Changes in Fatty Acids Profile, Health Indices, and Physical Characteristics of Organic Eggs from Laying Hens at the Beginning of the First and Second Laying Cycles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Egg Quality and Fatty Acid Analyses
2.3. Calculations
2.4. Statistical Analysis
3. Results
3.1. Egg Quality Characteristics
3.2. Fatty Acid Composition and Indices
4. Discussion
4.1. Egg Quality Characteristics
4.2. Fatty Acid Composition and Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krunt, O.; Zita, L.; Kraus, A.; Okrouhlá, M.; Chodová, D.; Stupka, R. Guinea fowl (Numida meleagris) eggs and free-range housing: A convenient alternative to laying hens’ eggs in terms of food safety? Poult. Sci. 2021, 100, 101006. [Google Scholar] [CrossRef]
- Rey, A.I.; De-Cara, A.; Rebolé, A.; Arija, I. Short-Term Spirulina (Spirulina platensis) supplementation and Laying Hen Strain Effects of Eggs’ Lipid Profile and Stability. Animals 2021, 11, 1944. [Google Scholar] [CrossRef] [PubMed]
- Abo Ghanima, M.M.; Elsadek, M.F.; Taha, A.E.; El-Hack, A.; Mohamed, E.; Alagawany, M.; Ahmed, B.M.; Elshafie, M.M.; El-Sabrout, K. Effect of housing system and rosemary and cinnamon essential oils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zita, L.; Tůmová, E.; Štolc, L. Effects of genotype, age and their interaction on egg quality in brown-egg laying hens. Acta Vet. Brno 2009, 78, 85–91. [Google Scholar] [CrossRef]
- Kraus, A.; Zita, L.; Krunt, O.; Härtlová, H.; Chmelíková, E. Determination of selected biochemical parameters in blood serum and egg quality of Czech and Slovak native hens depending on the housing system and hen age. Poult. Sci. 2021, 100, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Maertens, L.; Ampe, B.; Buyse, J.; Kempen, I.; Zoons, J.; Delezie, E. Changes in egg quality traits during the last phase of production: Is there potential for an extended laying cycle? Br. Poult. Sci. 2016, 57, 842–847. [Google Scholar] [CrossRef]
- Windhorst, H.W. EU egg production since the exit from conventional cages: Housing systems affect the volume of production. Lohmann Inf. 2019, 53, 4–7. [Google Scholar]
- Sokołowicz, Z.; Krawczyk, J.; Dykiel, M. Effect of alternative housing system and hen genotype on egg quality characteristics. Emir. J. Food Agric. 2018, 30, 695–703. [Google Scholar] [CrossRef]
- Huopalahti, R.; López, F.R.; Anton, M.; Schade, R. Bioactive Egg Compounds; Springer: Berlin/Heidelberg, Germany, 2007; 298p. [Google Scholar]
- Ziemlański, Ś. Fats in human nutrition. Żyw. Człow. Metab. 1997, 24, 35–48. [Google Scholar]
- Pisulewski, P. Wartość odżywcza jaj kurzych oraz współczesne metody jej kształtowania. In Jajczarstwo, Nauka, Technologia, Praktyka; Akademii Rolniczej: Wrocław, Poland, 2000; pp. 189–217. [Google Scholar]
- Chow, C.K. Fatty Acids in Foods and Their Health Implications, 3rd ed.; CRC Press: New York, NY, USA, 2007; 1296p. [Google Scholar]
- Sauerwald, T.U.; Hachey, D.L.; Jensen, C.L.; Chen, H.; Anderson, R.E.; Heird, W.C. Intermediates in endogenous synthesis of C22:6 omega 3 and C20:4 omega 6 by term and preterm infants. Ped. Res. 1997, 41, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Poławska, E.; Horba ńczuk, J.O.; Pierzchała, M.; Strzałkowska, N.; Jóźwik, A.; Wójcik, A.; Pomian owski, J.; Gutkowska, K.; Wierzbicka, A.; Hoffman, L.C. Effect of dietary linseed and rapeseed supplementation on fatty acid profiles in the ostrich. Part 1. Muscles. Anim. Sci. Pap. Rep. 2013, 31, 239–248. [Google Scholar]
- Lopez-Bote, C.J.; Arias, R.S.; Rey, A.I.; Castano, A.; Isabel, B.; Thos, J. Effect of free-range feeding on n? 3 fatty acid and ?-tocopherol content and oxidative stability of eggs. Anim. Feed Sci. Technol. 1998, 72, 33–40. [Google Scholar] [CrossRef]
- De Caterina, R. n–3 Fatty acids in cardiovascular disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.Y.; Hu, X.; Zhang, F.; Chen, J.; Gao, Y. Omega-3 polyunsaturated fatty acids in the brain: Metabolism and neuroprotection. Front. Biosci. 2011, 16, 2653–2670. [Google Scholar] [CrossRef] [Green Version]
- Yannakopoulos, A.L.; Tserveni-Gousi, A.S. Quality characteristics of quail eggs. Br. Poult. Sci. 1986, 27, 171–176. [Google Scholar] [CrossRef]
- Folch, J.M.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Okrouhlá, M.; Stupka, R.; Čítek, J.; Šprysl, M.; Brzobohatý, L. Effect of dietary linseed supplementation on the performance, meat quality, and fatty acid profile of pigs. Czech J. Anim. Sci. 2013, 58, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.E.; Tharrington, J.B.; Curtis, P.A.; Jones, F.T. Shell characteristics of eggs from historic strains of single comb white leghorn chickens and relationship of egg shape to shell strength. Int. J. Poult. Sci. 2004, 3, 17–19. [Google Scholar] [CrossRef]
- Haugh, R.R. The Haugh unit for measuring egg quality. US Poult. Mag. 1937, 43, 552–573. [Google Scholar]
- Heiman, V.; Carver, J.S. The albumen index as a physical measurement of observed egg quality. Poult. Sci. 1936, 15, 141–148. [Google Scholar] [CrossRef]
- Cicek, T.; Kartalkanat, A. Comparason of village eggs and commercial eggs in terms of egg quality. J. Anim. Vet. Adv. 2009, 8, 2542–2545. [Google Scholar]
- Dottavio, A.M.; Canet, Z.E.; Faletti, C.; Álvarez, M.T.; Font, M.T.; Di Masso, R.J. Yolk: Albumen ratio in experimental hybrid layers with different paternal genotype. Arch. Zootec. 2005, 54, 87–95. [Google Scholar]
- Thomson, B.K.; Hamilton, R.M.G.; Grunder, A.A. The relationship between laboratory measures of eggshell quality and breakage in commercial egg washing and candling equipment. Poult. Sci. 1985, 64, 901–909. [Google Scholar] [CrossRef]
- Ahmed, A.M.H.; Rodriguez-Navarro, A.B.; Vidal, M.L.; Gautron, J.; Garcia-Ruiz, J.M.; Nys, Y. Changes in eggshell mechanical properties, crystallographic texture and in matrix proteins induced by moult in hens. Br. Poult. Sci. 2005, 46, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Dutta, R.K. Egg quality traits of indigenous, exotic and crossbred chickens (Gallus domesticus L.) in Rajshahi, Bangladesh. J. Life Earth Sci. 2010, 5, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Petroni, M.L.; De Luca, P.P.; Andreoli, A.; Morini, P.; Jacopino, L.; Innocente, J.; Perriello, G. Use of Quality control indices in moderately hypocaloric Mediterranean diet for treatment of obesity. Diabetes Nutr. Metab. 2001, 14, 181–188. [Google Scholar]
- Arakawa, K.; Sagai, M. Species differences in lipid peroxide levels in lung tissue and investigation of their determining factors. Lipids 1986, 21, 769–775. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Fernández, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; de la Hoz, L. Fatty acid compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]
- Crosara, F.S.G.; Pereira, V.J.; Lellis, C.G.; Barra, K.C.; Santos, S.K.A.; Souza, L.C.G.M.; Morais, T.A.; Litz, F.H.; Limão, V.A.; Braga, P.F.S.; et al. Is the eggshell quality influenced by the egg weight or the breeder age? Braz. J. Poult. Sci. 2019, 21, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dunn, I.C. Long Life Layer; genetic and physiological limitations to extend the laying period. In Proceedings of the 19th European Symposium on Poultry Nutrition, Potsdam, Germany, 26–29 August 2013. [Google Scholar]
- Kowalska, E.; Kucharska-Gaca, J.; Kuźniacka, J.; Lewko, L.; Gornowicz, E.; Biesek, J.; Adamski, M. Egg quality depending on the diet with different sources of protein and age of the hens. Sci. Rep. 2021, 11, 2638. [Google Scholar] [CrossRef]
- Rizzi, C.; Chiericato, G.M. Organic farming production. Effect of age on the productive yield and egg quality of hens of two commercial hybrid lines and two local breeds. Ital. J. Anim. Sci. 2005, 4, 160–162. [Google Scholar] [CrossRef] [Green Version]
- Laudadio, V.; Tufarellim, V. Influence of substituting dietary soybean meal for dehulled-micronized lupin (Lupinus albus cv. Multitalia) on early phase laying hen’s production and egg quality. Livest. Sci. 2010, 140, 184–188. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Suri, M.F.K.; Ahmed, S.; Nasar, A.; Divani, A.A.; Kirmani, J.F. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med. Sci. Monit. 2006, 13, CR1–CR8. [Google Scholar]
- Kucukyilmaz, K.; Bozkurt, M.; Herken, E.N.; Cinar, M.; Cath, A.U.; Bintas, E.; Coven, F. Effects of rearing systems on performance, egg characteristics and immune response in two-layer hen genotype. Asian-Australas. J. Anim. Sci. 2012, 25, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, F.; Rahimi, F. Factors affecting quality and quantity of egg production in laying hens: A review. World Appl. Sci. J. 2011, 12, 372–384. [Google Scholar]
- Batkowska, J.; Drabik, K.; Brodacki, A.; Czech, A.; Adamczuk, A. Fatty acids profile, cholesterol level and quality of table eggs from hens fed with the addition of linseed and soybean oil. Food Chem. 2021, 334, 127612. [Google Scholar] [CrossRef]
- Lešić, T.; Krešić, G.; Cvetnić, L.; Petrović, M.; Pleadin, J. The influence of hen age on fatty acid composition of commercial eggs. Croat. J. Food Sci. Technol. 2017, 9, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Koppenol, A.; Delezie, E.; Aerts, J.; Willems, E.; Wang, Y.; Franssens, L.; Everaert, N.; Buyse, J. Effect of the ratio of dietary n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid on broiler breeder performance, egg quality, and yolk fatty acid composition at different breeder ages. Poult. Sci. 2014, 93, 564–573. [Google Scholar] [CrossRef]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary enrichment of eggs with omega-3 fatty acids: A review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Samman, S.; Kung, F.P.; Carter, L.M.; Foster, M.J.; Ahmad, Z.I.; Phuyal, J.L.; Petocz, P. Fatty acid composition of certified organic, conventional and omega-3 eggs. Food Chem. 2009, 116, 911–914. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016, 10, 2464–2471. [Google Scholar] [CrossRef]
- Laudadio, V.; Tufarelli, V. Growth performance and carcass and meat quality of broiler chickens fed diets containing micronized-dehulled peas (Pisum sativum cv. Spirale) as a substitute of soybean meal. Poult. Sci. 2010, 89, 1537–1543. [Google Scholar] [CrossRef]
- Vitina, I.I.; Cerina, S.; Jansons, J.; Karstina, V.; Daugavietis, M.; Polis, O.; Korica, A.; Anenkova, R.; Lujane, B. Functional poultry meat enriched with biologically active substances from neutral extractives obtained from Spruce Needles. Krmiva 2012, 5, 151–158. [Google Scholar]
- Rozbicka-Wieczorek, A.J.; Wiesyk, E.; Brzoska, F.; Sliwinski, B.; Kowalczyk, J.; Czauderna, M. Efficiency of fatty acids accumulation into breast muscles of chickens fed diets with lycopene, fish oil and different chemical selenium forms. Afr. J. Biotechnol. 2014, 13, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Nutrient Composition (%) |
|---|---|
| Crude protein | 17.00 |
| Crude fat | 5.00 |
| Crude fibre | 6.00 |
| Ash | 13.10 |
| Methionine | 0.32 |
| Lysine | 0.80 |
| Calcium | 3.80 |
| Phosphorus | 0.55 |
| Sodium | 0.16 |
| ME (MJ/kg) | 11.0 |
| FA 1 (% of total FA) | |
| C14:0 2 | 0.19 |
| C16:0 3 | 13.84 |
| C18:0 4 | 3.98 |
| C20:0 5 | 0.35 |
| C22:0 6 | 0.18 |
| C16:1 n-7 7 | 0.21 |
| C18:1 n-9 8 | 27.41 |
| C20:1 n-9 9 | 0.61 |
| C18:2 n-6 10 | 51.64 |
| C18:3 n-3 11 | 4.30 |
| C18:3 n-6 12 | 0.38 |
| C20:4 13 | 0.21 |
| SFA 14 | 18.07 |
| MUFA 15 | 27.41 |
| n-6 PUFA 16 | 52.02 |
| n-3 PUFA 17 | 4.30 |
| n-6/n-3 PUFA | 12.32 |
| Parameter | Laying Cycle | SEM 2 | p-Value | |
|---|---|---|---|---|
| 1st | 2nd | |||
| Egg weight (g) | 61.21 b ± 4.39 | 71.10 a ± 5.64 | 0.527 | 0.0001 |
| Egg shape index (%) | 78.69 a ± 2.20 | 76.16 b ± 3.38 | 0.232 | 0.0001 |
| Egg surface area (cm2) | 72.49 b ± 3.47 | 80.10 a ± 4.26 | 0.405 | 0.0001 |
| Egg volume (cm3) | 56.48 b ± 3.90 | 65.55 a ± 6.13 | 0.510 | 0.0001 |
| Albumen proportion (%) | 66.18 a ± 2.18 | 64.72 b ± 2.78 | 0.194 | 0.0001 |
| Albumen index (%) | 10.29 a ± 2.38 | 8.04 b ± 1.80 | 0.178 | 0.0001 |
| Haugh unit | 87.61 a ± 8.34 | 78.96 b ± 8.55 | 0.706 | 0.0001 |
| Yolk proportion (%) | 23.70 b ± 1.99 | 25.46 a ± 2.45 | 0.178 | 0.0001 |
| Yolk index (%) | 47.63 a ± 3.31 | 44.02 b ± 2.63 | 0.260 | 0.0001 |
| Yolk to albumen ratio | 0.36 b ± 0.05 | 0.40 a ± 0.05 | 0.004 | 0.0001 |
| Yolk colour (DSM scale fan) | 8.77 a ± 1.48 | 8.22 b ± 0.95 | 0.094 | 0.0037 |
| Shell proportion (%) | 10.12 a ± 0.80 | 9.83 b ± 0.90 | 0.064 | 0.0210 |
| Shell thickness (mm) | 0.338 ± 0.03 | 0.336 ± 0.03 | 0.002 | 0.6500 |
| Shell strength (N.cm−2) | 46.65 a ± 7.28 | 42.48 b ± 9.75 | 0.658 | 0.0014 |
| Shell reflectivity (%) | 28.48 b ± 4.52 | 30.49 a ± 5.01 | 0.363 | 0.0052 |
| Shell index (g.100 cm−2) | 8.53 ± 0.64 | 8.70 ± 0.71 | 0.051 | 0.0968 |
| Parameter | Laying cycle | SEM 3 | p-Value | |
|---|---|---|---|---|
| 1st | 2nd | |||
| Myristic (C14:0) | 0.35 ± 0.07 | 0.32 ± 0.05 | 0.0097 | 0.1894 |
| Palmitic (C16:0) | 26.95 a ± 1.34 | 25.70 b ± 1.00 | 0.2102 | 0.0019 |
| Margaric (C17:0) | 0.26 ± 0.08 | 0.24 ± 0.05 | 0.0103 | 0.4128 |
| Stearic (C18:0) | 9.64 a ± 1.08 | 8.93 b ± 0.61 | 0.1480 | 0.0144 |
| Behenic (C22:0) | 0.11 ± 0.25 | 0.02 ± 0.04 | 0.0291 | 0.1007 |
| Lignoceric (C24:0) | 1.02 a ± 1.00 | 0.18 b ± 0.48 | 0.1399 | 0.0017 |
| Parameter | Laying Cycle | SEM 3 | p-Value | |
|---|---|---|---|---|
| 1st | 2nd | |||
| Palmitoleic (C16:1) | 2.24 ± 0.62 | 2.38 ± 0.48 | 0.0871 | 0.4244 |
| Margaroleic (C17:1) | 0.09 b ± 0.06 | 0.17 a ± 0.05 | 0.0106 | 0.0003 |
| Oleic (C18:1) | 30.48 b ± 1.58 | 36.74 a ± 1.67 | 0.5626 | 0.0001 |
| Gadoleic (C20:1) | 0.30 ± 0.06 | 0.29 ± 0.06 | 0.0095 | 0.5436 |
| Parameter | Laying Cycle | SEM 3 | p-Value | |
|---|---|---|---|---|
| 1st | 2nd | |||
| Linoleic (C18:2) | 23.71 a ± 2.62 | 21.04 b ± 2.20 | 0.4341 | 0.0013 |
| γ-Linolenic (ω-6) (C18:3) | 0.13 a ± 0.02 | 0.09 b ± 0.04 | 0.0061 | 0.0002 |
| α-Linolenic (ω-3) (C18:3(9)) | 1.04 a ± 0.11 | 0.73 b ± 0.13 | 0.0310 | 0.0001 |
| Eicosadienoic (C20:2) | 0.33 a ± 0.07 | 0.22 b ± 0.04 | 0.0121 | 0.0001 |
| Eicosatrienoic (C20:3) | 0.22 a ± 0.05 | 0.13 b ± 0.03 | 0.0104 | 0.0001 |
| Arachidonic (C20:4) | 2.11 a ± 0.31 | 1.89 b ± 0.25 | 0.0470 | 0.0201 |
| Parameter | Laying cycle | SEM 2 | p-Value | |
|---|---|---|---|---|
| 1st | 2nd | |||
| SFA 3 (% of total FA 4) | 38.53 a ± 1.51 | 35.54 b ± 1.39 | 0.3300 | 0.0001 |
| MUFA 5 (% of total FA) | 33.26 b ± 1.90 | 39.69 a ± 1.76 | 0.5890 | 0.0001 |
| PUFA 6 (% of total FA) | 28.16 a ± 2.48 | 24.68 b ± 2.15 | 0.4570 | 0.0001 |
| n-6 PUFA 7 (% of total FA) | 25.95 a ± 2.45 | 23.03 b ± 2.14 | 0.4290 | 0.0003 |
| n-3 PUFA 8 (% of total FA) | 1.64 a ± 0.36 | 1.30 b ± 0.26 | 0.0560 | 0.0015 |
| MUFA/SFA | 0.86 b ± 0.06 | 1.12 a ± 0.07 | 0.0226 | 0.0001 |
| PUFA/SFA | 0.73 ± 0.09 | 0.70 ± 0.08 | 0.0132 | 0.1645 |
| n-6/n-3 PUFA | 16.56 ± 3.99 | 18.24 ± 3.35 | 0.5910 | 0.1587 |
| AI 9 | 0.46 a ± 0.04 | 0.42 b ± 0.03 | 0.0061 | 0.0002 |
| TI 10 | 1.07 a ± 0.08 | 0.99 b ± 0.05 | 0.0124 | 0.0007 |
| SI 11 | 0.60 a ± 0.05 | 0.54 b ± 0.03 | 0.0080 | 0.0001 |
| HI 12 | 2.08 b ± 0.17 | 2.31 a ± 0.15 | 0.0309 | 0.0001 |
| HHR 13 | 2.10 b ± 0.17 | 2.32 a ± 0.15 | 0.0310 | 0.0001 |
| PI 14 | 40.93 a ± 3.54 | 36.32 b ± 2.67 | 0.6131 | 0.0001 |
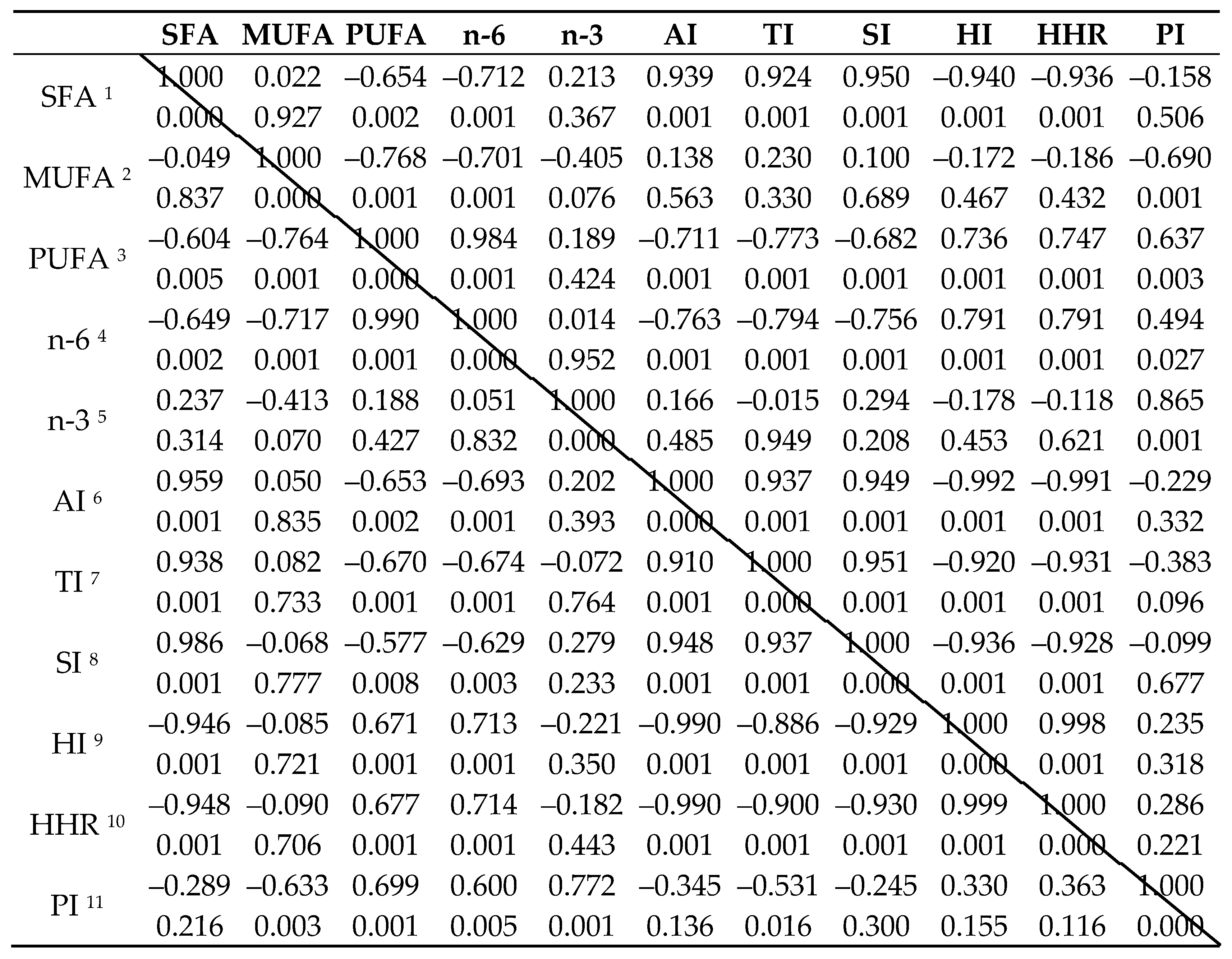 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zita, L.; Okrouhlá, M.; Krunt, O.; Kraus, A.; Stádník, L.; Čítek, J.; Stupka, R. Changes in Fatty Acids Profile, Health Indices, and Physical Characteristics of Organic Eggs from Laying Hens at the Beginning of the First and Second Laying Cycles. Animals 2022, 12, 125. https://doi.org/10.3390/ani12010125
Zita L, Okrouhlá M, Krunt O, Kraus A, Stádník L, Čítek J, Stupka R. Changes in Fatty Acids Profile, Health Indices, and Physical Characteristics of Organic Eggs from Laying Hens at the Beginning of the First and Second Laying Cycles. Animals. 2022; 12(1):125. https://doi.org/10.3390/ani12010125
Chicago/Turabian StyleZita, Lukáš, Monika Okrouhlá, Ondřej Krunt, Adam Kraus, Luděk Stádník, Jaroslav Čítek, and Roman Stupka. 2022. "Changes in Fatty Acids Profile, Health Indices, and Physical Characteristics of Organic Eggs from Laying Hens at the Beginning of the First and Second Laying Cycles" Animals 12, no. 1: 125. https://doi.org/10.3390/ani12010125
APA StyleZita, L., Okrouhlá, M., Krunt, O., Kraus, A., Stádník, L., Čítek, J., & Stupka, R. (2022). Changes in Fatty Acids Profile, Health Indices, and Physical Characteristics of Organic Eggs from Laying Hens at the Beginning of the First and Second Laying Cycles. Animals, 12(1), 125. https://doi.org/10.3390/ani12010125






