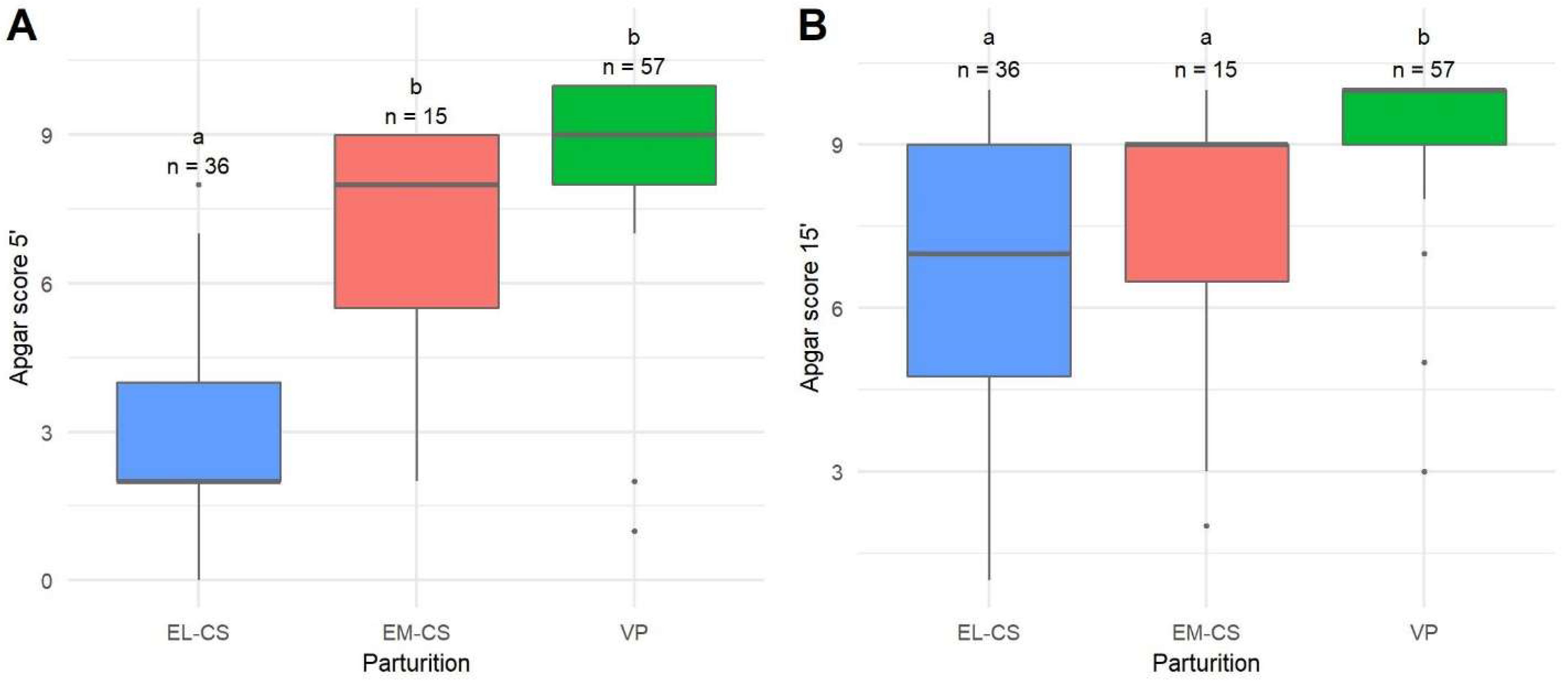
| Type of Parturition | Apgar Score 0–3: Severe Distress (%) | Apgar Score 4–6: Moderate Distress (%) | Apgar Score 7–10: No Distress (%) |
|---|---|---|---|
| 5 min after parturition | |||
| VP | 3 (5.3) | 0 (0) | 54 (94.7) |
| EM-CS | 2 (13.3) | 3 (20) | 10 (66.7) |
| EL-CS | 26 (72.2) | 3 (8.3) | 7 (19.4) |
| 15 min after parturition | |||
| VP | 1 (1.8) | 1 (1.8) | 55 (96.5) |
| EM-CS | 2 (13.3) | 2 (13.3) | 11 (73.3) |
| EL-CS | 7 (19.4) | 9 (25) | 20 (55.6) |
| 60 min after parturition | |||
| VP | 1 (1.7) | 0 (0) | 57 (98.3) |
| EM-CS | 0 (0) | 0 (0) | 15 (100) |
| EL-CS | 0 (0) | 0 (0) | 36 (100) |
| Type of Parturition | Number of Poorly Responsive Puppies (%) | Number of Moderately Responsive Puppies (%) | Number of Adequately Responsive Puppies (%) |
|---|---|---|---|
| Neonatal reflexes 5 min after parturition | |||
| VP | 3 (5.3) | 13 (22.8) | 41 (71.9) |
| EM-CS | 8 (53.3) | 2 (13.3) | 5 (33.3) |
| EL-CS | 28 (77.8) | 3 (8.3) | 5 (13.9) |
| Neonatal reflexes 15 min after parturition | |||
| VP | 2 (3.5) | 3 (5.3) | 52 (91.2) |
| EM-CS | 5 (33.3) | 2 (13.3) | 8 (53.3) |
| EL-CS | 19 (52.8) | 12 (33.3) | 5 (14.9) |
| Neonatal reflexes 60 min after parturition | |||
| VP | 1 (2) | 1 (2) | 56 (96) |
| EM-CS | 0 (0) | 1 (7) | 14 (93) |
| EL-CS | 0 (0) | 3 (8) | 33 (92) |
| AM Glucose | AM Lactate | Apgar 5 | Apgar 15 | AM Cortisol | Cortisol in Urine | UB Glucose | UB Lactate | |
|---|---|---|---|---|---|---|---|---|
| AM lactate | −0.1520 | / | ||||||
| Apgar 5 | 0.4953 * | −0.2141 | / | |||||
| Apgar 15 | 0.3952 | −0.1173 | 0.7400 *** | / | ||||
| AM cortisol | 0.2024 | 0.3513 | 0.0935 | 0.0376 | / | |||
| Cortisol in urine | 0.1744 | 0.4820 | 0.1115 | 0.1087 | 0.9199 *** | / | ||
| UB glucose | 0.5307 * | −0.1361 | 0.5536 ** | 0.5854 ** | 0.0680 | 0.0226 | / | |
| UB lactate | 0.0055 | 0.8741 *** | −0.1602 | −0.0013 | 0.4662 | 0.5778 ** | −0.0404 | / |
| Relative growth rate | 0.2062 | −0.5378 * | 0.0422 | 0.0350 | −0.2263 | −0.3657 | 0.0901 | −0.5876 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavec, T.; Knific, T.; Slapšak, A.; Raspor, S.; Lukanc, B.; Pipan, M.Z. Canine Neonatal Assessment by Vitality Score, Amniotic Fluid, Urine, and Umbilical Cord Blood Analysis of Glucose, Lactate, and Cortisol: Possible Influence of Parturition Type? Animals 2022, 12, 1247. https://doi.org/10.3390/ani12101247
Plavec T, Knific T, Slapšak A, Raspor S, Lukanc B, Pipan MZ. Canine Neonatal Assessment by Vitality Score, Amniotic Fluid, Urine, and Umbilical Cord Blood Analysis of Glucose, Lactate, and Cortisol: Possible Influence of Parturition Type? Animals. 2022; 12(10):1247. https://doi.org/10.3390/ani12101247
Chicago/Turabian StylePlavec, Tanja, Tanja Knific, Aleksandra Slapšak, Sara Raspor, Barbara Lukanc, and Maja Zakošek Pipan. 2022. "Canine Neonatal Assessment by Vitality Score, Amniotic Fluid, Urine, and Umbilical Cord Blood Analysis of Glucose, Lactate, and Cortisol: Possible Influence of Parturition Type?" Animals 12, no. 10: 1247. https://doi.org/10.3390/ani12101247
APA StylePlavec, T., Knific, T., Slapšak, A., Raspor, S., Lukanc, B., & Pipan, M. Z. (2022). Canine Neonatal Assessment by Vitality Score, Amniotic Fluid, Urine, and Umbilical Cord Blood Analysis of Glucose, Lactate, and Cortisol: Possible Influence of Parturition Type? Animals, 12(10), 1247. https://doi.org/10.3390/ani12101247







